Expression of phosphatidylinositol-3 kinase and effects of inhibitor Wortmannin on expression of tumor necrosis factor-α in severe acute pancreatitis associated with acute lung injury
2015-02-07
Department of Transfusion, Fifth Aff liated Hospital of Zhengzhou University, Zhengzhou 450052, China
Expression of phosphatidylinositol-3 kinase and effects of inhibitor Wortmannin on expression of tumor necrosis factor-α in severe acute pancreatitis associated with acute lung injury
Ming Wei, Yan-jie Gong, Ling Tu, Jia Li, Ying-hong Liang, Yi-hua Zhang
Department of Transfusion, Fifth Aff liated Hospital of Zhengzhou University, Zhengzhou 450052, China
BACKGROUND:Acute lung injury (ALI) is a common and serious complication of severe acute pancreatitis (SAP). The study aimed to investigate the protective effect and mechanism of phosphatidylinositol-3 kinase (PI3K) inhibitor Wortmannin in SAP associated with ALI.
METHODS:Ninety rats were randomly divided into three groups: sham operation (SO) group (n=30), SAP group (n=30), and SAP+Wortmannin (SAP+W) group (n=30). SAP model was induced by retrograde injection of 4% sodium taurocholate into the biliopancreatic duct of rats. The rate of lung water content, myeloperoxidase (MPO), matrix metalloproteinase 9 (MMP-9), protein kinase B (PKB), abdphosphorylation of protein kinase B (P-PKB) activity in the lung tissue were evaluated.
RESULTS:In the SAP group, the p-PKB expression in the lung tissue began to rise at 3 hours after modeling, and peaked at 12 hours (P<0.05); the rate of lung water content, MPO and TNF-α activity were also gradually increased, and the degree of lung lesion gradually increased (P<0.05). In the SAP+Wortmannin group, the p-PKB expression in the lung tissue began to rise at 3 hours after modeling, and peaked at 12 hours; it was higher than that in the SO group (P<0.05), but signif cantly lower than that in the SAP group (P<0.05). The rest indicators in the SAP+Wortmannin group were also signif cantly decreased as compared with the SAP group (P<0.05).
CONCLUSIONS:The expression of phosphatidylinositol-3 kinase/protein kinase B was elevated in severe pancreatitis rats with lung injury. This suggested that PI3K signal transduction pathway is involved in the control and release of proinf ammatory cytokines TNF-α, which may play an important role in the pathogenesis of severe acute pancreatitis associated with lung injury. This finding indicated that Wortmannin can block the PI3K signal transduction pathway, and inhibit the release of inf ammatory factor TNF-α.
Wortmannin; Phosphatidylinositol-3 kinase/protein kinase B; Severe acute pancreatitis; Acute lung injury
INTRODUCTION
Acute lung injury (ALI) is a common and serious complication of severe acute pancreatitis (SAP). It can develop into acute respiratory distress syndrome (ARDS) without timely intervention.[1]Wu et al[2–4]reported that ALI is a kind of medium disease. The activation, invasion and massive release of inf ammatory mediators of neutrophils can cause damage to lung tissue and capillaries, thus aggravaing lung injury. Procko et al[5,6]reported that the phosphatidylinosito l–3 kinase/protein kinase B (PI3K/PKB) pathway played an important role in the activation of neutrophils. Waterman penicillin(Wortmannin) is an inhibitor of PI3K, and few studies reported its ability to reduce the damage to lung tissue in SAP. Through establishing SAP-ALI models in rats, we studied the activity of the PI3K/PKB pathway in SAP, and observed changes caused by lung injury after using Wortmannin to block this pathway, attempting to determine whether Wortmannin can protect lung tissue from injury in rats with SAP.
METHODS
Animal group and the establishment of SAPALI model
Ninety healthy adult SD rats, body weight 320–360 g, purchased from laboratory animal center of Zhengzhou University, were randomly divided into three groups: sham operation group (n=30) (SO group), SAP group (n=30), and SAP plus Wortmannin group (n=30) (SAP+W group). The rats in the SAP group and SAP+W group were respectively divided into three subgroup (n=10) by 3 hours, 6 hours and 12 hours.
The rats were fasted for 12 hours before operation, but they were free access to water. The rats in the SAP+W group were given with 1.4 mg/kg Wortmannin by intraperitoneal injection within 2 hours before operation, while the rats in the SO group and SAP group were given with an equal amount of 0.9% NaCl. After general anesthesia and sterilization, surgical olrape was used. The BD needle with a diameter of 0.75 mm was used to poke into the bowel from the lateral duodenal wall via an abdominal incision at the center of the upper abdomen. Then the needle core was pulled out. Along the direction of the nipple, the needle went on into the pancreatic duct by 1.0–1.5 cm. The SAP model was established by injection of 4% sodium taurocholate (purchased from Sigma Company) into the biliopancreatic duct of rats with 0.1 mL per 100 g at the rate of 0.2 mL/min. The rats in the SAP group and SAP+W group were killed respectively at 3, 6, and 12 hours postoperation for tissue collection, while the rats in the SO group were only killed at 12 hours postoperation.
Measurement of water content of the lung
Filter paper was used to blot the drainage and blood in the surface of lower right lobectomy after it was taken out from the rats. The lung tissue was weighted and the wet weight was obtained. The lung tissue was weighted after it was put in the oven at 80 °C for 48 hours, and the dry weight was obtained. The rate of water content was calculated with the following formula:
The rate of water content (%)=(wet weight–dry weight)/dry weight×100%.
Paraff ned tissue was sectioned and HE staining was performed. The Schmidt[7]and Mayer[8]criteria were used to evaluate the tissue injury severity of the pancreas and lung.
Determination of myeloperoxidase (MPO) activity in the lung
Lung tissue homogenate was prepared to detect the activity of myeloperoxidase (MPO). The test was conducted strictly according to the instructions of kits (Nanjing Jiancheng Biological Reagent Company, China).
Detection of tissue tumor necrosis factor (TNF) in the lung
Immune histochemical method was used to detect the TNF-α in lung tissue. Rabbit anti-mouse monoclonal antibody was offered by Cell Signaling Technology Company. Three structural integrity sections per rat were viewed under a 100×microscope, and images were captured by using Olympus DE70CCD. Positive standard: yellow granules appeared in cytoplasm or nuclear in alveolar epithelial cells and vascular epithelial cells. The image-pro plus 6.0 immunohistochemical color image analysis system was applied for quantitative analysis. Based on one positive point in the image (a single cell or cell mass), the positive point number, area, integral absorbance value (AI) in the whole picture and other parameters were detected.
Determination of the expression of PKB and p-PKB by Western blot
One mL total protein extract was added into the 0.5 mg lung tissue homogenate. The protein was extracted under the condition of ice bath and ultrasonic wave. Protein quantitative analysis was carried out by using the internal reference method. PKB and p-PKB were detected by Western blot. Firstly, SDS-PAGE was conducted (25 µL per hole) for 2 hours, and the protein bands were transferred to the nitrocellulose (NC) membrane. The rabbit anti-mouse PKB monoclonal antibody (1:40 dilution) and rabbit anti-mouse p-PKB monoclonal antibody (1:1 000 dilution) were added at the condition of 4 °C for the night. Horseradish peroxidase labeling sheep anti-rabbit monoclonal antibody was used as the second antibody (1:40 000 dilution). Enhanced chemiluminescence (ECL) was applied to display the results, and the X-ray film was exposed for the image. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)was used as an internal reference. The Gelpro version 4.0 gel absorbance analysis software and Image-ProPlus 6.0 software were used to analyze the results. The corresponding stripe grey value showed the relative content of the protein.
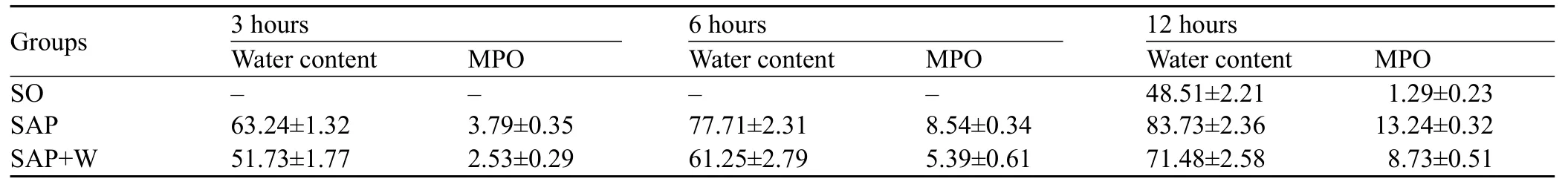
Table 1. The results of water content and MPO activity (mean±SD)
Statistical analysis
The SPSS 16.0 statistical software was used for statistical analysis. Measurement data were shown by mean±standard deviation and compared by one-way analysis of variance. The linear correlation analysis was performed for the correlation test.
RESULTS
Water content of the lung and myeloperoxidase (MPO) activity
The water content was increased more significantly at different time points in the SAP group than in the SO group (P<0.05). The pulmonary MPO activity was enhanced obviously and increased gradually (P<0.05). The water content and MPO activity peaked at 12 hours in the SAP group. The water content and MPO activity were also increased more signif cantly in the SAP group than in the SO group (P<0.05), but they decreased more signif cantly than in the SAP+W group (P<0.05) (Table 1).
Examination of the pancreas and lung
There was no obvious abnormity in the pancreatic tissue under a microscope in the SO group. While in the SAP group, there were hemorrhage, necrosis, inflammatory cells infiltration in the pancreases tissue and fat necrosis in the peripancreatic tissue, and the severity increased gradually with the extension of time (Figure 1). There were also different degree of damage in the SAP+W group, and the damage was more severe in the SO group, but less severe in the SAP group. At 3 hours, pulmonary interstitial hyperemia, edema, and infiltration of inflammatory cells around small vessels were observed in the SAP group. At 6 and 12 hours, typical ALI pathological changes appeared, such as different alveoli and interstitial edema, hemorrhage,focal or flake atelectasis, further broadening of the alveolar interval, infiltration of inflammatory cells, and destruction of pulmonary tissue structure (Figure 2). The damage was improved more signif cantly in the SAP+W group than in the SAP group (Table 2).
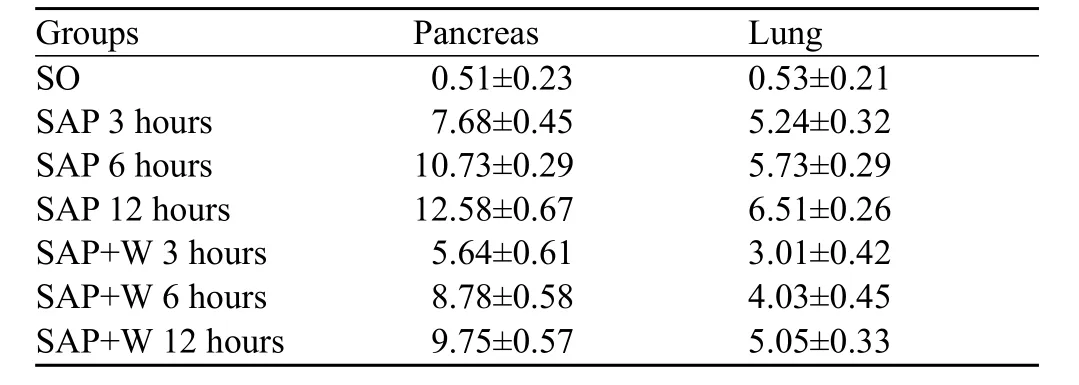
Table 2. Pathological grading of the pancreas and lung (mean±SD)
TNF-αstained area and IA value of the rat lung
There was no obvious staining in the alveolar epithelial cells or on endothelial cell surface in the SO group. Hence there was no expression of TNF-α in the SO group. At 3 hours, there were brown granules in part of vascular endothelial cell surface in the SAP group; at 6 hours, there were brown granules in the vascular endothelial cells or on alveolar epithelial cell surface (Figure 3); at 12 hours, there were lots of brown or tan particles on the pulmonary vascular endothelial cell surface, and thick brown granules were visible in the alveolar epithelial cells. The expression of TNF-α decreased in the SAP+W group compared with the SAP group. IPP immunohistochemical image analysis showed that the positive staining area and IA value in the SAP group increased gradually at different time point, thus pulmonary tissue pathogenesis gradually strengthened the expression of TNF-α. The expression of TNF-α increased in the SAP+W group compared with the SO group, but decreased compared with the SAP group (Table 3).
Western blot for p-PKB activity
PKB activity was low in the SO group, but it was increased more rapidly in the SAP group than in the SO group (P<0.05). In the SOP+W group, the PKB activity increased gradually with the disease progression. Even though the level of PKB activity in the SOP+W groupwas higher than that in the SO group (P<0.05), it was markedly lower than that in the SPA group (P<0.05) (Table 4, Figure 4).

Table 3. Comparison of TNF-α stained area and IA value (mean±SD)
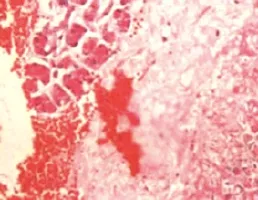
Figure 1. The pancreas tissue in the SAP group at 6 hours (original magnif cation×400).

Figure 3. TNF-α of pulmonary tissue in the SAP group at 6 hours (original magnif cation×400).
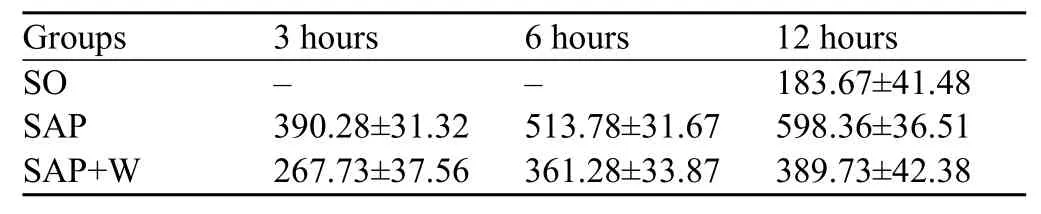
Table 4. Comparison of p-PKB activity in different groups and different time points (mean±SD)
DISCUSSION
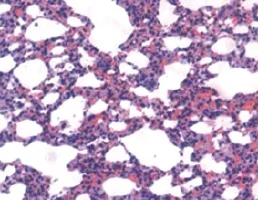
Figure 2. The pulmonary tissue in the SAP group at 6 hours (original magnif cation×400).
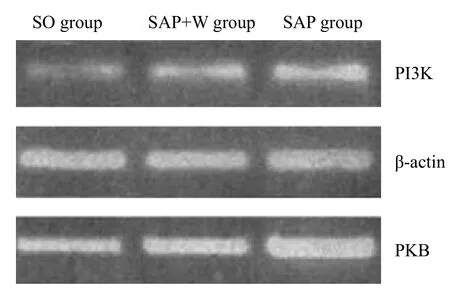
Figure 4. P13K, PKB mRNA PCR in the pulmonary tissue at 12 hours.
Acute lung injury (ALI) is the most common complication of severe acute pancreatitis (SAP). When lung injury occurs, a large number of inflammatory cells migrate to the lung and release large amounts of inflammatory mediators. This produces injury to the pulmonary capillaries and alveolar epithelium, causes pulmonary edema and atelectasis, and then develops into ARDS.[9]PI3K is present in the cell, and its biological effects are extensive. It is shown that PI3K can be activated to participate in signal transduction by a variety of inflammatory factors in inflammation; and PKB is an important protease located downstream of PI3K, closely related to PI3K. When activated by inf ammatory factors, PI3K can convert Ptdlns (4 and 5) P2 into a second messenger Ptdlns (3, 4, and 5) P3 (also known as PIP3). PIP3 can combine with the PH domain of PKB, phosphorylate it, and further cause changes of the target genes in the nucleus, and thereby regulate neutrophilsactivation, migration and the expression of inf ammatory factors. Therefore, phosphorylation of PKB can be used as an activity indicator of PI3K.[10]It has been reported that when SAP occurred, the activity of PI3K/ PKB in the lung tissue increased in accordance with the degree of infiltration of neutrophils and the damage of lung tissue.[11]This suggested that this pathway played an important role in mediating the neutrophils activation while aggravating lung injury. In our study, the expression of PKB was marked in rat lung tissue, but varied in degrees of PKB phosphorylation. Moreover, the phosphorylation was lower in the SO group, whereas it was increased signif cantly in the SAP group and peaked at 12 hours. Thus, the PI3K pathway was gradually activated in SAP, and the activated PI3K could enhance the phosphorylation of PKB, further transfer PKB from the cytoplasm to the cell membrane and obtain catalytic activity, and thus complete the inflammatory factormediated signal transduction.[12]The expression of MPO and TNF-α was signif cantly increased in the SAP group compared with the SO group and this f nding was consistent with the dynamic changes. Hence, when SAP occurred, the PI3K/PKB pathway was activated, thereby mediated the activation of a large number of neutrophils. The distinctively increased activation of MPO in the lung tissue indicated that neutrophils invaded the lung tissue and aggravated the inf ammation.[13]
TNF-α is an important proinflammatory cytokine. It has been proved that the activated PI3K/Akt signaling pathway could enhance the transcription of proinf ammatory cytokines such as TNF-α and IL-1β genes, increase the production and release of TNF-α and IL-1β, and then result in inflammation.[14]More importantly, TNF-α can also promote the release of other cytokines, involve in the formation of the cytokines network, expand a chain of inflammatory reaction, and lead to persistence of inf ammation.[15]
With the progress of moisture and the histopathology of the lung, the degree of lung injury and pulmonary edema was aggravated, indicating that the PI3K/PKB pathway plays an important role in the pathogenesis of ASP-ALI. After use of PI3K blocker Wortmanning, the phosphorylation level in the SAP+W group was signif cantly lower than that in the SAP group; the lung tissue MPO and TNF-α was also declined markedly, the moisture content of the lung and the pulmonary pathology were improved. Thus the phosphorylation of PKB was inhibited after blockage of PI3K. Its activation was reduced, the activation of neutrophils alleviated, and the lung injury relieved, all of which proved that Wortmanning plays a role in protecting the lung tissue.
In conclusion, the excessive activation of the PI3K/ PKB signaling pathway is an important step in lung injury when ASP occurs. This activation can mediate a large number of pulmonary neutrophils infiltrating the lung tissue, release inflammatory mediators, and aggravate the lung injury. This effect may be one of the important pathogeneses in SAP complicated by acute lung injury. Wortmanning blocked intracellular signal transduction by inhibiting the activity of the PI3K pathway, relieved the inflammation while protecting the lung tissue. As ALI is a continuous process from mild to severe, and multi-signaling pathways and multiinflammatory factors are involved, further studies are needed to investigate the interactions between the various signaling pathways. Thus, early and effective inhibition of the activities of these pathways will help to control the deterioration of ALI.
Funding:None.
Ethical approval:This study was conducted according to the principles of the Animal Care and Use Committee of the Fifth Aff liated Hospital of Zhengzhou University, Zhengzhou, China.Conf icts of interest:No any benef ts have been received from a commercial party related directly or indirectly to the study.
Contributors:Wei M proposed and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts.
1 De Campos T, Deree J, Coimbra R. From acute pancreatitis to end organ injury: mechanisms of acute lung injury. Surg Infect (Larchmt) 2007; 8: 107–120.
2 Wu Y, Lv J, Feng D, Jiang F, Fan X, Zhang Z, et al. Restoration of alveolar type II cell function contributes to simvastatininduced attenuation of lung ischemia-reperfusion injury. Int J Mol Med 2012; 30: 1294–1306.
3 Gao Z, Xu J, Sun D, Zhang R, Liang R, Wang L, et al. Traditional Chinese medicine, Qing Ying Tang, ameliorates the severity of acute lung injury induced by severe acute pancreatitis in rats via the upregulation of aquaporin-1. Exp Ther Med 2014; 8: 1819–1824. Epub 2014 Sep 22.
4 Zhu HM, Guo SQ, Liao XM, Zhang L, Cai L. Embryonic natural orif ce transluminal endoscopic surgery in the treatment of severe acute pancreatitis complicated by abdominal compartment syndrome.World J Emerg Med 2015; 6: 23–28.
5 Procko E, McColl SR. Leukocytes on the move with phosphoinositide 3-kinase and its downstream effectors. BioEssays 2005; 27: 153–163.
6 Singh VP, Saluja AK, Bhagat L, van Acker GJ, Song AM, Soltoff SP, et al. Phosphatidylinosito1–3 kinase dependent activation of trypsinogen modulates the severity of acute of pancreatitis. J Clin Invest 2001; 108: 1387–1395.
7 Wang YZ, Wang SW, Zhang YC, Sun ZJ. Protective effect of exogenous IGF-I on the intestinalmucosal barrier in rats with severe acute pancreatitis.World J Emerg Med 2012; 3: 213–220.
8 Mayer J, Ran B, Gansauge F. Inf ammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut 2000; 47: 546–552.
9 Pezzilli R, Bellacosa L, Felicani C. Lung injury in acute pancreatitis. J Pancreas 2009; 10: 481–484.
10 Lin CH, Yeh SH, Lin CH. A role for the PI-3 kinase signaling path-way in fear conditioning and synaptic plasticity in the amygdale. Neuron 2001; 31: 841–851.
11 Kang X, Wang LZ, Wang YG, Liu L, Fan ZW, Bai LZ, et al. Expression and significance of phosphatidylinositol 3-kinase/ protein kinase B signal transduction pathway in severe acute pancreatitis-associated lung injury. Zhonghua Yi Xue Za Zhi 2010; 90: 732–737.
12 Lee KS, Lee HK, Joel S. Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness inmurine asthma model. FASEB J 2006; 20: 455–465.
13 Keck T, Balcom JH 4th, Fernández-del Castillo C, Antoniu BA, Warshaw AL. Matrix metallopmteinase-9 promotes neutrophil migration and alveolar capillaryleakage in pancreatitisassociated lung injury in the rat. Gastroenterology 2002; 122: 188–201.
14 Gustin JA, Ozes ON, Akca H, Pincheira R, Mayo LD, Li Q, et al. Cell type-specific expression of the IkappaB kinases determines the significance of phosphatidylinositol 3 kinase/ Akt signaling to NF-kappa B activation. J Biol Chem 2004; 279: 1615–1620.
15 Yang SK, Eckmann L, Panja A, Kagnoff MF. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterolog 1997; 113: 1214–1223.
Received April 11, 2015
Accepted after revision September 3, 2015
Ming Wei, Email: gushiweiming@126.com
World J Emerg Med 2015;6(4):299–304
10.5847/wjem.j.1920–8642.2015.04.009
