茶树根细胞壁不同组分对铅的吸附性能及其功能团的傅里叶红外光谱学研究
2015-03-11段德超于明革施积炎
王 梦,段德超,徐 辰,于明革,2,*,施积炎
1 浙江大学环境与资源学院, 杭州 310058 2 杭州市环境集团有限公司, 杭州 310022
茶树根细胞壁不同组分对铅的吸附性能及其功能团的傅里叶红外光谱学研究
王 梦1,段德超1,徐 辰1,于明革1,2,*,施积炎1
1 浙江大学环境与资源学院, 杭州 310058 2 杭州市环境集团有限公司, 杭州 310022
选择我国重要的经济作物之一茶树(CamelliasinensisL.)为研究对象,考察了茶树根细胞壁中不同多糖组分在吸附铅(Pb)过程中的作用差异以及与其发生交互作用的主要功能团。结果表明,在茶树根细胞壁吸附Pb过程中,绝大多数的Pb(68.42%)是吸附在纤维素以及木质素上;其次是果胶(20%)、半纤维素2类(5.26%);半纤维素1类的贡献可以忽略不计。同时,通过细胞壁不同组分吸附Pb前后的傅立叶红外光谱表征结果得出,在吸附Pb的过程中,果胶中起作用的功能团主要有羟基、羧基;半纤维素1类中起作用的功能团主要是羧基;半纤维素2类中起作用的功能团主要为羟基。
Pb毒; 细胞壁组分; 傅立叶红外; 功能团; 茶树
茶树(CamelliasinensisL.)是我国重要的经济作物之一,相关的研究报道表明,我国茶叶产品Pb含量已呈现逐年升高的趋势[1],是茶叶质量安全监控的重要指标之一[2-4]。在茶树根系吸收Pb的过程中,细胞壁是Pb离子跨膜进入细胞质的第一道屏障,在茶树吸收累积Pb的过程中起着重要的作用。康孟利等通过组分分级提取法研究发现,浙农117品种茶树根、叶细胞中的Pb主要分布在细胞壁中[5]。徐劼等采用亚细胞分离方法发现,龙井43和迎霜品种茶树根细胞壁累积的Pb高达细胞总含量的51.2%[6]。
细胞壁中存在金属离子,早在植物细胞与金属离子作用的研究初期就有所报道[7-11],近期的研究表明细胞壁也是积累金属离子的重要亚细胞组分之一[12-16],可以容纳大量的金属离子[15, 17-20]。植物细胞壁是由糖、蛋白质和芳香族化合物等组成,其中糖含量占到初生壁总量的90%[21],可见,糖类对细胞壁功能有着至关重要的作用。研究表明,在重金属胁迫下,细胞壁组分,如半纤维素、多糖、蛋白质、胼胝质等物质含量显著提高,从而增强了对重金属的吸附固定能力[22]。细胞壁中各种化学组分(果胶、纤维素、半纤维素、木质素、结构蛋白等)中的负电基团通过沉淀、吸附、络合等作用将金属离子固定在细胞壁中,从而减少金属离子通过跨膜运输进入原生质体,在一定程度上降低了金属胁迫对植物正常生理活动的干扰[23-24]。而细胞壁结合二价金属离子的能力取决于富含官能团羧基、羟基和巯基 的多糖数量[25-28]。然而目前关于植物重金属污染方面的研究工作大部分限于农作物、经济作物等生长期短的草本植物,关于木本植物也有关于红树植物的相关报道[29],但是针对茶树的研究则鲜见报道。
因此,本文选择木本植物茶树为研究对象,分别通过吸附动力学和傅立叶红外光谱表征,探究了茶树根细胞壁中不同多糖组分在吸附Pb过程中的作用差异以及与其发生交互作用的主要功能团,揭示茶树根系细胞壁组分吸收累积Pb的内在机制,从而阐明茶树根细胞壁Pb累积的适应性分子机制。
1 材料与方法
1.1 细胞壁的提取
试验所用样品来自浙江省新昌县某未污染茶园。选取植株高度在10 cm到20 cm之间长势均一的茶树幼苗,收集根部用去离子水冲洗后,根据Zhong和Lauchi的方法稍加修改提取细胞壁[30]。冰冻的根在研钵中加液氮磨碎,用冰乙醇(75%)浸提植物粉末,每次冰乙醇用量为10 mL/g根鲜重,浸提3次,混合物置于50 mL离心管,混匀,静置20 min后于4 ℃下1000 g离心10 min。沉淀物再用1∶7(根重(g)/体积(mL))的丙酮(4 ℃)、甲醇-三氯甲烷混合液(体积分数1∶1)及甲醇溶液依次洗涤,每次洗涤后,悬浮液在4 ℃下1000 g离心10 min。弃去上清液,沉淀冷冻干燥后,加液氮磨碎,作为粗细胞壁,保存于4 ℃备用。
1.2 细胞壁多糖组分的分离和测定
参照Zhong和Lauchi的方法进行部分修改,称取干燥的细胞壁样品,加入超纯水(5 mg干CW/1 mL提取剂),沸水浴1 h,然后17000 g离心10 min,取上清液,重复3次后认为果胶提取完全,上清液即为果胶提取组分;沉淀用去离子水冲洗2次,用4% NaOH(内含0.1% NaHB4)于室温下分3次提取,上清液即为半纤维素1类(HC1);沉淀用去离子水冲洗2次,用24% NaOH(内含0.1% NaHB4)于室温下分3次提取,上清液即为半纤维素2类(HC2)。
1.3 Pb的吸附动力学实验
本实验分4部分进行,其实验主体分别为茶树根细胞壁(CW)、细胞壁去果胶pectin(CW-pectin(CW-1))、细胞壁去果胶去半纤维素1类HC1(CW-pectin-HC1(CW-2))、细胞壁去果胶去半纤维素1类HC1去半纤维素2类HC2(CW-pectin-HC1-HC2(CW-3))。
吸附溶液为15 μmol/L Pb(NO3)2溶液,0.01 mol/L NaNO3溶液作为电解液(pH 5.0)。4个实验主体分别取0.05 g置于底部有滤纸的滤头中,滤头上下均有接口用以连接吸附溶液或收集流出液。吸附溶液用蠕动泵以8 mL/10 min的流速泵入小管中,经细胞壁后用自动收集器收集,每10 min收集1管,直到流出液中Pb浓度与吸附液中Pb浓度相同,本研究中约在400 min时达到吸附平衡。用原子吸收光谱仪AAS测定每管中Pb含量。动力学实验重复3次,取平均值做曲线,为防止图中曲线不清晰,误差线未在图中标出,误差均在10%之内。
1.4 细胞壁多糖组分的傅立叶红外光谱(FTIR)测定
利用傅立叶变换红外光谱仪(FTIR-IR Prestige-21 岛津)对Pb吸附前后的根细胞壁样品CW、CW-1、CW-2和CW-3进行红外光谱表征。分别称取1 mg冻干后根细胞壁样品,按1∶150的比例加入150 mg KBr于玛瑙研钵中充分研磨均匀,压片后放入FTIR样品工作室,在相同条件下测定红外光谱图。光谱分辨率为4 cm-1,记录样品在4000—500 cm-1范围内的红外光谱信号。
2 结果
2.1 细胞壁不同多糖组分对Pb的吸附动力学

图1 茶树根细胞壁不同组分对Pb2+的吸附动力学 Fig.1 Pb adsorption kinetics of different CW components in tea tree roots
图1是茶树根细胞壁不同组分对Pb2+的吸附曲线。从图中可以看出,茶树根细胞壁组分对Pb2+的吸附过程分为3个阶段:初期是快速过程,100 min后,吸附速率明显减慢,进入中速过程,200 min后进入慢速过程,吸附400 min后基本达到平衡。吸附平衡后,与CW相比去果胶后的CW-1吸附量降低了26.31%,可知果胶的吸附量占整个细胞壁吸附量的26.31%。同理可知,绝大多数的Pb(68.42%)是吸附在纤维素以及木质素上;HC2的吸附量占有率为5.26%;HC1的贡献可以忽略不计。
2.2 不同细胞壁组分的红外光谱分析
图2为对照组不同的茶树根细胞壁组分的FTIR图谱,自上而下,按顺序分别为茶树根CW、 CW-1、CW-2和CW-3。

图2 茶树根细胞壁不同组分对Pb2+的吸附动力学 Fig.2 The FTIR spectra of different CWcomponents in tea tree roots

根据FTIR谱图进行半定量分析,对每个谱图分别以2924 cm-1处—HC3中C—H特征吸收峰的吸光度(A2924)为基准,其他特征峰如3385 cm-1、1738 cm-1、1651 cm-1和1512 cm-1等处的吸光度A与A2924的比值,来半定量分析茶树根细胞壁不同组分的功能团含量差异。即可以通过A/A2924比值的变化来半定量分析官能团数目变化[32]。
茶树根细胞壁不同组分含量差异如表1和图2所示。由A/A2924比值可知,茶树根CW中含量最高的为纤维素多糖链C—C,其次为羟基、羧基和氨基。同CW相比,CW-1、CW-2和CW-3的A3385/A2924和A1258/A2924比值依次降低,由此可知茶树根细胞壁果胶、HC1和HC2中均含有一定量的羟基和羧基。其中,羟基和羧基均以纤维素中含量最多;羟基含量除纤维素以外以果胶中最多;其次为HC1和HC1;羧基在果胶、HC1和HC2 3个组分中含量相当。
表1 茶树根细胞壁不同组分(CW:细胞壁;CW-1:细胞壁去果胶;CW-2细胞壁去果胶去半纤维素1类;CW-3:细胞壁去果胶去半纤维素1和2类)红外光谱特征峰的半定量分析(峰的序号对应于图2)
Table 1 Semi_quantitative analysis of FTIR spectra of different CW components in tea tree roots (CW: cell wall,CW-1: CW-pectin, CW-2: CW-pectin-hemicellulose 1(HC1), CW-3: CW-pectin-HC1-HC2)

序号Number官能团FunctiongroupCW波数/cm-1WavenumberA/A2924细胞壁不同组分CWcomponentsCW-1CW-2吸光度比值A/A2924A/A2924CW-3A/A29241羟基/氨基—OH/—NH33851.891.391.231.082甲基—CH3292411113酯基C O17380.790.840.670.714酰胺1带C—N16511.460.791.040.995酰胺2带N—N15121.081.021.041.026羧酸盐C O不对称收缩震动14271.120.981.051.057硫酸盐或羧基或磷酸盐12581.181.11.050.988多糖链C—C10572.851.71.591.33
A: 吸光度Adsorption
2.3 不同多糖组分在吸附Pb过程中起主要作用的官能团
FTIR谱图可以表征那些能够离子化的基团如羧基、羟基、氨基等在重金属吸附过程中的作用。当官能团参与金属结合时,其吸收峰会发生偏移,因此可以通过峰位的位移来判断参与Pb结合的官能团。例如,谷壳吸附Pb2+以后,由于取代了部分羟基中的氢,引起羟基伸缩振动峰的位置向高波数移动[33]。
茶树根细胞壁不同组分在Pb2+处理前后FTIR谱图(图3—图6)和表2所示。CW的羟基伸缩振动峰位与对照组相比向高频位移动了23 cm-1,CW-1和CW-2分别移动了10 cm-1和11 cm-1,CW-3移动了4 cm-1,可见羟基的作用随着果胶和HC2的去除是依次减弱。Pb处理后CW和CW-1的羧基分别向低频位移动了5 cm-1和7 cm-1,而随着果胶和HC1的去除这种变化也相应减弱。处理后的CW-1和CW-2的蛋白质氨基中的C—N分别向低频位移动了6 cm-1和4 cm-1同时CW-3中蛋白质氨基中的N—N向高频位移动了10 cm-1,可见随着果胶的去除CW中蛋白质氨基的作用也随之加强。处理后的CW-2和CW-3的糖类C—C伸缩振动峰位变化显著增强,分别移动了-8 cm-1和19 cm-1,说明随着果胶、HC1和HC2的顺次去除,它们的作用逐渐突显出来。
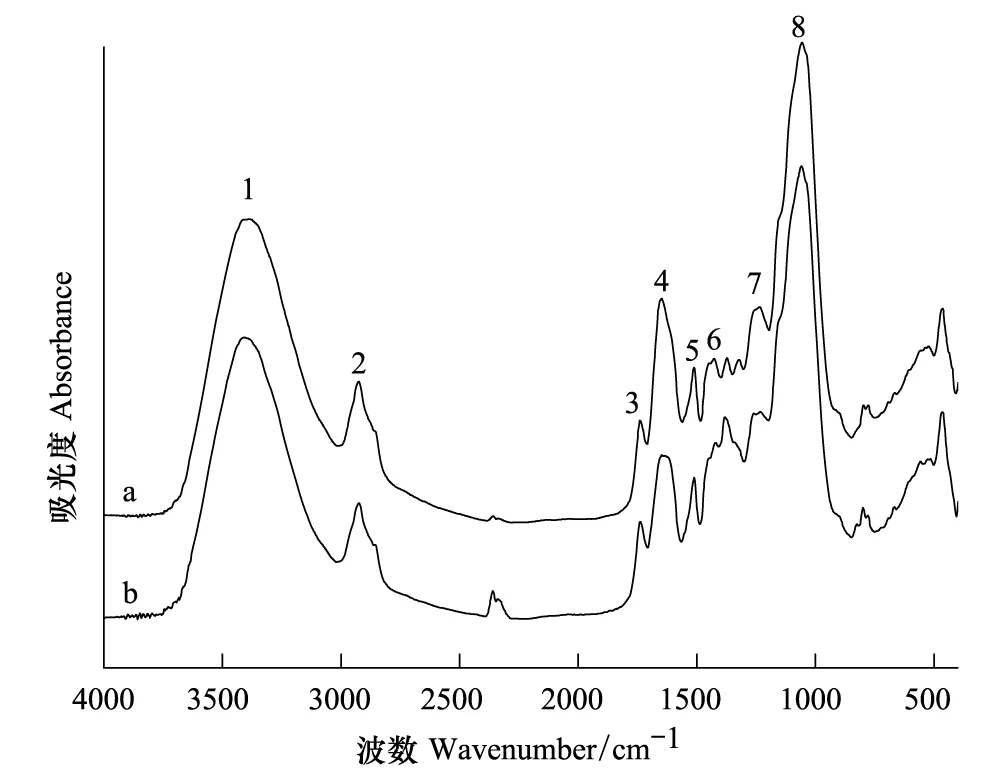
图3 细胞壁(CW)Pb2+处理前(a)后(b)的红外光谱图Fig.3 The FTIR spectra of cell wall (CW) before (a) and after (b) Pb2+adsorption
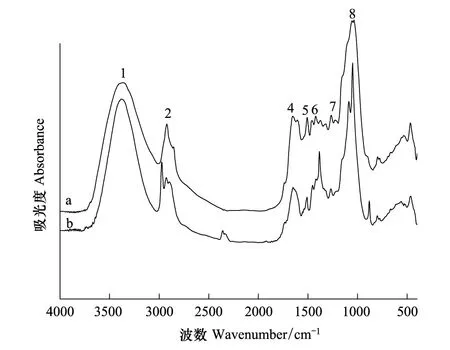
图5 细胞壁去果胶去半纤维素1类(CW-2)Pb2+处理前(a)后(b)的红外光谱图Fig.5 The FTIR spectra of CW-pectin —hemicellulose 1 (HC1) (CW-2) before (a) and after (b) Pb2+ adsorption
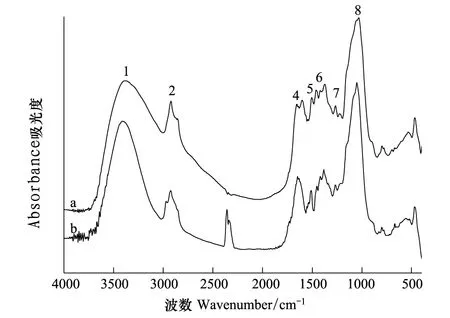
图6 细胞壁去果胶去半纤维素1和2类(CW-3)Pb2+处理前(a)后(b)的红外光谱图Fig.6 The FTIR spectra of CW-pectin —HC1-HC2 (CW -3) before (a) and after (b) Pb2+ adsorption
综上所述,在吸附Pb的过程中,果胶中起作用的功能团主要有羟基、羧基;HC1中起作用的功能团主要是羧基;HC2起作用的功能团主要是羟基。纤维素和木质素中起作用的官能团有纤维素多糖链中的C—C以及蛋白质氨基中的N—N。但在完整CW吸附Pb的过程中,它们并无明显变化。推测是在当果胶、HC1和HC2去除后由于羟基和羧基的减少使得残留的蛋白质中的氨基和纤维素糖链中的C—C成为除羟基外重要的Pb结合位点。
表2 茶树根细胞壁不同组分(CW:细胞壁;CW-1:细胞壁去果胶;CW-2细胞壁去果胶去半纤维素1类(HC1);CW-3:细胞壁去果胶去半纤维素1和2类)吸附Pb2+前后红外光谱表征(峰的序号对应于图2—图5)
Table 2 FTIR spectra of different CW components in tea tree roots (CW: cell wall,CW-1: cell wall-pectin, CW-2: CW-pectin-hemicellulose 1(HC1), CW-3: CW-pectin-HC1-HC2 before and after Pb2+adsorption)

序号Number官能团Functiongroup对照CW Pb处理CWCk-CWPb-CW波数cm-1Wavenumber偏移量cm-1Offset对照CW-1Pb处理CW-1Ck-CW-1Pb-CW-1波数cm-1Wavenumber偏移量cm-1Offset对照CW-2Pb处理CW-2Ck-CW-2Pb-CW-2波数cm-1Wavenumber偏移量cm-1Offset对照CW-3 Pb处理CW-3Ck-CW-3 Pb-CW-3波数cm-1Wavenumber偏移量cm-1Offset1羟基/氨基—OH/—NH3385340823338533951033663377113389339342甲基—CH329262924-229242922-229222928629242926-23酯基C O17381738017381734-4------4酰胺1带C—N16471647016531647-616531649-41653164725酰胺2带N—N15121512015101510015081510215101420106羧酸盐C O不对称收缩振动14271418-914271420-714221420-21427137527硫酸盐或羧基或磷酸盐1258126021260126111265126941260126128多糖链C—C10571057210571057010571049-81057105719
表中“-”表示未检出; 表中 Ck(control chech)-CW、Ck -CW-1、Ck -CW-2、Ck -CW-3分别表示对照处理的CW、CW-1、 CW-2、CW-3; 表中 Pb-CW、Pb-CW-1、Pb-CW-2、Pb-CW-3分别表示加Pb处理的CW、CW-1、 CW-2、CW-3
3 讨论
细胞壁多糖主要包含4个组分,分别为果胶、HC1、HC2和纤维素。通过Pb的吸附动力学实验表明,在茶树根细胞壁中,绝大多数的Pb(68.42%)吸附在纤维素以及木质素上。金属离子可以与细胞壁高分子物质结合在1987年就有报道,Nishizono等人发现在禾秆蹄盖蕨根系细胞壁中的Cu、Zn和Cd含量占整个植株累积量的70%—90%,并且认为它们中的大部分是与纤维素以及木质素结合,以结合状态存在[34]。茶树根细胞壁果胶中Pb的吸附量占整个CW的26.31%,可见在茶树根细胞壁吸附Pb的过程中,果胶是除纤维素外起最重要作用的多糖组分。与本研究结论相似,Zheng等人(2004)报道细胞壁中的果胶是细胞壁阳离子结合的主要位点之一[35]。目前对于果胶研究较多的是与Al3+[36]、Cd2+[37]、Cu2+[38]以及其他金属阳离子[11, 39]的结合作用。除果胶和纤维素外,半纤维素也起到了一定的作用,其中HC2的贡献率为5.26%,HC1的贡献则可以忽略不计。
对茶树根细胞壁各个多糖组分FTIR谱图进行半定量分析得知,茶树根细胞壁中含量最高的为纤维素多糖链C—C,其次依次为羟基、羧基和氨基。对比各个多糖组分所含的功能团差异,羟基和羧基均以纤维素中含量最多。在果胶、HC1和HC2中,果胶中羟基含量最多,其次是HC1和HC2;羧基含量在此三个组分中差异不大。由此可见,果胶、HC1、HC2和纤维素均有吸附Pb2+的潜力,而果胶是除纤维素外最具潜力的多糖组分。
研究表明,细胞壁结合二价金属离子的能力取决于富含官能团羧基,羟基和巯基的多糖数量[25-28]。结合吸附Pb前后的CW和CW-3的FTIR谱图结果可知,在纤维素中含有丰富的羟基基团,在其吸附Pb的过程中,并没有发挥显著的作用。这是由于纤维素是由1,4-糖苷键组成的直链多糖,这种高分子结构上有大量的羟基存在,这使其分子链间和分子链的内部形成了氢键,这种羟基覆盖的结构影响了其反应活性[40]。同时发现在CW-3组分(纤维素、木质素和蛋白质)吸附Pb的过程中纤维素多糖链中的C—C以及蛋白质氨基中的N-N作用显著,但是在完整CW吸附Pb的过程中它们并没有发挥作用。推测当果胶、HC1和HC2去除后由于羟基和羧基的减少,使得纤维素和木质素中残留的蛋白质中的氨基[41]和纤维素糖链中的C-C成为除羟基外重要的Pb结合位点。在CW吸附Pb的过程中,纤维素中富含的羟基、羧基等功能团与Pb的交互作用并不显著,由此可知纤维素对Pb的主要吸附作用并不是离子交换、螯合等化学吸附作用。而从物理结构来看纤维素是一种纤维状多毛细管的分子聚合物,具有多孔和比表面积大的特点,具有吸附金属离子的能力。因此推测在完整CW中纤维素对Pb的吸附作用是以物理吸附为主。然而,另外3种多糖组分都存在与Pb发生明显交互作用功能团。其中,果胶中起作用的功能团主要有羟基、羧基;HC1中起作用的功能团主要是羧基;HC2起作用的功能团主要是羟基。研究表明,果胶是胞间层以及初生细胞壁的主要组分之一,含有很多负电基团,如羟基、羧基、醛基、氨基等[23]能结合多种金属离子。其基本化学成分主要有同型半乳糖醛酸聚糖(Homogalacturonan(HGA)),鼠李半乳糖醛酸聚糖Ⅰ(RhamnogalacturonanⅠ(RGⅠ))和鼠李半乳糖醛酸聚糖Ⅱ(RhamnogalacturonanⅡ(RGⅡ))。其中绑定二价和三价金属离子的主要成分是HGA。研究表明在低甲酯化的HGA中,两个自由羟基间会互通形成钙离子通道,被称为蛋壳结构[42]从而导致,钙凝胶形成和细胞壁的硬化[21, 43]。通过金属离子与果胶的结合能力 Al3+> Cu2+> Pb2+> Zn2+= Ca2+或者 Cu2+= Pb2+> Cd2+= Zn2+> Ca2+[25]可以发现Pb2+的结合能力要强于Ca2+,所以Ca2+可以被 二价的Pb2+取代[25]。同时Pb2+也可以直接取代羟基中的氢,引起羟基伸缩振动峰的移动[33]。研究表明,半纤维素的主要成分之一阿拉伯木聚糖链上富含葡萄糖残基结构,这使其带一定的负电荷[44]对金属阳离子具有一定的吸附能力。另外,Marcus等发现,果胶中的HGA成分的存在掩盖了半纤维素的抗原决定簇[45],从而可能掩盖了半纤维素上的Pb结合位点,随着果胶的去除半纤维素上的结合位点得到了有效的暴露,从而可以结合一定量的Pb。因此,推测在CW中Pb2+主要是通过离子交换、螯合等化学吸附作用与果胶以及半纤维素结合。在草本植物根系细胞壁中,与金属离子发生作用的多糖组分主要是果胶。果胶对于金属阳离子的作用最早发现于笋瓜(Cucurbitamaxima) 根系中[46],果胶与铝耐性的关系也一直备受关注[47-48],并且Kasia等发现拟南芥(Arabidopsisthaliana)中果胶对Pb2+作用显著[49]。
综上所述,与绝大多数草本植物不同,茶树根细胞壁是以纤维素和木质素在吸附总量上作用最为突出。其次是果胶,起作用的功能团主要有羟基和羧基。半纤维素2类也起到一定吸附作用,有效功能团主要为羟基;而半纤维素1类的作用可以忽略不计。
[1] 韩文炎, 韩国柱, 蔡雪雄. 茶叶铅含量现状及其控制技术研究进展. 中国茶叶, 2008, 30(3): 16-17.
[2] Han W Y, Zhao F J, Shi Y Z, Ma L F, Ruan J Y. Scale and causes of lead contamination in Chinese tea. Environmental Pollution, 2006, 139(1): 125-132.
[3] 陈宗懋, 阮建云, 蔡典雄, 章力建. 茶树生态系中的立体污染链与阻控. 中国农业科学, 2007, 40(5): 948-958.
[4] Karak T, Bhagat R M. Trace elements in tea leaves, made tea and tea infusion: A review. Food Research International, 2010, 43(9): 2234-2252.
[5] 康孟利, 薛旭初, 骆耀平, 陈惠云, 石元值, 马立峰, 韩文炎. 茶树与土壤中铅的存在形态与分布. 浙江农业科学, 2006, (3): 280-282.
[6] 徐劼, 于明革, 陈英旭, 傅晓萍, 段德超. 铅在茶树体内的分布及化学形态特征. 应用生态学报, 2011, 22(4): 891-896.
[7] Malone C, Koeppe D E, Miller R J. Localization of lead accumulated by corn plants. Plant Physiology, 1974, 53(3): 388-394.
[8] Wozny A, Zatorska B, Mlodzianowski F. Influence of lead on the development of lupin seedllings and ultrastructural localization of this metal in the roots. Acta Societatis Botanicorum Poloniae, 1982, 51(3/4): 345-351.
[9] Wierzbicka M. Lead in the apoplast ofAlliumcepaL. root tips-ultrastructural studies. Plant Science, 1998, 133(1): 105-119.
[10] Samardakiewicz S, Woz′ny A. The distribution of lead in duckweed (LemnaminorL.) root tip. Plant and Soil, 2000, 226(1): 107-111.
[11] Neumann D, zur Nieden U. Silicon and heavy metal tolerance of higher plants. Phytochemistry, 2001, 56(7): 685-692.
[12] Wójcik M, Vangronsveld J, D′Haen J, Tukiendorf A. Cadmium tolerance inThlaspicaerulescens-II. Localization of cadmium inThlaspicaerulescens. Environmental and Experimental Botany, 2005, 53(2): 163-171.
[13] Islam E, Yang X E, Li T Q, Liu D, Jin X F, Meng F H. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes ofElsholtziaargyi. Journal of Hazardous Materials, 2007, 147(3): 806-816.
[14] Malecka A, Piechalak A, Morkunas I, Tomaszewska B. Accumulation of lead in root cells ofPisumsativum. Acta Physiologiae Plantarum, 2008, 30(5): 629-637.
[15] Konno H, Nakashima S, Katoh K. Metal-tolerant mossScopelophilacataractaeaccumulates copper in the cell wall pectin of the protonema. Journal of Plant Physiology, 2010, 167(5): 358-364.
[16] Meyers D E R, Kopittke P M, Auchterlonie G J, Webb R I. Characterization of lead precipitate following uptake by roots of brassica juncea. Environmental Toxicology and Chemistry, 2009, 28(11): 2250-2254.
[17] Kopittke P M, Asher C J, Blamey F P C, Auchterlonie G J, Guo Y N, Menzies N W. Localization and chemical speciation of Pb in roots of signal grass (Brachiariadecumbens) and Rhodes grass (Chlorisgayana). Environmental Science and Technology, 2008, 42(12): 4595-4599.
[18] Kopittke P M, Asher C J, Kopittke R A, Menzies N W. Toxic effects of Pb2+on growth of cowpea (Vignaunguiculata). Environmental Pollution, 2007, 150(2): 280-287.
[19] Krzeslowska M, Lenartowska M, Mellerowicz E J, Samardakiewicz S, Woz′ny A. Pectinous cell wall thickenings formation-A response of moss protonemata cells to lead. Environmental and Experimental Botany, 2009, 65(1): 119-131.
[20] Krzeslowska M, Lenartowska M, Samardakiewicz S, Bilski H, Woz′ny A. Lead deposited in the cell wall of Funaria hygrometrica protonemata is not stable -A remobilization can occur. Environmental Pollution, 2010, 158(1): 325-338.
[21] Gibeaut D M, Carpita N C. Glucan synthesis in membranes fromZeamaysandGlycinemax: interaction of ER and Golgi membranes. Plant Physiology, 1993, 102(1): 51-51.
[23] Haynes R J. Ion exchange properties of roots and ionic interactions within the root apoplasm: their role in ion accumulation by plants. Botanical Review, 1980, 46(1): 75-99.
[24] Allan D L, Jarrell W M. Proton and copper adsorption to maize and soybean root cell walls. Plant Physiology, 1989, 89(3): 823-832.
[25] Dronnet V M, Renard C M G C, Axelos M A V, Thibault J F. Characterisation and selectivity of divalent metal ions binding by citrus and sugar beet pectins. Carbohydrate Polymers, 1996, 30(4): 253-263.
[26] Kartel M T, Kupchik L A, Veisov B K. Evaluation of pectin binding of heavy metal ions in aqueous solutions. Chemosphere, 1999, 38(11): 2591-2596.
[27] Davis T A, Volesky B, Mucci A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Research, 2003, 37(18): 4311-4330.
[28] Pelloux J, Rustérucci C, Mellerowicz E J. New insights into pectin methylesterase structure and function. Trends in Plant Science, 2007, 12(6): 267-277.
[29] 张凤琴, 王友绍, 殷建平, 董俊德. 红树植物抗重金属污染研究进展. 云南植物研究, 2005, 27(3): 225-231.
[30] Zhong H L, Lauchli A. Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. Journal of Experimental Botany, 1993, 44(4): 773-778.
[31] 潘燕飞. 傅立叶红外光谱法用于茶叶品质的鉴定. 烟台大学学报: 自然科学与工程版, 2008, 21(4): 266-272.
[32] 张晓斌, 刘鹏, 李丹婷, 徐根娣, 蒋敏姣. 铬诱导植物根细胞壁化学成分变化的FTIR表征. 光谱学与光谱分析, 2008, 28(5): 1067-1070.
[33] 韩润平, 等. 化学改性与吸附Pb离子前后谷壳的红外光谱分析比较. 南昌大学学报: 理科版, 2006,30:1304-1306.
[34] Nishizono H, Ichikawa H, Suziki S, Ishii F. The role of the root cell wall in the heavy metal tolerance ofAthyriumyokoscense. Plant and Soil, 1987, 101(1): 15-20.
[35] Zheng S J, Lin X Y, Yang J L, Liu Q, Tang C X. The kinetics of aluminum adsorption and desorption by root cell walls of an aluminum resistant wheat (TriticumaestivumL.) cultivar. Plant and Soil, 2004, 261(1/2): 85-90.
[36] Schmohl N, Horst W J. Cell wall pectin content modulates aluminium sensitivity ofZeamays(L.) cells grown in suspension culture. Plant, Cell and Environment, 2000, 23(7): 735-742.
[37] Lozano-Rodriguez E, Hernandez L E, Bonay P, Carpena-Ruiz R O. Distribution of cadmium in shoot and root tissues of maize and pea plants: Physiological disturbances. Journal of Experimental Botany, 1997, 48(306): 123-128.
[38] Konno H, Nakato T, Nakashima S, Katoh K.Lygodiumjaponicumfern accumulates copper in the cell wall pectin. Journal of Experimental Botany, 2005, 56(417): 1923-1931.
[39] Bringezu K, Lichtenberger O, Leopold I, Neumann D. Heavy metal tolerance ofSilenevulgaris. Journal of Plant Physiology, 1999, 154(4): 536-546.
[40] 郑庆锋, 郑建军, 陈小娟. 新型螯合纤维对重金属离子吸附性能的研究. 水处理技术, 2004, 30(4): 211-212, 223-223.
[41] Neumann D, Nieden U Z, Lichtenberger O, Leopold I. How doesArmeriamaritmatolerate high heavy metal concentrations? Journal of Plant Physiology, 1995, 146(5/6): 704-717.
[42] Grant G T, Morris E R, Rees D A, Smith P J C, Thom D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Letters, 1973, 32(1): 195-198.
[43] Caffall K H, Mohnen D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydrate Research, 2009, 344(14): 1879-1900.
[44] Carpita N C, Gibeaut D M. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant Journal, 1993, 3(1): 1-30.
[45] Marcus S E, Verhertbruggen Y, Hervé C, Ordaz-Ortiz J J, Farkas V, Pedersen H L, Willats W G T, Knox J P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BioMed Centrol Plant Biology, 2008, 8: 60-60.
[46] Le Van H, Kuraishi S, Sakurai N. Aluminum-induced rapid root inhibition and changes in cell-wall components of squash seedlings. Plant Physiology, 1994, 106(3): 971-976.
[47] Chang Y C, Yamamoto Y, Matsumoto H. Accumulation of aluminium in the cell wall pectin in cultured tobacco (NicotianatabacumL.) cells treated with a combination of aluminium and iron. Plant, Cell and Environment, 1999, 22(8): 1009-1017.
[48] Eticha D, Stass A, Horst W J. Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant Cell and Environment, 2005, 28(11): 1410-1420.
[49] Polec′-Pawlak K, Ruzik R, Lipiec E, Ciurzyńska M, Gawrońska H. Investigation of Pb(II) binding to pectin inArabidopsisthaliana. Journal of Analytical Atomic Spectrometry, 2007, 22(8): 968-972.
Adsorption ability of cell wall (CW) components in roots of Tea Plant (CamelliasinensisL.) to Pb and FTIR spectra of their functional groups
WANG Meng1,DUAN Dechao1,XU Chen1,YU Mingge1,2,*,SHI Jiyan1
1CollegeofEnvironmentandResource,ZhejiangUniversity,Hangzhou310058,China2HangzhouEnvironmentalGroupCompanyLimited,Hangzhou310022,China
Tea is one of the most important economic crops in China and the relevant research suggests that every year, Pb content in tea products has shown an increasing trend. Pb is also one of the most important indicators of quality and safety monitoring. Recent, research mostly focused on the resistance of plants to heavy metals was limited to short growing herbs. Reports on the study of woody plants are very rare. On the basis of these conditions tea tree was selected as research work. The objective of the present study is to find out the molecular mechanism of Pb stress resistance in roots CW of tea plants.Tea trees grown in the clean tea garden were selected for our experimental design. Tea roots were collected and washed with deionized water. Then extraction of crude CW and subsequent fractionation of CW components were carried out. Adsorption kinetics was carried out to determine the adsorption ability of different CW components to Pb stress. A total of 5 mg of CW materials or its corresponding residues was placed into a 2-ml column equipped with a filter at the bottom.The solution consisted of 15 μmol/L Pb(NO3)2in 0.01 mol/L NaNO3at pH 5.0.The solution was sipped by a peristaltic pump set a speed of 8 ml per 10 min after running through a 2-ml column holding the CW samples. The adsorption solutions were collected at 10-min intervals and Pb in the adsorption solutions was measured by Atomic Absorption Spectroscopy (AAS). At last the Fourier Transform infrared spectroscopy (FTIR) spectra of different CW components before and after Pb2+adsorption was carried out to study the difference in functional groups those can interact with Pb between different CW components.The results of this study shown that the vast majority of Pb (68.42%) adsorbed in the cellulose and lignin,followed by pectin (20%) and hemicellulose2 (HC2) (5.26%).While, the contribution of HC1 was negligible. These results indicated that in the CW of roots cellulose and lignin has a greater ability to accumulate Pb as compared to pectin, HC1 and HC2. But the adsorption capacity of pectin was also very considerable. According to the FTIR spectra of CW-pectin-HC1-HC2 (CW-3) before and after Pb2+adsorption it was found that although the cellulose and lignin contain a mass of —OH and —COOH, when they adsorbed Pb, their characteristic peaks′ positions had no obvious changes. However, the positions of characteristic peaks′ of C—C in cellulose polysaccharide and N—N in protein amino changed significantly, but these didn′t appear at the complete CW. In summary, it was found that when CW adsorbed Pb all these functional groups in cellulose and lignin had hardly interacted with Pb. However, it was different in pectin, HC1 and HC2. For instance in pectin, when CW adsorbed Pb, —OH and —COOH characteristic peaks′ positions changed significantly. It can be indicated that Pb was fixed through interactions with functional groups like —OH and —COOH in pectin. The same approach had yielded the main functional groups in HC1 and HC2 was —COOH and —OH respectively.
lead toxicity; cell wall components; FTIR; functional groups; tea tree
国家自然科学基金(41201319); 新世纪优秀人才支持计划(NCET-11-0455)
2013-05-29;
日期:2014-04-25
10.5846/stxb201305291222
*通讯作者Corresponding author.E-mail: mgyu_369@163.com
王梦,段德超,徐辰,于明革,施积炎.茶树根细胞壁不同组分对铅的吸附性能及其功能团的傅里叶红外光谱学研究.生态学报,2015,35(6):1743-1751.
Wang M,Duan D C,Xu C,Yu M G,Shi J Y.Adsorption ability of cell wall (CW) components in roots of Tea Plant (CamelliasinensisL.) to Pb and FTIR spectra of their functional groups.Acta Ecologica Sinica,2015,35(6):1743-1751.
