Nanopore-based Fourth-generation DNA Sequencing Technology
2015-02-06YanxiaoFengYuechuanZhangCuifengYingDeqiangWangChunleiDue
Yanxiao Feng,Yuechuan Zhang,Cuifeng Ying,Deqiang Wang*, Chunlei Due
1Chongqing Key Laboratory of Multi-scale Manufacturing Technology,Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Sciences,Chongqing 400714,China
2School of Physical Electronics,University of Electronic Science and Technology of China,Chengdu 611731,China
3MOE Key Laboratory of Weak-light Nonlinear Photonics,School of Physics,Nankai University,Tianjin 300071,China
4University of Chinese Academy of Sciences,Beijing 100049,China
Nanopore-based Fourth-generation DNA Sequencing Technology
Yanxiao Feng1,4,a,Yuechuan Zhang1,2,b,Cuifeng Ying1,3,c,Deqiang Wang1,4,*,d, Chunlei Du1,4,e
1Chongqing Key Laboratory of Multi-scale Manufacturing Technology,Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Sciences,Chongqing 400714,China
2School of Physical Electronics,University of Electronic Science and Technology of China,Chengdu 611731,China
3MOE Key Laboratory of Weak-light Nonlinear Photonics,School of Physics,Nankai University,Tianjin 300071,China
4University of Chinese Academy of Sciences,Beijing 100049,China
Nanopore;
DNA sequencing;
Single base;
Single molecule
Nanopore-based sequencers,as the fourth-generation DNA sequencing technology,have the potential to quickly and reliably sequence the entire human genome for less than$1000,and possibly for even less than$100.The single-molecule techniques used by this technology allow us to further study the interaction between DNA and protein,as well as between protein and protein. Nanopore analysis opens a new door to molecular biology investigation at the single-molecule scale. In this article,we have reviewed academic achievements in nanopore technology from the past as well as the latest advances,including both biological and solid-state nanopores,and discussed their recent and potential applications.
Introduction
DNA,a molecule that encodes genetic instructions,is the blueprint of life.Accurate and rapid DNA sequencing technologywould have profound impacts on human diseases and personalized medicine.The non-nanopore DNA sequencing technologies currently on the market require a great deal of sample preparation and complicated algorithms for data processing. Therefore,they have limitations such as low throughput,high cost,and short read lengths.Fortunately,several academic and commercial efforts have been made to develop inexpensive DNA sequencers.After the development of three generations, DNA sequencing technology is now entering the era of singlemolecule nanopore technology.In the 1990s,Church et al.and Deamer and Akeson separately proposed that it is possible to sequence DNA using nanopore sensors[1,2].Since 1996, beginning with the frst nanopore paper published in PNAS [3],nanopore-based detection of single molecules has emergedas one of the most powerful sequencing technologies.The signifcant advantages of nanopores include label-free,ultra-long reads(104–106bases),high throughput,and low material requirement.Each of these greatly simplifes the experimental process and can be easily used for DNA sequencing applications.The nanopore approach will be one option for the fourth-generation low-costand rapid DNA sequencing technology.
Nanopore-based technologies originated from the Coulter counter and ion channels[4,5].With the application of an external voltage,particles with sizes slightly smaller than the pore size are passed through the pore.The nanometer-sized pores are either embedded in a biological membrane or formed in solid-state flm,which separates the reservoirs containing conductive electrolytes intocisandtranscompartments. Electrodes are immersed in each chamber as shown inFigure 1.Under a biased voltage,electrolyte ions in solution are moved through the pore electrophoretically,thereby generating an ionic current signal.When the pore is blocked by an analyte,such as a negatively-charged DNA molecule added into thecischamber,current fowing through the nanopore would be blocked,interrupting the current signal.The physical and chemical properties of the target molecules can be calculated by statistically analyzing the amplitude and duration of transient current blockades from translocation events [6].
Nanopores as single-molecule sensing technologies have great potential applications in many areas,such as analysis of ions,DNA,RNA,peptides,proteins,drugs,polymers, and macromolecules,as previously reviewed[7–10].This review describes the concept and attributes of biological and solid-state nanopores,as well as the latest academic advances and commercial achievements in DNA sequencing and other applications.
Type of nanopores
Nanopore technologies can be broadly divided into two categories:biological and solid-state.Several groups have verifed that both types of nanopores can be used to detect biological and chemical molecules at the single-molecule level.Table 1categorizes many of the available biological and solid-state nanopores for DNA sequencing.
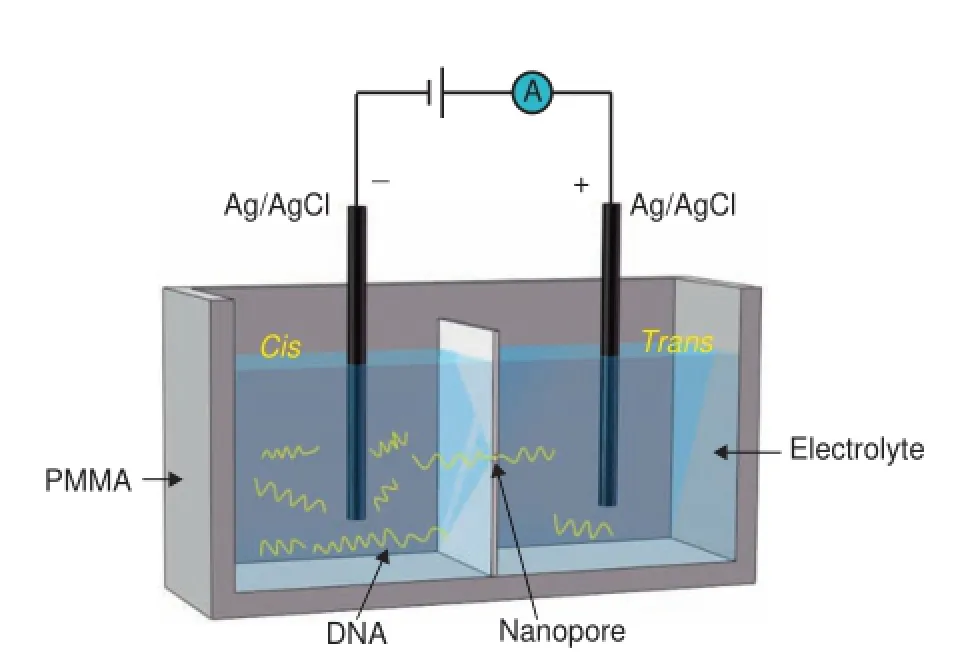
Figure 1 Schematic view of nanopore fuidic setup comprising a DNA molecule through the nanopore
Biological nanopores have been widely used in single-molecule detection,disease diagnosis,and DNA sequencing.Recent advances in nanotechnology have facilitated the rise of solidstate nanopore sensors[11,12].In combination with other devices such as a feld-effect transistor,these synthetic nanopores can be further integrated on a circuit chip,which offers the potential for miniature,portable DNA sequencing devices. More recently,hybrid nanopores have been proposed to take advantage of the features of both biological and solid-state nanopores[13].Nanopore DNA sequencing technology is developing rapidly.Although it has a very high error rate(over 90%),an instrument based on nanopore technology that sequences DNA at the scale of a single molecule is currently available on the market[14].
Biological nanopores
Biological nanopores,also called transmembrane protein channels,are usually inserted into a substrate,such as planar lipid bilayers,liposomes,or other polymer flms.The advantages of biological nanopores include their well-defned and highly-reproducible nanopore size and structure.More importantly,biological nanopores can be modifed easily with modern molecular biology techniques,such as mutating the nucleotide sequence to change the amino acid residue at a specifc site.In this section,three well-studied biological nanopores are discussed.
α-Hemolysin
α-Hemolysin(α-HL,also called α-toxin)is the frst and most commonly used biological nanopore,holding a tremendous value in the feld of DNA sequencing.α-HL is an exotoxin secreted by the bacteriumStaphylococcus aureus,a human pathogen.This mushroom-shaped heptamer is a 232.4-kDa transmembrane channel,consisting of a 3.6-nm diameter cap and a 2.6-nm diameter transmembrane β-barrel(Figure 2A) [15].The external dimensions of the pores are 10 nm×10 nm.In vivo,an α-HL unit can quickly insert itself into planar bilayers and form a nanochannel with a width of 1.4 nm at the narrowest point.The inner diameter of the α-HL channel and the size of a single-stranded DNA(ssDNA)molecule are very close in size(diameter~1.3 nm).Thus,the α-HL nanopore is able to discriminate single nucleotides using ionic current inside the nanopore[16].This makes α-HL a very promising tool for analyzing biomolecular interactions and structures at the single-molecule level.Furthermore,the nanopore structure can remain functionally stable at temperatures close to 100°C [17]within a wide pH range(pH 2–12)[17].
Although α-HL pores are extensively used in biological experiments,the limited pore size(~1.4 nm)has restricted its application in the analysis of ssDNA,RNA,or small molecules.Moreover,the β-barrel of its nanopore is too long to distinguish individual nucleotides from single long-chain DNA molecules directly.
MspA
Mycobacterium smegmatisporin A(MspA)is a promising and powerful nanopore for reading information from four nucleotides simultaneously,as reported by Butler et al.in 2008[7].As shown inFigure 2B,the channel of the MspA octamer is~1 nm in diameter at the minimal point,which is relativelysmall and narrow,compared to that of α-HL.Thus,it can improve the spatial resolution of ssDNA sequencing.In addition,MspA is very robust and keeps the channel active under extreme experimental conditions,such as varying the pH value from 0 to 14 and maintaining the temperature at 100°C for 30 min[18].Laszlo et al.has shown that the MspA nanopore can accurately sequence the phi X 174 genome up to 4500 bases in length[19].

Table 1 Comparison of the schematics and structural features for the major types of nanopore

Figure 2 Side and top views of three biological nanopores
Bacteriophage phi29
Phi29,another biological nanopore,has generated a great deal of interest.Wendell et al.frst demonstrated that doublestranded DNA(dsDNA)could pass through the phi29 pore [20].As shown inFigure 2C,the bacteriophage phi29 DNA-packaging motor has a 12-subunit gp10 connector[21],six copies of ATP-binding DNA packaging RNA(pRNA)[22], and an ATPase protein,gp16[23],which provides the chemical energy required for DNA translocation.The connector protein can easily self-assemble to form a stable and repetitive dodecameric structure in solution.The length is about 7 nm,while the cross-sectional area of the channel is about 10 nm2(3.6 nm in diameter)at one end and 28 nm2(6 nm in diameter)at the other end[24].The phi29 connector channel shows stable channel properties in a voltage range from-150 mV to +150 mV,even under a wide range of pH conditions[25].
Compared to α-HL and MspA,the phi29 pore has a larger diameter,which allows for the measurement of larger molecules,such as dsDNA,complexes of DNA,and proteins. The larger phi29 pore also provides more fexibility for biochemical modifcations.
Commercial biological nanopore sequencers
Oxford Nanopore Technologies(ONT),founded by Hagan Bayley and Gordon Sanghera,has been developing nanopore-based DNA sequencing systems for commercial use. ONT released the preliminary experimental results from its larger GridION system at the Advances in Genome Biology& Technology meeting(AGBT)in Marco Island,Florida in February 2012[26].ONT’s GridION system can be extended with additional cartridges,each of which contains arrays of nanopores.Each GridION node and cartridge can produce several gigabytes of raw data per day.The system is designed for fexible run times ranging from a few minutes up to several days depending on the data requirements of the experiments.

Figure 3 The portable MinION––the frst handheld nanopore DNA sequencer
ONT has miniaturized the commercial nanopore sequencing instruments to develop the MinION[26],frst launched at February’s AGBT conference in Marco Island,Florida (shown inFigure 3)in November 2013.The MinION is a single-use DNA sequencing device with the size of a USB memory stick,which is designed for general applications of DNA sequencing.More recently,it was announced at AGBT 2014 [27]that the average read length using the MinION is about 5.4 kb up to 10 kb.This is much longer than the average read lengths of other DNA sequencing technologies,which are hundreds of base pairs.Several groups have used the MinION to sequenceEscherichia coliK-12 substr.[28],the λ phage genome,and amplicons from a snake venom gland transcriptome. However,the error rates of these analyses were over 90%[14]. Although the MinION is still a long way from use in a wide range of applications,these results are very encouraging for nanopore-based DNA sequencing technology.
Solid-state nanopore
Although biological nanopores have shown very exciting experimental results for ssDNA sequencing,these protein pores have a constant pore size,profle and lack of stability [29].Furthermore,they suffer from the fragility of traditional supported lipid membranes.To adjust for these defciencies, various synthetic nanopores have been fabricated using different methods and applied to DNA and RNA analysis [12,18,30–32].
In 2001,Li et al.confrmed that solid-state nanopores can be used to study the molecule translocation process[11]. With thedevelopmentofmicrofabrication technologies, solid-state nanopores have attracted increasing attention. Solid-state nanopores have many superior advantages over their biological counterparts,such as chemical,thermal,and mechanicalstability,size adjustability,and integration [6,11,18].In addition,solid-state nanopores can work properly under a wide variety of experimental conditions and can be mass produced using conventional semiconductor processes. In recent years,solid-state nanopores have been applied as a new method in various felds,including DNA sequencing,protein detection,molecule translocation process,and disease diagnosis[33].Several primary techniques are often used to fabricate nanopores in silicon nitride(Si3N4)[30],silicon dioxide(SiO2)[12],aluminum oxide(Al2O3)[34],boron nitride (BN)[35],graphene[36],polymer membranes[37],and hybrid materials[38].Methods of fabricating nanopores include the ion milling track-etch method[11],electron beam based decomposition sputtering[12,30],focused ion beam(FIB) techniques[39],the laser ablation method[40],electron-beam lithography[38],helium ion microscopy[41],and the latest dielectric breakdown methods[42,43].Numerous groups have studied ultrathin membranes using chemical vapor deposition [38,44],and there are also studies on biomolecule transport through nanopores in graphene[33,45].The exclusive electrical and geometric properties of solid-state nanopores give them a distinct advantage over their biological counterparts;however, for these nanopores to make reliable devices,their chemical and thermal stabilities still need to be improved[33,40] (Figure 4).
Si3N4and SiO2nanopores
Si3N4and SiO2flms have been widely used as substrates because of their low mechanical stress and high chemical stability[46].They can be manufactured with the complementary metal oxide semiconductor(CMOS)-compatible industrial integrated circuit processes[16,40,47–49].Nanopores are often drilled by electron or ion beam sculpting in a free-standing membrane window,which can be controlled by standard photolithography [48,50]and wet-etching techniques with micrometer precision[11,40,49,51–53].Si3N4and SiO2substrates also exhibit good performance in high concentrations of an electrolyte solution.Immersion in an electrolyte solution has been shown to change the size of the Si3N4and SiO2pores over time[54].Two groups have reported that Si3N4and SiO2pores were used to detect DNA molecules,although DNA sequencing has not yet been demonstrated[18,55].
Al2O3membranes
Compared to SiO2and Si3N4flms,Al2O3flms have improved electrical performance,a higher signal-to-noise ratio,and lower noise during DNA translocation[6].Atomic-layer deposition (ALD)can be used to fabricate Al2O3membranes at single atomic-level thickness.Focused ion beam(FIB)and transmission electron microscopy(TEM)can be used to manufacture nanopores in metal oxide membranes[6,40].The DNA translocation speed was slower through Al2O3nanopores than that seen through Si3N4nanopores,which is attributed to the strong electrostatic interaction between the positively-charged surface of Al2O3and the negatively-charged dsDNA molecules[40].
Single-layer membranes
Although solid-state nanopores fabricated in insulating membranes have been widely applied in DNA and protein translocation processes,they do not have enough spatial and temporal resolution to obtain structural information of molecules at the single-base level.Graphene membrane,a single atomic layer of carbons with extraordinary electrical and mechanical properties,has recently been used as an alternative to traditional solid-state membranes[33].BN and molybdenum disulfde(MoS2)have also generated a great deal of interest[35,38,51,56].Nanopores can be fabricated in suspended single-layer membranes by controlled electron-beam exposure via TEM[45].One particular advantage of using nanopores in ultrathin membranes is that the minimal thickness of the membrane(~0.335 nm)is equivalent to the distance betweentwo bases in a DNA chain[33].Single layer membranes may hold the potential to achieve extraordinarily high spatial resolution for DNA sequencing.
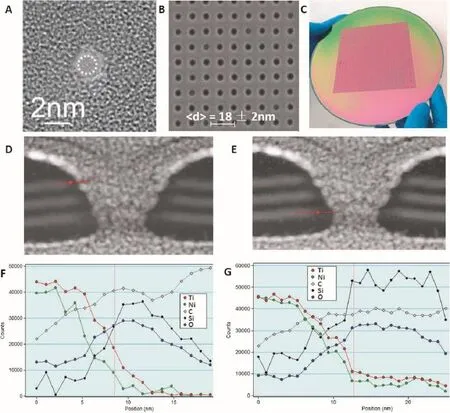
Figure 4 Solid state nanopores
Hybrid biological/solid-state nanopores
Amajordrawbackofsolid-statenanoporesatpresentisthelack ofchemicaldifferentiationfromthetargetmoleculesofapproximately the same size.This chemical specifcity can be improved by functionalizing surfaces[38]or attaching specifc recognition sequences and receptors to the nanopores[34,57].Nanopores functionalized with hairpin DNAs or other receptors have the potentialtouniquelyidentifynucleotidesinsequencingapplications[7,57].The synthetic nanopores can be coated with a fuid lipidbilayertocontrolproteintranslocations[58].Thethickness and surface chemistry of the coating surface can be accurately controlled by various lipids.Venkatesan et al.have reported vesicle fusion techniques to form fuid lipid bilayers with high impedance on single Al2O3nanopore sensors[34].These nanoporesensors exhibitexcellent electricalproperties andenhanced mechanical stability and,therefore,may fnd broader applications in nano-biotechnology.
Applications
Nanopore-based DNA sequencing
ssDNA detection
In 1996,Kasianowicz et al.demonstrated that an α-HL channel embedded in planar phospholipid bilayers has the ability to electrically detect individual ssDNA and ssRNA molecules [3],which marked the beginning of the era of single-molecule nanopore DNA sequencing.Recently,Clarke et al.demonstrated that α-HL with a covalently-attached adapter molecule can be used to continuously identify unlabeled single nucleotides(dAMP,dCMP,dGMP,and dTMP)through nanopore-based resistive current measurements[59].Besides the α-HL channel,the MspA nanopore,containing a single 1.2-nm wide and 0.6-nm long constriction,also allows for the detection of ssDNA.In 2010,Derrington et al.demonstrated that the MspA nanopore has a high signal-to-noise ratio and can be used to distinguish single nucleotides of ssDNA,while duplex DNA sections pause the translocation [13].
Many groups have used a solid-state nanopore to study the ssDNA translocation process.InFigure 5,the nanopore group at IBM was able to slow down the translocation of ssDNA with a 6-nm diameter solid-state nanopore immersed in different concentrations of a glycerol solution[60].Iqbal et al.functionalized a silicon-on-insulator solid-state nanopore using hairpin-loop DNA as a selected probe for single molecule DNA detection[57].Their results show a shorter translocation time of the target molecules,which exhibit single molecule mismatch than that of the perfectly-complementary DNA molecules,which is the opposite of the experimental result using bio-nanopores.These functionalized nanopores have the potential to be stable and robust and can be implemented in an array.
However,real-time DNA sequencing is currently a major challenge because longitudinal current detection cannot distinguish individual nucleotides due to the thickness of membrane(>10 bases)and the fast translocation of a single base[45].Many experiments have focused on measuring the transverse tunneling current or capacitance when a single ssDNA molecule is driven through a solid-state nanopore with embedded electrodes[7,45,61,62].Ventra et al.were able to detect the transverse charge transport to the backbone axis of ssDNA between two embedded electrodes inside of the nanopore[61].Each nucleotide provides a unique electrical signature determined by its orientation and charge properties. It is also slightly affected by neighboring nucleotides if the lateral dimension of the electrode is comparable with the spatial gap between the two bases.Although this approach seems to provide a potential method for DNA sequencing, there are several challenges that must be addressed:(1)the thickness of the embedded electrode must be comparable with the‘size’of the bases[45];(2)the device must be able to control the orientation and position of each base between metal electrodes as the tunneling current is exponentially sensitive to atomic scale changes of orientations and distance [62];and(3)unidirectional translocation must be controlled so that each nucleobase remains between the tunneling probes for at least 0.1 ms to sample over inevitable noise and molecule motion[7].
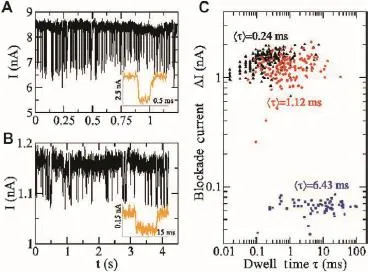
Figure 5 Experimental results on ssDNA translocation through a 6-nm nanopore
dsDNA segment detection
Most biological pores have small channels,which only allow small molecules to pass through,such as ssDNA and ssRNA.The bacteriophage phi29 DNA-packaging motor has a minimal 3.6-nm diameter channel that allows for the translocation of ssDNA,dsDNA,and small proteins.In 2009, Wendell et al.reported that modifcation of this connector protein with distinct regions of hydrophilicity allows for the translocation of dsDNA when reconstituted into liposomes and inserted into a planar lipid bilayer[20].Haque et al.frst demonstrated that as a native wild type protein channel,the phi29 connector channel exhibits a rectifying behavior with regard to DNA translocation;that is,it only allows dsDNA to translocate from the N-terminal entrance(narrower-end) to the C-terminal exit(wider-end).This one-way traffc property has been demonstrated by voltage ramping, electrode polarity switching,and sedimentation force assessment[25].
The possibility of using solid-state nanopores for DNA translocation was frst shown by Li and colleagues[11,18]. They demonstrated a solid-state nanopore microscope capable of electronically characterizing dsDNA molecules.The folding confgurations of dsDNA could also be revealed by the ionic current blockade induced by the DNA molecule.In 2009, Bashir et al.studied the properties of nanopores in Al2O3membranes formed via the atomic layer deposition process [6,34,63].These nanopores showed better noise performance and higher mechanical properties over their silicon-substrate counterparts.Furthermore,the average translocation velocities of dsDNA through these alumina nanopores were an order of magnitude less than those measured through Si3N4/SiO2nanopores[63].InFigure 6,the nanopore group at IBM has shown that a nanopore functionalized with a self-assembledmonolayer(SAM)can potentially regulate the transport of a DNA molecule by changing the hydrophobility and hydrophobicity of SAM.They found that an enhanced interaction between DNA and a SAM-coated nanopore can slow down the translocation speed of DNA molecules and increase the DNA capture rate in the hydrophobic state[38].Dimitrov et al.at the University of Illinois at Urbana–Champaign was able to trap single λ phage DNA for about 20 s extremely with a 2.6-nm diameter solid-state nanopore[44].DNA detection based on graphene nanopores was frst demonstrated by Golovchenkoand then studied bymanyothergroups [36,64,65].In these devices,graphene acts as the membrane allowing for single base resolution in the ionic current due to its single-atom thickness.The noise of the ionic current of graphene nanopores was several orders of magnitude larger than that of silicon-based nanopores.
Xie et al.described a novel sensor that combined solid-state nanopores with silicon nanowire feld-effect transistors(FETs) on a Si3N4membrane,in which detection is locally self-aligned at the nanopore(Figure 4A)[47].Both FET signals and block ionic current signals are observed simultaneously during dsDNA translocation.It has been demonstrated that nanowire FET could achieve high speed and sensitivity as chemical and biological sensors[7].Signifcantly,if combined with nanopores,the nanowire FET would offer the possibility of DNA sequencing characterized by high sensitivity and high throughput.Radenovic et al.recently reported nanopore DNA detection based on a graphene FET[33].A lower translocation rate at a 200-mV transmembrane bias was achieved,and the longer DNA translocation time facilitated the event analysis.For the frst time,they demonstrated that translocation events of DNA segments are detected by electrical means other than the ionic current itself using a novel device based on the integration of graphene ribbons with solid-state Si3N4nanopores.
Other applications
The engineered transmembrane protein pores of α-HL have been used as stochastic sensing elements for the identifcation and quantifcation of a wide variety of substances,including cations[66],anions[11],organic molecules[67],enantiomers [68],chemical and biological terrorism agents[69–72],DNA [3,59,73–78],peptides[70,79–82],proteins[66,83–85],and microRNAs[86].The α-HL nanopore was able to detect 2,4,6-trinitrotoluene(TNT)in an aqueous feld following the introduction of aromatic side chains at position 113 of the α-HL polypeptide using site-directed mutagenesis [69]. Furthermore,via weak non-covalent bonding interactions, various peptides may be identifed and differentiated using engineered protein pores with different surface functional groups.For example,a series of short peptides,consisting of mainly aromatic amino acids and of various lengths,is able to be analyzed with a(M113Y)7pore,which contains an aromatic binding site with seven aromatic tyrosine chains.In addition,due to differences in blockage residual currents and mean residence timesoftranslocation events(Figure7),the sequences of short peptides such as PYWF,YPWF,YWPF, and YPFW could also be differentiated from each other [70,87].
It is well known that β-cyclodextrin(β-CD)can be used as a composite host to capture and sense organic molecules[88]. Therefore,pinacolyl methylphosphonic acid(PMPA)and cyclohexyl methylphosphonic acid(CMPA),the hydrolysis products of the nerve agents soman(GD)and cyclohexylsarin (GF),respectively,could be detected using the engineered α-HL(M113F/K147N)7pore and a host β-CD molecule(as depicted inFigure 8)as a molecular adapter[71].When β-CD is bound to the α-HL pore,the channel is partially blocked,and the open channel current drops.A guest molecule(PMPA or CMPA)added in the channel would be captured by β-CD,blocking the channel and greatly reducing the current.

Figure 6 Experimental set-up
Challenges
Although the nanopore-based sequencing technology has emerged to be a promising tool,several problems remain to be solved.One of the more troublesome among these is the rapid DNA translocation velocity(~1–3 μs/base),which limits the identifcation of single nucleotide bases via the currently available single-channel recording technique[89].In the past several years,various strategies have been developed to slow down the translocation rate,including immobilization of DNA polynucleotides with streptavidin[90],formation of DNA-hemolysin rotaxane[77],and binding to an enzyme [91–94].Furthermore,a modifed α-HL channel conjugated with a molecular adaptor β-CD via disulfde linkage was able to successfully capture and identify four single nucleotide bases [59,89].The optimization of experimental physical conditions is also useful to control the DNA translocation rate,including reducing the voltage[3],decreasing the solution temperature [75],increasing the viscosity[75,95],and increasing the salt concentration[64].Recently,Manrao et al.demonstrated a new method to sequence ssDNA at single-nucleotide resolution using a mutant MspA nanopore and phi29 DNA polymerase[96],which can control the rate of DNA translocation with median durations of~28 ms and ionic current differences up to 40 pA.Atomic force microscopy has been developed to control the DNA translocation rate at less than 100 μs/base [97],allowing the ionic current and force signal to be detected simultaneously[98,99].This technique directly reveals how the position of a single DNA molecule could affect the ionic current of the nanopore when the DNA is moved around the entrance and exit of the nanopore.It can also be used to study the friction interaction between single DNA molecules and different pore surfaces.
As addressed previously,α-HL and MspA nanopores can sequence DNA molecules up to a few kilobases in length by reading three or four nucleotides simultaneously with a large error rate.One of the most promising methods is reading the tunneling current through a single base between two electrodes.However,the orientation of single bases and distances between two electrodes are diffcult to control.The size and thickness of the two electrodes should be designed carefully. Teams at the Arizona State University and IBM have used a multi-layer structure to fx the gap between the top and bottom electrodes for single base detection[53].However,reading single nucleotide information from a long chain of DNA through a nanopore or nanogap continues to present a challenge.
The use of nanopore array sensors has become an active research area,and methods of fabricating the sensors have been improving as well.With multi-dimensional signals,a nanopore array can increase the capability to distinguish analytes compared to a single pore.This allows for identifcation of a target molecule from a mixture and offers the potential for monitoring multiple targets simultaneously[87].Using the reactive ion etching(RIE)method instead of TEM drilling, an average size of 18±2 nm nanopore arrays were fabricated in a membrane with an embedded 3-level metal[38].Thesenanopore devices are electrically and structurally functional and use semiconductor processes that are compatible with integrated circuits.For the commercialization of a DNA sequencing instrument or sensor based on solid-state nanopores,it will be necessary to develop new approaches to mass production of nanopores smaller than 20 nm in size.
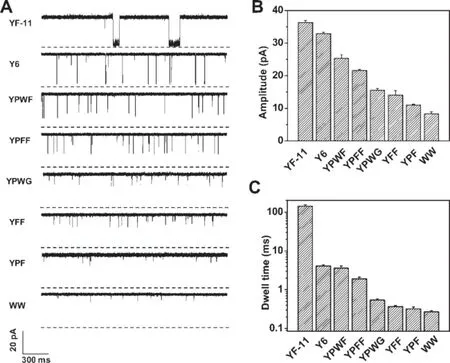
Figure 7 Effects of peptide length and structure on the transport of peptides through a single(M113Y)7pore
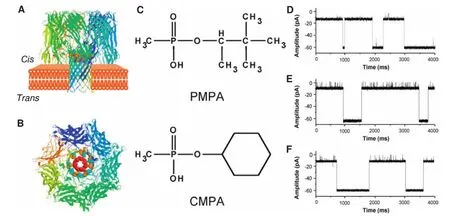
Figure 8 Molecular graphic representation of the staphylococcalα-HL protein withβ-CD lodged in the lumen of the channel
Summary
Detection of single molecules using nanopore-based technology has been used for the identifcation and quantifcation of a wide variety of analytes.As the fourth-generation sequencing technique,nanopores have the potential to become a labelfree,rapid,and low-cost DNA sequencing technology and thus may achieve the$1000-per-human-genome goal set by the National Institutes of Health of the United States.At the same time,nanopores provide several advantages,including minimal sample preparation,elimination of the need for amplifcation or modifcation(nucleotides,polymerases or ligases),and long read lengths(10,000–50,000 bases).Although nanopore DNA sequencing has good maneuverability,there are still signifcant challenges remaining to be overcome.Among them,a key limitation of nanopore-based DNA sequencing at the single-molecule level is the requirement of ultra-precise,high-speed DNA detection beyond the spatial and temporal resolutions of existing optical and electrical technologies.Therefore,single base recognition and slowing down the rate of DNA velocity are still the principal challenges.Nevertheless,nanopore technology will have a tremendous impact on DNA sequencing and the future of personal health and disease diagnosis.
Competing interests
The authors have declared no competing interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China–China(Grant No.61471336),and by the Joint-Scholar of West Light Foundation of the Chinese Academy ofSciencesawarded to DW and Shanghai Synchrotron Radiation Facility.CY was supported by China PostdoctoralScience Foundation – China (GrantNo. 2014M551011).
[1]Deamer DW,Akeson M.Nanopores and nucleic acids:prospects for ultrarapid sequencing.Trends Biotechnol 2000;18:147–51.
[2]Church G,Deamer DW,Branton D,Baldarelli R,Kasianowicz J. Measuring physical properties.US5795782;1998.
[3]Kasianowicz JJ,Brandin E,Branton D,DeamerDW. Characterization of individual polynucleotide molecules using a membrane channel.Proc Natl Acad Sci U S A 1996;93:13770–3.
[4]Coulter WH.Means for counting particles suspended in a fuid. US2656508;1953.
[5]Cornell BA,BraachMaksvytis VLB,King LG,Osman PDJ, Raguse B,Wieczorek L,et al.A biosensor that uses ion-channel switches.Nature 1997;387:580–3.
[6]Venkatesan BM,Dorvel B,Yemenicioglu S,Watkins N,Petrov I, Bashir R.Highly sensitive,mechanically stable nanopore sensors for DNA analysis.Adv Mater 2009;21:2771.
[7]Branton D,Deamer DW,Marziali A,Bayley H,Benner SA, Butler T,et al.The potential and challenges of nanopore sequencing.Nat Biotechnol 2008;26:1146–53.
[8]Wanunu M.Nanopores:a journey towards DNA sequencing. Phys Life Rev 2012;9:125–58.
[9]Maitra RD,Kim J,Dunbar WB.Recent advances in nanopore sequencing.Electrophoresis 2012;33:3418–28.
[10]Dekker C. Solid-state nanopores. Nat Nanotechnol 2007;2:209–15.
[11]Cheley S,Gu LQ,Bayley H.Stochastic sensing of nanomolar inositol 1,4,5-trisphosphate with an engineered pore.Chem Biol 2002;9:829–38.
[12]Storm AJ,Chen JH,Ling XS,Zandbergen HW,Dekker C. Fabrication ofsolid-state nanoporeswith single–nanometre precision.Nat Mater 2003;2:537–40.
[13]Derrington IM,Butler TZ,Collins MD,Manrao E,Pavlenok M, Niederweis M,et al.Nanopore DNA sequencing with MspA. Proc Natl Acad Sci U S A 2010;107:16060–5.
[14]Mikheyev AS,Tin MMY.A frst look at the Oxford Nanopore MinION sequencer.Mol Ecol Resour 2014;14:1097–102.
[15]Song LZ,Hobaugh MR,Shustak C,Cheley S,Bayley H,Gouaux JE.Structure of staphylococcal alpha-hemolysin,a heptameric transmembrane pore.Science 1996;274:1859–66.
[16]Cherf G,Lieberman K,Rashid H,Lam C,Karplus K,Akeson M. Automated forward and reverse ratcheting of DNA in a nanopore at 5-a precision.Nat Biotechnol 2012;30:344–8.
[17]Kang XF,Gu LQ,Cheley S,Bayley H.Single protein pores containing molecular adapters at high temperatures.Angew Chem Int Ed Engl 2005;44:1495–9.
[18]Abiola O,Angel JM,Avner P,Bachmanov AA,Belknap JK,BennettB,et al.The nature and identifcation of quantitative trait loci:a community’s view.Nat Rev Genet 2003;4:911–6.
[19]Laszlo AH,Derrington IM,Ross BC,Brinkerhoff H,Adey A, Nova IC,et al.Decoding long nanopore sequencing reads of natural DNA.Nat Biotechnol 2014;32:829–33.
[20]WendellD,JingP,GengJ,Subramaniam V,LeeTJ, Montemagno C,et al.Translocation of double-stranded DNA through membrane-adapted phi29 motor protein nanopores.Nat Nanotechnol 2009;4:765–72.
[21]Guasch A,Pous J,Ibarra B,Gomis-Ruth FX,Valpuesta JM, Sousa N,et al.Detailed architecture of a DNA translocating machine:the high-resolution structure of the bacteriophage phi 29 connector particle.J Mol Biol 2002;315:663–76.
[22]Guo PX,Zhang CL,Chen CP,Garver K,Trottier M.Inter-RNA interaction of phage phi 29 pRNA to form a hexameric complex for viral DNA transportation.Mol Cell 1998;2:149–55.
[23]Lee TJ,Guo PX.Interaction of gp16 with pRNA and DNA for genome packaging by the motor of bacterial virus phi29.J Mol Biol 2006;356:589–99.
[24]Xiang Y,Morais MC,Battisti AJ,Grimes S,Jardine PJ, Anderson DL,et al.Structuralchangesofbacteriophage phi29 upon DNA packaging and release.EMBO J 2006; 25:5229–39.
[25]Haque F,Guo P.Membrane-embedded channel of bacteriophage phi29 DNA-packaging motor for translocation and sensing of double-stranded DNA.In:IqbalSM,BashirR,editors. Nanopores.New York:Springer US;2011.p.77–106.
[26]Eisenstein M.Oxford Nanopore announcement sets sequencing sector abuzz.Nat Biotechnol 2012;30:295–6.
[27]Hayden EC.Data from pocket-sized genome sequencer unveiled. Nature 2014.http://dx.doi.org/10.1038/nature.2014.14724.
[28]Quick J,Quinlan A,Loman N.A reference bacterial genome dataset generated on the MinIONTM portable single-molecule nanopore sequencer.GigaScience 2014;3:22.
[29]Deamer DW,Branton D.Characterization of nucleic acids by nanopore analysis.Acc Chem Res 2002;35:817–25.
[30]Heng JB,Ho C,Kim T,Timp R,Aksimentiev A,Grinkova YV, et al.Sizing DNA using a nanometer-diameter pore.Biophys J 2004;87:2905–11.
[31]Heng JB,Aksimentiev A,Ho C,Marks P,Grinkova YV,Sligar S, et al.Stretching DNA using the electric feld in a synthetic nanopore.Nano Lett 2005;5:1883–8.
[32]Heng JB,Aksimentiev A,Ho C,Marks P,Grinkova YV,Sligar S, et al.The electromechanics of DNA in a synthetic nanopore. Biophys J 2006;90:1098–106.
[33]Traversi F,Raillon C,Benameur SM,Liu K,Khlybov S,Tosun M,et al.Detecting the translocation of DNA through a nanopore using graphene nanoribbons.Nat Nanotechnol 2013;8:939–45.
[34]Venkatesan BM,Bashir R.Nanopore sensors for nucleic acid analysis.Nat Nanotechnol 2011;6:615–24.
[35]Liu S.Boron nitride nanopores:highly sensitive DNA singlemolecule detectors.Adv Mater 2013;25:4549–54.
[36]Garaj S,Hubbard W,Reina A,Kong J,Branton D,Golovchenko JA.Graphene as a subnanometre trans-electrode membrane. Nature 2010;467:190–3.
[37]Menestrina J,Yang C,Schiel M,Vlassiouk IV,Siwy ZS.Charged particles modulate local ionic concentrations and cause formation of positive peaks in resistive-pulse based detection.J Phys Chem C 2014.http://dx.doi.org/10.1021/jp412135v.
[38]Bai JW,Wang DQ,Nam SW,Peng HB,Bruce R,Gignac L,et al. Fabrication of sub-20 nm nanopore arrays in membranes with embedded metal electrodes at wafer scales. Nanoscale 2014;6:8900–6.
[39]Bayley H.Sequencing single molecules of DNA.Curr Opin Chem Biol 2006;10:628–37.
[40]Haque F,Li JH,Wu HC,Liang XJ,Guo PX.Solid-state and biological nanopore for real-time sensing of single chemical and sequencing of DNA.Nano Today 2013;8:56–74.
[41]Marshall MM,Yang J,Hall AR.Direct and transmission milling of suspended silicon nitride membranes with a focused helium ion beam.Scanning 2012;34:101–6.
[42]Harold K,Kyle B,Vincent T-C.Nanopore fabrication by controlled dielectric breakdown.PLoS One 2013:19.
[43]Yanagi I,Akahori R,Hatano T,Takeda K-I.Fabricating nanopores with diameters of sub-1 nm to 3 nm using multilevel pulse-voltage injection.Sci Rep 2014;4.http://dx.doi.org/10.1038/ srep05000.
[44]Dimitrov V,Mirsaidov U,Wang DQ,Sorsch T,Mansfeld W, Miner J,et al.Nanopores in solid-state membranes engineered for single molecule detection.Nanotechnology 2010;21:065502.
[45]Liu S,Zhao Q,Xu J,Yan K,Peng HL,Yang FH,et al.Fast and controllable fabrication of suspended graphene nanopore devices. Nanotechnology 2012;23:6.
[46]Kim MJ,McNally B,Murata K,Meller A.Characteristics of solid-state nanometre pores fabricated using a transmission electron microscope.Nanotechnology 2007;18:5.
[47]Gao N,Xie C.Experimental demonstration of free-space optical vortex transmutation with polygonal lenses. Opt Lett 2012;37:3255–7.
[48]Dai L,Gao X,Guo Y,Xiao J,Zhang Z.Bioinformatics clouds for big data manipulation.Biol Dir 2012;7:43[discussion].
[49]Gao N,Li H,Zhu X,Hua Y,Xie C.Quasi-periodic gratings: diffraction orders accelerate along curves. Opt Lett 2013;38:2829–31.
[50]Gao N,Xie C.High-order diffraction suppression using modulated groove position gratings.Opt Lett 2011;36:4251–3.
[51]Zhou S,Xie C,Yang Y,Hu S,Xu X,Yang J.Moire-based phase imaging for sensing and adjustment of in-plane twist angle.IEEE Photonics Technol Lett 2013;25:1847–50.
[52]Gao N, Xie C. Parabolic scaling beams. Opt Lett 2014;39:3619–22.
[53]Pang P,Ashcroft BA,Song W,Zhang P,Biswas S,Qing Q,et al. Fixed-gap tunnel junction for reading DNA nucleotides.ACS Nano 2014;8:11994–2003.
[54]Hall AR,Scott A,Rotem D,Mehta KK,Bayley H,Dekker C. Hybrid pore formation by directed insertion of[alpha]-haemolysin into solid-state nanopores.Nat Nanotechnol 2010;5:874–7.
[55]Storm AJ,Chen JH,Zandbergen HW,Dekker C.Translocation of double-strand DNA through a silicon oxide nanopore.Phys Rev E Stat Nonlinear Soft Matter Phys 2005;71:10.
[56]Farimani AB,Min K,Aluru NR.DNA base detection using a single-layer MoS2.ACS Nano 2014;8:7914–22.
[57]Iqbal SM,Akin D,Bashir R.Solid-state nanopore channels with DNA selectivity.Nat Nanotechnol 2007;2:243–8.
[58]Yusko EC,Johnson JM,Majd S,Prangkio P,Rollings RC,Li JL, et al.Controlling protein translocation through nanopores with bio-inspired fuid walls.Nat Nanotechnol 2011;6:253–60.
[59]Clarke J,Wu HC,Jayasinghe L,Patel A,Reid S,Bayley H. Continuous base identifcation for single-molecule nanopore DNA sequencing.Nat Nanotechnol 2009;4:265–70.
[60]Luan Binquan,Wang Deqiang,Zhou Ruhong,Harrer Stefan, Peng Hongbo,Stolovitzky Gustavo.Dynamics of DNA translocation in a solid-state nanopore immersed in aqueous glycerol. Nanotechnology 2012;23:455102.
[61]Zwolak M,Di Ventra M.Electronic signature of DNA nucleotides via transverse transport.Nano Lett 2005;5:421–4.
[62]Zhang XG,Krstic PS,Zikic R,Wells JC,Fuentes-Cabrera M. First-principles transversal DNA conductance deconstructed. Biophys J 2006;91:L04–6.
[63]Venkatesan BM,Shah AB,Zuo JM,Bashir R.DNA sensing using nanocrystalline surface-enhanced Al2O3nanopore sensors. Adv Funct Mater 2010;20:1266–75.
[64]Merchant CA,Healy K,Wanunu M,Ray V,Peterman N,Bartel J,et al.DNA translocation through graphene nanopores.Nano Lett 2010;10:2915–21.
[65]Schneider GF,Kowalczyk SW,Calado VE,Pandraud G, Zandbergen HW, Vandersypen LMK, et al. DNA translocation through graphene nanopores.Nano Lett 2010;10: 3163–7.
[66]Movileanu L,Howorka S,Braha O,Bayley H.Detecting protein analytes that modulate transmembrane movement of a polymer chain within a single protein pore.Nat Biotechnol 2000;18: 1091–5.
[67]Gu LQ,Braha O,Conlan S,Cheley S,Bayley H.Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter.Nature 1999;398:686–90.
[68]Kang XF,Cheley S,Guan XY,Bayley H.Stochastic detection of enantiomers.J Am Chem Soc 2006;128:10684–5.
[69]Guan XY,Gu LQ,Cheley S,Braha O,Bayley H.Stochastic sensing of TNT with a genetically engineered pore.Chembiochem 2005;6:1875–81.
[70]Jayawardhana DA,Crank JA,Zhao Q,Armstrong DW,Guan XY.Nanopore stochasticdetection ofa liquid explosive component and sensitizers using boromycin and an ionic liquid supporting electrolyte.Anal Chem 2009;81:460–4.
[71]Wang D,Zhao Q,Zoysa RSSD,Guan X.Detection of nerve agent hydrolytes in an engineered nanopore.Sens Actuators B Chem 2009;139:440–6.
[72]Wu HC,Bayley H.Single-molecule detection of nitrogen mustards by covalent reaction within a protein nanopore.J Am Chem Soc 2008;130:6813–9.
[73]Maglia G,Henricus M,Wyss R,Li QH,Cheley S,Bayley H. DNA strands from denatured duplexes are translocated through engineered protein nanoporesatalkalinepH.Nano Lett 2009;9:3831–6.
[74]Maglia G,Restrepo MR,Mikhailova E,Bayley H.Enhanced translocation of single DNA molecules through alpha-hemolysin nanopores by manipulation of internal charge.Proc Natl Acad Sci U S A 2008;105:19720–5.
[75]Meller A,Nivon L,Brandin E,Golovchenko J,Branton D.Rapid nanopore discrimination between single polynucleotide molecules. Proc Natl Acad Sci U S A 2000;97:1079–84.
[76]Howorka S,Cheley S,Bayley H.Sequence-specifc detection of individualDNA strandsusingengineered nanopores.Nat Biotechnol 2001;19:636–9.
[77]Sanchez-Quesada J,Saghatelian A,Cheley S,Bayley H,Ghadiri MR.Single DNA rotaxanes of a transmembrane pore protein. Angew Chem Int Ed Engl 2004;43:3063–7.
[78]Stoddart D,Heron AJ,Mikhailova E,Maglia G,Bayley H. Single-nucleotide discrimination in immobilized DNA oligonucleotides with a biological nanopore.Proc Natl Acad Sci U S A 2009;106:7702–7.
[79]Mohammad MM,Movileanu L.Excursion of a single polypeptide into a protein pore:simple physics,but complicated biology.Eur Biophys J 2008;37:913–25.
[80]Movileanu L,SchmittschmittJP,ScholtzJM,Bayley H. Interactionsofpeptideswith a protein pore.BiophysJ 2005;89:1030–45.
[81]Stefureac R,Long YT,Kraatz HB,Howard P,Lee JS.Transport of alpha-helical peptides through alpha-hemolysin and aerolysin pores.Biochemistry 2006;45:9172–9.
[82]Wolfe AJ,Mohammad MM,Cheley S,Bayley H,Movileanu L. Catalyzing the translocation of polypeptides through attractive interactions.J Am Chem Soc 2007;129:14034–41.
[83]Cheley S,Xie HZ,Bayley H.A genetically encoded pore for the stochastic detection of a protein kinase. Chembiochem 2006;7:1923–7.
[84]Howorka S,Nam J,Bayley H,Kahne D.Stochastic detection of monovalent and bivalent protein–ligand interactions.Angew Chem Int Ed Engl 2004;43:842–6.
[85]Xie HZ,Braha O,Gu LQ,Cheley S,Bayley H.Single-molecule observation of the catalytic subunit of cAMP-dependent protein kinase binding to an inhibitor peptide.Chem Biol 2005;12:109–20.
[86]Wang Y,Zheng DL,Tan QL,Wang MX,Gu LQ.Nanoporebased detection of circulating microRNAs in lung cancer patients. Nat Nanotechnol 2011;6:668–74.
[87]Zhao QT,Wang D,Jayawardhana DA,Guan XY.Stochastic sensing of biomolecules in a nanopore sensor array. Nanotechnology 2008;19:8.
[88]Loftsson T,Duchene D.Cyclodextrins and their pharmaceutical applications.Int J Pharm 2007;329:1–11.
[89]Astier Y,Braha O,Bayley H.Toward single molecule DNA sequencing:direct identifcation of ribonucleoside and deoxyribonucleoside 5′-monophosphates by using an engineered protein nanopore equipped with a molecular adapter.J Am Chem Soc 2006;128:1705–10.
[90]Purnell RF,Mehta KK,Schmidt JJ.Nucleotide identifcation and orientation discrimination of DNA homopolymers immobilized in a protein nanopore.Nano Lett 2008;8:3029–34.
[91]Olasagasti F,Lieberman KR,Benner S,Cherf GM,Dahl JM, Deamer DW,et al.Replication of individual DNA molecules under electronic controlusing a protein nanopore.Nat Nanotechnol 2010;5:798–806.
[92]Benner S,Chen RJA,Wilson NA,Abu-Shumays R,Hurt N, Lieberman KR,et al.Sequence-specifc detection of individual DNA polymerase complexes in real time using a nanopore.Nat Nanotechnol 2007;2:718–24.
[93]Hornblower B,Coombs A,Whitaker RD,Kolomeisky A,Picone SJ,Meller A,et al.Single-molecule analysis of DNA–protein complexes using nanopores.Nat Methods 2007;4:315–7.
[94]Cockroft SL,Chu J,Amorin M,Ghadiri MR.A single-molecule nanopore device detects DNA polymerase activity with singlenucleotide resolution.J Am Chem Soc 2008;130:818.
[95]Fologea D,Uplinger J,Thomas B,McNabb DS,Li JL.Slowing DNA translocation in asolid-statenanopore.Nano Lett 2005;5:1734–7.
[96]Manrao EA,Derrington IM,Laszlo AH,Langford KW,Hopper MK,Gillgren N,et al.Reading DNA at single-nucleotide resolution with a mutant MspA nanopore and phi29 DNA polymerase.Nat Biotechnol 2012;30:349–53.
[97]Hyun C,Kaur H,Rollings R,Xiao M,Li J.Threading immobilized DNA molecules through a solid-state nanopore at>100 μs per base rate.ACS Nano 2013;7:5892–900.
[98]Kurz V,Nelson EM,Shim J,Timp G.Direct visualization of single-molecule translocations through synthetic nanopores comparable in size to a molecule.ACS Nano 2013;7:4057–69.
[99]Nelson EM,Li H,Timp G.Direct,concurrent measurements of the forces and currents affecting DNA in a nanopore with comparable topography.ACS Nano 2014;8:5484–93.
Received 4 January 2015;revised 14 January 2015;accepted 23 January 2015
Available online 2 March 2015
Handling Editor:Fei Chen
*Corresponding author.
E-mail:dqwang@cigit.ac.cn(Wang D).
aORCID:0000-0002-2513-860X.
bORCID:0000-0003-0941-2370.
cORCID:0000-0002-7279-1388.
dORCID:0000-0002-3151-6769.
eORCID:0000-0001-9024-0881.
Peer review under responsibility of Beijing Institute of Genomics Chinese Academy of Sciences and Genetics Society of China.
http://dx.doi.org/10.1016/j.gpb.2015.01.009
1672-0229©2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Beijing Institute of Genomics,Chinese Academy of Sciences and Genetics Society of China.
This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Genomics,Proteomics & Bioinformatics的其它文章
- Exosome and Exosomal MicroRNA:Trafcking, Sorting,and Function
- YPED:An Integrated Bioinformatics Suite and Database for Mass Spectrometry-based Proteomics Research
- Web Resources for Mass Spectrometry-based Proteomics
- Web Resources for Stem Cell Research
- Databases and Web Tools for Cancer Genomics Study
- Web Resources for Pharmacogenomics
