重组人硫氧还蛋白对大鼠内毒素肺损伤保护作用
2015-02-24陈红武赵海燕胡旭初
陈红武, 赵海燕, 胡旭初
(1.南方医科大学南方医院新生儿科,广东广州510515;2.中山大学中山医学院寄生虫学教研室,广东广州510080)
重组人硫氧还蛋白对大鼠内毒素肺损伤保护作用
陈红武1, 赵海燕1, 胡旭初2
(1.南方医科大学南方医院新生儿科,广东广州510515;2.中山大学中山医学院寄生虫学教研室,广东广州510080)
目的:探讨重组人硫氧还蛋白1(rhTrx-1)对新生SD大鼠急性内毒素肺损伤的保护作用.方法:制备、鉴定rhTrx-1;将新生SD大鼠分为对照组、脂多糖(LPS)实验组和LPS+rhTrx-1干预组.腹腔注射LPS(质量分数为5 mg/kg),制备急性内毒素肺损伤模型,干预组于LPS注射前30 min腹腔注射rhTrx-1(质量分数为10 mg/kg),观察注射后24 h各组新生SD大鼠的一般情况以及死亡率;重复实验,每组分别于实验后的2、8 h随机处死大鼠,提取肺组织用于病理切片观察.结果:(1)成功制备rhTrx-1;(2)LPS组大鼠表现出活动少,反应差,精神萎靡,毛发无光泽,口周发绀等全身炎症反应症状,濒死状态时表现为毛色青灰,呼吸浅表;LPS+hTrx-1干预组动物症状明显减轻;阴性对照组、LPS实验组和LPS+rhTrx-1干预组大鼠24 h死亡率分别为0%,67%、17%(χ2=14.400,P=0.001);(3)LPS组肺组织损伤严重,可见肺组织结构不完整,肺泡间隔明显增宽,肺泡腔内有红细胞、炎症细胞及浆液渗出,血管周围组织可见白细胞浸润,毛细血管有明显充血及扩张表现,随时间延长有加重趋势,LPS+rhTrx-1干预组病理改变较内毒素组明显减轻,肺泡结构尚正常,轻度肺水肿,肺泡间隔稍增宽,红细胞渗出明显减少,肺泡及肺间质中白细胞浸润减少.结论:重组人硫氧还蛋白1对急性内毒素肺损伤的新生大鼠具有保护作用.
重组人硫氧还蛋白1; 急性肺损伤; 新生SD大鼠
新生儿急性肺损伤(acute lung injury,ALI)是高病死率的临床重症和急症之一,近年研究发现氧化应激(oxidativestress,OS)在ALI的病理发展过程中扮演重要的角色[1],炎症和抗炎、氧化和抗氧化失衡的理论已被人们广泛关注[2-3].肺是炎症反应的重要靶器官之一,炎症反应会严重损伤肺功能,导致呼吸障碍.硫氧还蛋白(thioredoxin,Trx)又称白细胞介素-1样细胞因子,普遍存在于生物体中[4-5].近年国内外研究发现,人硫氧还蛋白1与氧化应激、炎症性疾病关系密切[6].本研究通过克隆和表达有酶活性的Trx,探讨该重组蛋白对急性内毒素肺损伤新生大鼠的保护作用,为后期其抗炎作用相关机制研究奠定基础.
1 材料与方法
1.1 材料
(1)rhTrx-1与动物 ①人重组硫氧还蛋白(hTrx-1)cDNA的获得、引物设计、基因扩增 从人胎肝细胞中提取总RNA,逆转录为cDNA;从人cDNA文库中筛选出hTrx-1基因,根据其编码序列及原核载体PET-28a(+)的多克隆位点,利用DNA Club设计引物,上游引物P1:5′-AGAGAGCTCATGTCTTCAGGAAATGCTAAA-3′,下划线为SacI酶切位点,AGA为保护性碱基,下游引物P2:5′-TTTGTCGACCTTCTGCTTGGAGAAATA-TTC-3′,下划线为Sal I酶切位点,TTT为保护性碱基.以cDNA为模板,应用设计的特异引物,利用高保真的Ex TaqTM酶进行扩增,反应条件如下:95℃预变性5 min,循环参数是95℃1 min,50℃1 min,72℃1 min,共35个循环;最后72℃延伸10 min.扩增产物用质量分数1%的琼脂糖凝胶电泳进行鉴定回收.②hTrx-1基因的构建和鉴定 将聚合酶链式反应(PCR)产物和重组质粒PET-28a(+)经SacI和Sal I双酶切后转入大肠埃希菌DH5α感受态细胞,卡那霉素筛选阳性克隆,对阳性克隆提取质粒,进行双酶切、PCR和测序鉴定.将验证正确的阳性质粒转入大肠杆菌BL21菌中.③hTrx-1的大量表达及纯化 对阳性克隆进行大量诱导表达,收集沉淀 、超声裂解,参照His柱说明书,利用6×His的镍离子亲和层析柱进行蛋白纯化,收集蛋白洗脱液,透析后用Bradford法进行蛋白浓度测定,并于-80℃保存备用.
(2)动物与试剂 SPF级足月新生7 d SD大鼠(由中山大学实验动物中心提供);脂多糖(LPS)(美国Sigma公司)
1.2 方法
(1)建立新生SD大鼠急性内毒素肺损伤模型足月新生7 d SD大鼠为实验对象,建立内毒素急性肺损伤模型[4],质量分数为5 mg/kg LPS腹腔注射.
(2)实验动物分组 36只新生7 d大鼠逐一编号,按照随机数字表分为阴性对照组、LPS实验组和LPS+rhTrx-1干预组,各组12只,阴性对照组腹腔注射生理盐水0.1 mL;LPS实验组腹腔注射质量分数为5 mg/kg LPS;LPS+hTrx-1干预组于LPS注射前30 min腹腔注射rhTrx-1(质量分数为10 mg/kg).观察注射后24 h各组大鼠的一般情况和死亡率.
(3)每组分别于实验后的2、8 h随机处死3只,提取肺组织用于病理切片观察.
1.3 统计学分析
采用SPSS 13.0统计软件分析,3组间死亡率比较采用多个样本率χ2检验,P<0.05为有统计学意义.
2 结果
2.1 rhTrx-1全长基因及编码序列
rhTrx-1基因全长为600 bp,起始密码子为ATG,终止密码为TGA,编码198个氨基酸.
2.2 rhTrx-1基因的扩增和克隆
用特异性引物扩增出hTrx-1基因,经凝胶电泳检测在600 bp附近有特异条带,与理论值大小相符.将提取的重组质粒进行双酶切鉴定,测序结果表明插入序列与hTrx-1基因cDNA序列一致,上述结果说明重组原核表达质粒构建成功,如图1.

图1 重组质粒PET-28a(+)-hTrx-1的PCR及酶切鉴定Fig.1 The identification of the PET-28a(+)-hTrx-1 by PCR Amplification and digestion with restriction enzyme
2.3 蛋白表达纯化
将重组质粒转化到大肠埃希菌BL21/DE3中进行表达,SDS聚丙烯凝胶电泳(SDS-PAGE)电泳分析结果如图2中所示,在25~35 kDa有表达条带,与重组蛋白分子量27.9 kDa基本相符.对蛋白进行纯化,结果如图2中第7泳道,其位置与目的蛋白相符.测得纯化产物的蛋白质量浓度为1.2 mg/mL.
2.4 24 h各组新生大鼠的一般情况
阴性对照组大鼠活动良好,反应灵敏,饮食正常,毛发光泽,肤色红润;实验组动物注入LPS后,逐渐表现出活动少,反应差,精神萎靡,毛发无光泽,口周发绀等全身炎症反应症状,濒死状态时表现为毛色青灰,呼吸浅表;rhTrx-1干预组动物症状较实验组明显减轻.
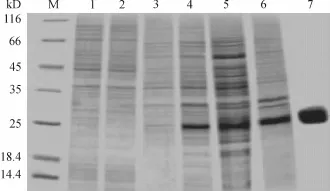
图2 重组质粒PET-28a(+)-hTrx-1在大肠埃希菌BL21/DE3的表达产物及其纯化产物的12%SDS-PAGE电泳分析Fig.2 Expression and purification of recombinant CsTP21.1 in Escherichia coli BL21(DE3)identified by 12% SDSPAGE
2.5 各组新生大鼠死亡率比较
如表1所示数据,对3组间死亡率差异采用χ2检验,对照组死亡率为0,实验组死亡率为67%,干预组死亡率为17%,各组死亡率差异有统计学意义(χ2=14.400,P=0.001),由此可以看出,rhTrx-1可明显降低内毒素急性肺损伤新生大鼠的死亡率,对新生大鼠具有保护作用.

表1 rhTrx-1对内毒素急性肺损伤新生大鼠的存活率的影响Table 1 Effect of rhTrx-1 on the survival rate of neonatal rats treated with acute lung injury
2.6 肺组织大体观察
实验组在LPS腹腔注射后,新生大鼠肺脏即可见点状肺出血,给药后1~8 h出血程度由局灶到弥漫,逐渐加重.与实验组相比,干预组的新生大鼠肺脏出血程度明显减轻.
2.7 肺组织病理形态学观察
实验组肺组织损伤严重,镜下可见肺组织结构不完整,肺泡间隔明显增宽,肺泡腔内有红细胞、炎症细胞及浆液渗出,血管周围组织可见白细胞浸润,毛细血管有明显充血及扩张,随时间延长有加重趋势(如图3和图5分别为LPS注射后2 h及8 h).干预组病理改变较内毒素组明显减轻,肺泡结构尚正常,轻度肺水肿,肺泡间隔稍增宽,红细胞渗出明显减少,肺泡及肺间质中白细胞浸润减少(如图4和图6分别为rhTrx-1干预后2 h及8 h).阴性对照组肺组织结构完整,肺泡腔清晰,肺泡间隔无水肿,间质及肺泡内见极少红细胞及炎症细胞浸润(如图7).

图3 实验组2 h HE染色×400Fig.3 2 h in LPS group staining of hematoxylin and Eosin×400

图4 干预组2 h HE染色×400Fig.4 2 h in rhTrx-1 group staining of hematoxylin and Eosin×400
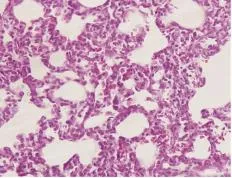
图5 实验组8 h HE染色×400Fig.5 8 h in LPS group staining of hematoxylin and Eosin×400

图6 干预组8 h HE染色×400Fig.6 8 h in rhTrx-1 group staining of hematoxylin and Eosin×400

图7 阴性对照组HE染色×400Fig.7 Control group staining of hematoxylin and Eosin×400
3 讨论
肺是内毒素最常累及靶器官,由于ALI导致机体缺氧、酸中毒和呼吸衰竭,死亡率高达40%~70%,当合并其他脏器损害时病死率会显著提高,是临床上常见的高死亡率疾病.ALI是多种致病因素导致的肺组织结构和功能的损伤,以肺部微血管和肺泡上皮弥漫性损伤、肺水肿及肺间质纤维化等临床综合征,严重者可发展为死亡率极高的ARDS.ALI发病的根本原因是肺内过度失控的炎症反应综合征(systemic inflammatory response syndrome,SIRS)、细胞凋亡、血管紧张素系统参与了的氧化抗氧化失衡等[7].因此,抑制体内过激的炎症反应和氧化反应是防治ALI的关键.近年国内外研究发现,Trx与氧化应激、炎症性疾病关系密切,但给予外源性的Trx是否对感染或缺氧导致的ALI具有保护作用尚未见报道.
Trx于1964年由Reichard首次发现,除具有基本的抗氧化功能外,还具有促生长、抗凋亡、抗炎和调节转录因子活性等作用[8].在急性肺损伤患者中,胞外Trx的水平与细胞因子IL-8呈正相关性,胞外Trx的水平可作为疾病炎症反应强度的判断指标[9].在急性肺损伤患者中,患者血浆、支气管肺泡灌洗液(BAL)、肺巨噬细胞及肺泡Ⅱ型上皮细胞中Trx的含量显著增加.腹腔注射重组人Trx-1可减弱博莱霉素或炎症因子IL-2和lL-18引起的间质性肺炎和肺纤维化[10],这提示外源性重组人Trx-1的适当补充可增强其抗炎作用,对机体组织及脏器等具有保护作用.LPS是细菌内毒素的主要成分,本研究中LPS注射后大鼠出现ALI,肺组织损伤严重,肺出血明显,光镜下可见肺组织结构不完整,肺泡间隔明显增宽,肺泡腔内有红细胞、炎症细胞及浆液渗出,血管周围组织可见白细胞浸润,毛细血管有明显充血及扩张表现,随时间延长,损伤有加重趋势.但给予外源性rhTrx-1干预后,内毒素急性肺损伤大鼠24 h死亡率明显降低,肺出血显著减轻,肺组织病理形态观察提示红细胞和炎症细胞的渗出及浸润减少,肺部炎症反应减轻,急性肺损伤的病理进程被阻断,这些均证实补充外源性rhTrx-1对内毒素导致急性肺损伤新生大鼠也具有保护作用.
给予外源性rhTrx-1能减少内毒素作用后新生大鼠的死亡,抑制肺组织的炎症反应.可能与其对于炎症反应的关键因子NF-κB有一定的调节作用有关,通过抑制氧化剂,提高DNA和NF-κB的连接,活化NF-κB,从而调控炎症因子的转录和激活,Trx的这种还原作用比L—Cys和还原型谷胱甘肽的作用更为强烈[11].胞外Trx除了调节细胞因子的分泌、表达及释放外,它还具有调节炎症信号转导过程的作用,如能抑制P38有丝分裂原蛋白激酶的活性[12],参与对炎症相关的转录因子如转录激活因子(AP-1)、核因子κB(NF-κB)、糖皮质激素受体的调节,抑制巨噬细胞迁移、抑制因子MIF(一种促炎因子)的活性与释放[11]等.Trx对于各种还原酶系统都有显著调节作用,例如超氧化物歧化酶(superoxidasedimutase,SOD),从而能抵抗体内的氧化反应.因此,Trx能抑制过激的炎症反应和氧化反应,起到保护脏器免受损害的作用
本研究动物实验证实,给予外源性rhTrx-1能抑制内毒素造成的过激炎症反应和氧化反应,抑制单核细胞和中性粒细胞等免疫细胞到达炎症反应部位,进而抑制促炎因子的表达和释放,减轻炎症反应及炎症损伤.胞外Trx具有抗炎特性及对过激炎症导致脏器损害的保护作用.因此,许多研究者开始合成rhTrx-1,进一步明确其抗炎效果以及相关机制,期待可以用于临床,治疗炎症类疾病.
[1] GUO R F,WARD P A.Role of oxidants in lung in jury during sepsis[J].Antioxid Redox Signal,2007,9(11):1991-2002.
[2] HAN F,LUO Y,LIY,et al.Seawater induces apoptosis in alveolar epithelial cells via the Fas/Fas L-mediated pathway[J].Respir Physiol Neurobiol,2012,82(2-3):71-80.
[3] CROSS C E,EISEFICH J P.Oxidative stress in acute lung injury:Dejavu or some thing new?[J].Crit Crae Med,2004,32(3):892-893.
[4] HOLMGREN A.Thioredoxin and glutaredoxin systems[J].JBiol Chem,1989,264(24):13963-13966.
[5] MEYER Y,BUEHANAN B B,VIGNOLSF,et al.Thioredoxins and glutaredoxins:unifying elements in redoxbiology[J].Annu Rev Genet,2009,43:335-367.
[6] CRISTINE S,MARIA J,PAOLO P,et al.Statins inhibit expression of Thioredoxin reductase 1 in rat and human liver and reduce tumor development[J].Biochem Biophys Res Commun,2012,417(3):1046-1051.
[7] ZHOU M T,CHEN C S,CHEN B C,et al.Acute lung injury and ARDS in acute pancreatitis:mechanisms and potential intervention[J].World J Gastroenterol,2010,16(17):2094-2099.
[8] GIROIR B P,JOHNSON JH,BROM T,et al.The tissue distributon of tumor necrosis factor biosynthesis during endotoxemia[J].JClin Invest,1992,,90(3):693-698.
[9] CALLISTER M E,BURKE G A,QUINLAN G J,et al.Extracellular thioredoxin levels are increased in patients with acute lung injury[J].Thorax,2006,61(6):521-527.
[10]HOSHINO T,NAKAMURA H,OKAMOTO M,et al.Redox-active protein thioredoxin prevents proinflammatory cytokine-or bleomycin-induced lung injury[J].Am JRespir Criti Care Med,2003,168(9):1075-1083.
[11]HEILMAN JM,BURKE T J,MECLAIN C J,et al.Transaetivation of gene expression by NF-κB is dependent on thioredoxin reductase activity[J].Free Radical Bio1.Med,2011,51(8):1533-1542.
[12]TAMAKI H,NAKAMURA H,NISHIO A,et al.Human thioredoxin-1 amelioratese experimental murine colitis in association with suppressed macrophase inhibitory factor production[J].Gastroenterology,2006,131(4):1110-1121.
[责任编辑:陈咏梅]
Protective effect of recombinant human thioredoxin-1 on LPS-induced acute lung injury of neonatal rats
CHEN Hongwu1, ZHAO Haiyan1, HU Xuchu2
(1.Department of Neonatology,the Nanfang Hospital,Southern Medical University,Guangzhou 510515,China;2.Department of Parasitology,the Zhongshan Medical College,Sun Yat-Sen University,Guangzhou 510080,China)
Aim:To study the protective effects of recombinant human thioredoxin-1 on LPS-induced acute lung injury of neonatal rats.Methods:Prepare recombinant human thioredoxin-1 and then prepare animal model of LPS-induced acute lung injury of newborn rats.The full-term newborn Sprague-Dawley rats aged seven days were randomly divided into three groups-negative control group,LPS group and rhTrx-1 intervention group.The rats of rhTrx-1 group were received intraperitoneal injection with rhTrx-1 10 milligram per kilogram,and then with LPS 5 milligram per kilogram in the same way half an hour later,while LPS group were received intraperitoneal injection with LPS 5 milligram per kilogram,negative control group with 0.9%saline 0.1 m l.Their general state and mortality of24 hour were observed.The lung morphology of 2 hour and 8 hour was observed by hematoxylin-eosin stain.Results:1.The prepara-tion of rhTrx-1 was successful.2.In the negative control group,everything was normal,no deaths occurred.In the LPS group,rats gradually showed a decrease in activity,drowsiness,perioral cyanosis,shortness of breath and some systemic inflammatory response symptoms after LPS injected.It turned grey in the skin and they had shallow breathing when they were moribund.The mortality was 67%.In rhTrx-1 group,the general condition of rats were obviously better than that of the control group.The mortality was 17%.The differences among the three groups had statistical significance.3.In the negative control group,the structure of the lung tissue was complete and there was no edema in alveolar septum.In LPS group,the structure of the lung tissue was incomplete and alveolar septum widened.There were a large number of red blood cells,inflammatory cells and serous exudates in the alveolar.Leukocyte infiltration,capillary hyperemia and expansion was visible in perivascular tissue.Compared with LPS group,pathological change was significantly reduced in the rhTrx-1 group.The edema was mild,alveolar septum widened slightly.Also,the leakage of red blood cell and the infiltration of leukocyte reduce significantly.Conclusion:The rhTrx-1 protein has protective effects on LPS-induced acute lung injury of neonatal rats.
recombinant human thioredoxin-1; acute lung injury; neonatal rat
R365.563
A
1000-9965(2015)03-0256-05
10.11778/j.jdxb.2015.03.012
2015-03-13
广东省科技计划基金项目(2012B031800140)
陈红武(1966-),主任医师,研究方向:新生儿危重症
陈红武,女,主任医师,Mobile:13682257393;E-mail:13682257393@163.com
