阻抗在细胞检测中的应用进展
2015-02-02胡清清何品刚
胡清清,崔 垚,张 帆,何品刚
(华东师范大学化学与分子工程学院,上海200241)
阻抗在细胞检测中的应用进展
胡清清,崔 垚,张 帆*,何品刚*
(华东师范大学化学与分子工程学院,上海200241)
细胞阻抗传感技术是细胞培养和阻抗技术的结合,将细胞培养在工作电极上,通过施加微小的电压,收集流经包括细胞在内的整个系统的电流信号来检测细胞的状态。阻抗技术由于实时、连续、不破坏性、无需标记等优点,已被广泛应用于细胞性质测定、癌细胞检测和抗癌药物筛选等方面。该文综述了阻抗技术在细胞检测中的应用和进展,包括:1、阻抗检测电极的设计;2、阻抗监测细胞生理状态;3、阻抗在细胞毒性和药物筛选中的应用;4、阻抗区分正常细胞与癌细胞。
阻抗;细胞检测;综述
0 引言
阻抗技术是一种以较小振幅正弦波电势 (电流)作为扰动信号,使电极系统产生近似于线性关系响应、测量频率很宽的阻抗值。由于电路中的物质对电流的阻碍作用不同,导致检测到的阻抗值不同,从而反映物质的相关性质。
细胞阻抗传感技术是将细胞培养和阻抗技术相结合,可在细胞培养的过程中对细胞的状态进行实时、连续地监测,能够及早发现细胞状态的变化,被认为是定量研究细胞行为最有前景的技术之一[1-2]。利用阻抗技术检测细胞是将细胞培养在工作电极上,通过施加微小的电压,收集流经包括细胞在内的整个系统的电流信号来检测细胞的状态。细胞被磷脂双分子层构成的细胞膜包裹着,使得细胞成为电的不良导体,直流电会被旁路,当施加一定频率的交流电时,电流流经细胞就会受到一定阻碍,从而引起整个体系阻抗的变化。这种阻碍与细胞的性质和状态密切相关,通过收集到的电流信号就可以分析细胞信息。由于施加的电压非常小,电流非常微弱,不会对细胞造成损伤,所以阻抗检测可以贯穿细胞培养的整个过程[3-7]。细胞阻抗传感技术由Giaever和Keese于1984年建立,他们将哺乳动物的成纤
维细胞(fibroblasts)培养在培养皿中,培养皿底部有两个共面的金电极,细胞在工作电极上生长,当施加4 kHz的正弦交流电场时,通过锁相放大器获得流经细胞的电流信号,实验结果表明粘附在电极上的细胞对电流有明显的阻碍作用,并且随时间发生变化[8]。该文综述了阻抗技术近年来在细胞检测中的应用进展。
1 阻抗检测电极的设计
利用阻抗技术进行细胞检测,第一步是要将细胞培养和阻抗检测结合于一体,这就涉及到阻抗检测装置的构建,其中最关键的是培养基底要导电,通常是将细胞培养在导电的工作电极上,由培养液连接对电极,进行检测。根据工作电极数目的不同,阻抗检测装置可以分为单电极体系和阵列电极体系。
1.1 单电极
单电极是指阻抗检测体系只含有一个工作电极和一个对电极,如图1。通常情况下,选用导电性好的、面积小的电极作为工作电极,面积大的电极作为对电极,两个电极的面积比值往往小于0.01,这就保证了体系阻抗的变化主要取决于工作电极表面的改变[9]。单电极系统应用广泛[10-14],工作电极材料可选用金、铂、铟锡氧化物(ITO)等,而对电极则多采用铂。

图1 单电极阻抗检测体系Fig.1 ECIS with mono work electrode
1.2 阵列电极
阵列电极体系包含多个工作电极,其中可分为叉指电极和普通阵列电极。
1.2.1 叉指电极
叉指电极是阵列电极的一种特殊构型,是指具有梳状的、面内有周期性图案的电极,由多组并列的条形电极组成,其特点是由一个共同的末端相连接,形成类似手指交叉的电极阵列结构。在叉指电极中,并列的条形电极大小一样,材质相同,因此电极的阻抗在总阻抗中所占的比例相等,不区分工作电极和对电极[15-17],如图2。叉指电极易于微型化,但是无法进行单细胞分析。

图2 叉指电极示意图[17]Fig.2 Sketch map showing the layout of the ECIS sensor electrodes
1.2.2 普通阵列电极
普通阵列电极体系含有多个通道,每一个通道含一个工作电极,这些工作电极是相互独立的,共用一个对电极,即每一个独立的电路都含一套完整的工作电极和对电极,利用开关可在工作电极间切换。Applied Biophysics(Troy,NY)公司采用光刻等技术,在基底上形成了由8个独立的工作电极(0.057 mm2)和1个共用的对电极(7×46 mm2)组成的阻抗检测体系,可进行多通道的同时测量[18-24],如图3所示。

图3 阵列电极系统示意图[20]Fig.3 Schematic diagram of the ECIS system
2 阻抗监测细胞生理状态
细胞阻抗技术可以实时、原位、长时间地反映电极表面细胞发生的变化,因此被广泛应用于各种细胞研究,包括细胞粘附和铺展[25-33]、细胞
繁殖[34-38]、细胞迁移与修复[39-43]等。
2.1 粘附和铺展
不同种类贴壁性细胞在电极表面的粘附能力不同,粘附所需时间也不一样,不同的电极对细胞粘附的影响也有差异。对于不易粘附的电极,需要在电极表面附着一层粘附蛋白。Wegener等运用细胞阻抗技术检测MDCK细胞在不同蛋白处理过的金电极表面的贴附情况,在他们的研究中提到,MDCK细胞在涂有粘附蛋白的电极表面更容易贴壁,并提出高频电容是反映MDCK细胞早期粘附和铺展最灵敏的参数[26]。Liu等利用多通道微阵列电极测定了细胞粘附和细胞形态的变化,他们将人类食道癌细胞(KYSE 30)培养在涂有粘连蛋白的电极表面,通过细胞粘附和铺展引起的形态改变均可将阻抗值的变化体现出来,如图4,细胞分别粘附在纤连蛋白预处理的和未处理的电极表面,当细胞粘附在电极表面时,阻抗值有增加[27]。Asphahani等在金电极表面进行小鼠成纤维细胞的图案化,细胞通过lysinearginine-glycine-aspartic acid(KRGD)粘附肽固定在电极表面,这种粘附机理依赖于共价结合和物理吸附,结果表明单细胞图案化能够改进细胞传感器的阻抗特性[30]。对于细胞粘附而导致的阻抗变化,Giaever和Keese认为有细胞时比没细胞时体系阻抗之所以增大,是因为细胞粘附到电极表面后,导致空白电极面积减少,电子传递受阻,表现为阻抗增加[33]。
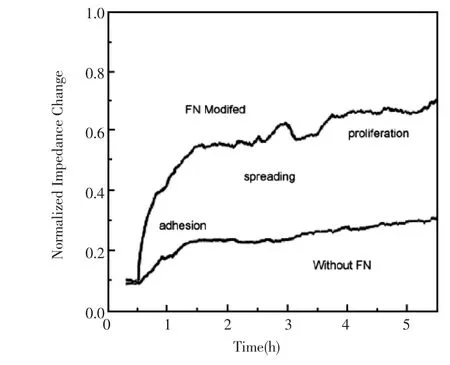
图4 微电极阵列单个通道中癌细胞分别在有无纤连蛋白时粘附的阻抗值随时间的变化曲线,检测频率为1 kHz[27]Fig.4 One channel impedance detection of the microelectrode array for cancer cells adhesion.Time course of the impedance magnitude at a sampling frequency of 1 kHz for fibronectin(FN)modified and non-modified electrode surfaces
2.2 细胞繁殖
细胞在电极上繁殖会导致阻抗增大,因此可根据阻抗的变化间接检测细胞数目的变化[34]。Shih等利用自制的数字微流控系统对HeLa、NIH-3T3、CHO-K1等三种细胞进行了4天的阻抗检测,分析得到了细胞的繁殖速率,这种方法使分析试剂用量减少近1000倍[35]。Brischwein等将小鼠成纤维细胞(L929)培养在阻抗传感器上,检测细胞引起的阻抗改变,结果表明,细胞增殖导致阻抗显著增大,在一定培养时间内,阻抗随时间逐渐增大[37]。
2.3 细胞迁移与修复
Keese等将细胞培养在金薄膜电极表面,正常模式下,1 μA,4 kHz的交流阻抗测试电流不会对细胞造成影响,当施加2.5 V,40 kHz高压脉冲时,细胞产生控制性损害,从电极上脱落,引起阻抗的下降,然后随着细胞的迁移和修复,阻抗又恢复到融汇时的水平,文章优化了脉冲作用时间、电极面积和细胞的种类对损伤再生测试的影响[39]。Wang等通过自组装单层膜(selfassembled monolayers,SAMs)的脱落以使得细胞脱离电极表面,模拟细胞损伤,并对比了不同细胞的迁移差异[40]。Stolwijk等利用原位电穿孔技术 (In situ electroporation,ISE)对细胞进行损伤后,再施加药物,研究细胞修复程度。当施加损伤信号时,细胞膜被电穿孔破坏后导致细胞死亡。损伤电流施加时间不同会引起细胞损伤程度以及细胞再生过程的差异,这些均可通过细胞不同时期的阻抗值反映出来[41]。
3 阻抗在细胞毒性和药物筛选中的应用
细胞的生长过程是动态的,并且对周围环境十分敏感,尤其是毒物。阻抗技术可做到实时表征细胞对周围环境的反应,细胞培养在电极上,体系中加入毒物作用以后,阻抗值下降,且毒物浓度越高,作用时间越长,阻抗值下降越明显。根据药物作用对象的不同,研究可分为两类:毒物对正常细胞的毒性[44-47]和抗癌药物对癌细胞的
作用[48-54]。
3.1 毒物对正常细胞的毒性
通过传统的生物化学测定方法,如基于细胞膜通透性改变的台盼蓝染色法[55-56]、对细胞核染色的碘化丙啶法[57]、测定细胞酶活性的 MTT法[58-61]、外加标记物等[62],确定细胞的存活率,通常会破坏细胞的连续培养,难以实现实时分析。阻抗技术则突破了这些技术的局限,能够在细胞的培养过程中,进行动态观察和原位检测,记录毒物作用的整个过程。Xiao等将氯化镉(CdCl2),苯扎氯铵(BAK),砷酸钠(Na2HAsO4)三种物质作用于中国仓鼠肺细胞(V79),利用阻抗技术测定这些物质对细胞的毒性,如图5,当施加毒性物质作用后,细胞的阻抗值明显下降,其结果与中性红标准方法的结果一致[44]。这个课题组又将上述三种物质以及氯化汞(HgCl2)和三硝基苯(TNB)作用于V79,前四种物质作为急性毒物的代表,而TNB作为长期慢性毒物的代表,利用阻抗技术检测毒物浓度和作用时间对V79细胞的影响,为细胞对毒物的动态反应提供了信息[45]。其他课题组也做了很多相关研究,如用阻抗方法评估镉[46],Triton X-100[47]对细胞的影响等。

图5 三种毒物(氯化镉、苯扎氯铵和砷酸钠)对成纤维V79细胞的影响[44]Fig.5 Responses(Ω per cell)of fibroblastic V79 cells to three cytotoxic chemicals(μmol/L):cadmium chloride,(a) 2.9,(b)4.6,(c)6.2,and(d)8.1;benzalkonium chloride,(a) 15.2,(b)18.3,(c)21.3,and(d)30.4;sodium arsenate,(a) 45,(b)60,(c)140,and(d)200
3.2 抗癌药物对癌细胞的作用
许多抗癌药物通过诱导细胞凋亡来杀死癌细胞。对细胞死亡的可靠评估,在发展有效、安全的抗癌药物治疗中显得尤为重要。细胞凋亡过程中,细胞形态、细胞间的连接等发生改变,而细胞阻抗传感技术能够实时检测这些变化,降低了检测成本,加快了测定流程,为抗癌药物筛选提供了一种新的评估方法。清华大学周玉祥课题组通过细胞阻抗技术检测阿司匹林诱导人结肠腺癌细胞(HT29)的改变,同时,通过透射电子显微镜成像佐证细胞形态改变,阐明了细胞阻抗技术在药物毒性测试和药物研发中的重要作用[48]。浙江大学王平课题组研究了癌症治疗的常用化学药物-顺铂对人食管鳞癌细胞(KYSE30)的作用,细胞阻抗技术用于监测细胞生长行为并探讨了抗癌药物的化学选择性[27]。Pradhan等报导了利用阻抗技术评估抗癌药物ZD6474对乳腺癌细胞(T47D、MCF-7)的作用,优化了电极尺寸和测试频率,测试结果与传统的流式细胞仪测定结果一致[49-50]。
4 阻抗技术区分正常细胞与癌细胞
目前,临床医学领域诊断癌症的方法包括超声、放射和活检等,活检是确定癌变阶段和程度的最精确的方法,然而有时也有假阴性判断,而且需要诸如免疫组织化学 (IH)、显色原位杂交(CISH)或荧光原位杂交(FISH)等程序[63]。近年来,微管吸吮(MA)、蠕变压缩、原子力显微镜(AFM)、光镊技术等也被用来评估细胞的癌变程度[64]。然而,比较经济、非侵害、更加快速的诊断方法,是应用阻抗技术来探知细胞的相关信息[65-71]。Han等将四种细胞:正常乳腺细胞(MCF-10A)、早期乳腺癌细胞(MCF-7)、侵入性人类乳腺癌细胞株(MDA-MB-231)和转移性人类乳腺癌细胞株 (MDA-MB-435),捕获到空腔
内,并测定它们的阻抗,结果表明,与正常乳腺细胞相比,癌细胞的电容值分别下降4.1%,16.0%和19.1%[65]。Kang等在此基础上,提高了检测装置的稳定性和灵敏度,测定出正常乳腺细胞(MCF-10A)和早期乳腺癌细胞(MCF-7)阻抗实部和相位角的平均差异分别是 44.4 Ω和1.41°[66],如图6。之后,这个课题组又利用相同的装置区分了正常前列腺细胞(RWPE-1)和其癌细胞(PC-3),两者的导纳和电纳分别相差54.55%和54.59%,重现性优异[67]。Chandra等报道了基于功能化的磁性纳米颗粒制成的生物阻抗传感器,进行宫颈癌的早期检测。宫颈癌细胞可以有选择性地通过修饰电极检测到,这在癌症的监测和临床治疗方面有巨大的发展前景[68]。利用阻抗技术区分正常细胞和癌细胞已受到广泛关注,如何进一步提高准确度和灵敏度,为癌症早期检测提供预警信息,是目前的研究重点。
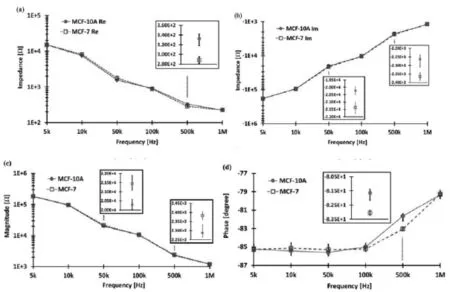
图6 乳腺癌细胞MCF-10A和正常乳腺细胞MCF-7阻抗值和相位角随频率变化的差异:(a)阻抗实部;(b)阻抗虚部;(c)阻抗模值;(d)相位角[66]Fig.6 Electrical impedance responses of MCF-10A and MCF-7 as a function of frequency:(a)real part(b) imaginary part;(c)magnitude and(d)phase angle.The vertical bars represent the error defined by maximum and minimum values.The insets show the precise values when the target cells are distinguished at each signal
5 展望
目前,细胞阻抗传感技术已在细胞测定方面展示出了巨大前景,其无需标记、非破坏性、操作简便等特点使得其广泛应用于临床和科研领域,包括细胞状态和行为的监测、药物测试、正常细胞与癌细胞的区分等。然而,目前的检测体系大多是将很多细胞培养在一个电极上,因此阻抗响应反映的是细胞的整体状态,而无法提供单细胞信息,如何发展超灵敏电极传感平台以实现单细胞的检测是今后研究的重点。
随着检测技术的快速发展,细胞阻抗传感装置也逐渐商业化,性能更加稳定,操作更加方便,然而可以进一步微型化、自动化,设计成各种功能化芯片,供应各种检测需要,降低成本,减少检测时间,实现高通量检测。
[1]Lei K F.Review on impedance detection of cellular responses in micro/nanoenvironment[J].Micromachines, 2014,5(1):1-12.
[2]Coffman F D,Cohen S.Impedance measurements in the biomedical sciences[J].Studies in health technology and informatics,2012,185:185-205.
[3]Qiu Y,Liao R,Zhang X.Real-time monitoring primary cardiomyocyte adhesion based on electrochemical impedance spectroscopy and electrical cell-substrate impedance sensing[J].Analytical chemistry,2008,80 (4):990-996.
[4]Spegel C,Heiskanen A,Skjolding L H D,et al.Chip based electroanalytical systems for cell analysis[J]. Electroanalysis,2008,20(6):680-702.
[5]Gu W,Zhao Y.Cellular electrical impedance spectroscopy:an emerging technology of microscale biosensors[J].Expert review of medical devices,2010,7(6): 767-779.
[6]K'Owino I O,Sadik O A.Impedance spectroscopy:a powerful tool for rapid biomolecular screening and cell culture monitoring[J].Electroanalysis,2005,17(23):2101-2113.
[7]Liu Q,Wu C,Cai H,et al.Cell-based biosensors and their application in biomedicine[J].Chemical reviews, 2014,114(12):6423-6461.
[8]Giaever I,Keese C R.Monitoring fibroblast behavior in tissue culture with an applied electric field[J].Proceedings of the National Academy of Sciences,1984,81(12): 3761-3764.
[9]Hug T S.Biophysical methods for monitoring cell-substrate interactions in drug discovery[J].Assay and drug development technologies,2003,1(3):479-488.
[10]Tlili C,Reybier K,Géloën A,et al.Fibroblast cells:a sensing bioelement for glucose detection by impedance spectroscopy[J].Analytical chemistry,2003,75(14): 3340-3344.
[11]Nwankire C E,Venkatanarayanan A,Glennon T,et al. Label-free impedance detection of cancer cells from whole blood on an integrated centrifugal microfluidic platform[J].Biosensors and Bioelectronics,2015,68: 382-389.
[12]Haddad S,Zanina N,Othmane A,et al.Polyurethane films modified by antithrombin–heparin complex to enhance endothelialization:An original impedimetric analysis[J].Electrochimica Acta,2011,56(21):7303-7311.
[13]Lo C M,Keese C R,Giaever I.Impedance analysis of MDCK cellsmeasured by electric cell-substrate impedance sensing[J].Biophysical journal,1995,69(6): 2800-2807.
[14]Park G,Choi C K,English A E,et al.Electrical impedance measurements predict cellular transformation [J].Cell biology international,2009,33(3):429-433.
[15]Moore E,Rawley O,Wood T,et al.Monitoring of cell growth in vitro using biochips packaged with indium tin oxide sensors[J].Sensors and Actuators B:Chemical, 2009,139(1):187-193.
[16]Lin C Y,Teng N C,Hsieh S C,et al.Real-time detection of β1 integrin expression on MG-63 cells using electrochemical impedance spectroscopy[J].Biosensors and Bioelectronics,2011,28(1):221-226.
[17]Wang L,Wang H,Mitchelson K,et al.Analysis of the sensitivity and frequency characteristics of coplanar electrical cell–substrate impedance sensors[J].Biosensors and Bioelectronics,2008,24(1):14-21.
[18]Merrill D R,Tresco P.Impedance characterization of microarray recording electrodes in vitro[J].Biomedical Engineering,IEEE Transactions on,2005,52(11):1960-1965.
[19]Hondroulis E,Liu C,Li C Z.Whole cell based electrical impedance sensing approach for a rapid nanotoxicityassay[J].Nanotechnology,2010,21(31):315103.
[20]Xiao C,Lachance B,Sunahara G,et al.An in-depth analysis of electric cell-substrate impedance sensing to study the attachment and spreading of mammalian cells [J].Analytical Chemistry,2002,74(6):1333-1339.
[21]Yang X,Zhou Z,Xiao M,et al.Research on electric impedance spectroscopy of living cell suspensions by a chip with microelectrodes[J].XiyouJinshuCailiaoyu-Gongcheng (Rare Metal Materials and Engineering), 2006,35:429-432.
[22]McCoy M H,Wang E.Use of electric cell-substrate impedance sensing as a tool for quantifying cytopathic effect in influenza A virus infected MDCK cells in realtime[J].Journal of Virological Methods,2005,130(1): 157-161.
[23]肖名飞,杨兴,周兆英,等.细胞阻抗谱检测芯片的设计及实验[J].纳米技术与精密工程,2008,5(4):302-306.
[24]Lin S P,Kyriakides T R,Chen J JJ.On-line observation of cell growth in a three-dimensional matrix on surfacemodified microelectrode arrays[J].Biomaterials,2009, 30(17):3110-3117.
[25]Yu H,Wang J,Liu Q,et al.High spatial resolution impedance measurement of EIS sensors for light addressable cell adhesion monitoring[J].Biosensors and Bioelectronics,2011,26(6):2822-2827.
[26]Wegener J,Keese C R,Giaever I.Electric cell–substrate impedance sensing(ECIS)as a noninvasive means
to monitor the kinetics of cell spreading to artificial surfaces[J].Experimental cell research,2000,259(1): 158-166.
[27]Liu Q,Yu J,Xiao L,et al.Impedance studies of bio-behavior and chemosensitivity of cancer cells by microelectrode arrays[J].Biosensors and Bioelectronics, 2009,24(5):1305-1310.
[28]Luong J H T,Habibi-Rezaei M,Meghrous J,et al.Monitoring motility,spreading,and mortality of adherent insect cells using an impedance sensor[J].Analytical chemistry,2001,73(8):1844-1848.
[29]Keese C R,Giaever I.Substrate mechanics and cell spreading[J].Experimental cell research,1991,195(2): 528-532.
[30]Asphahani F,Thein M,Veiseh O,et al.Influence of cell adhesion and spreading on impedance characteristics of cell-based sensors[J].Biosensors and Bioelectronics, 2008,23(8):1307-1313.
[31]Tiruppathi C,Malik A B,Del Vecchio P J,et al.Electrical method for detection of endothelial cell shape change in real time:assessment of endothelial barrier function [J].Proceedings of the National Academy of Sciences, 1992,89(17):7919-7923.
[32]Atienza J M,Zhu J,Wang X,et al.Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays[J].Journal of biomolecular screening,2005,10 (8):795-805.
[33]Giaever I,Keese C R.Use of electric fields to monitor the dynamical aspect of cell behavior in tissue culture[J]. Biomedical Engineering,IEEE Transactions on,1986,2: 242-247.
[34]向常洲,袁泉,夏应清.新型全数字化细胞阻抗传感器的设计与实现[J].计算机测量与控制,2007,15(10): 1412-1414.
[35]Shih S C C,Barbulovic-Nad I,Yang X,et al.Digital microfluidics with impedance sensing for integrated cell culture andanalysis[J].Biosensors and Bioelectronics, 2013,42:314-320.
[36]Soley A,Lecina M,Gámez X,et al.On-line monitoring of yeast cell growth by impedance spectroscopy[J].Journal of biotechnology,2005,118(4):398-405.
[37]Brischwein M,Herrmann S,Vonau W,et al.Electric cell-substrate impedance sensing with screen printed electrode structures[J].Lab on a Chip,2006,6(6):819-822.
[38]Wegener J,Sieber M,Galla H J.Impedance analysis of epithelial and endothelial cell monolayers cultured on gold surfaces[J].Journal of biochemical and biophysical methods,1996,32(3):151-170.
[39]Keese C R,Wegener J,Walker S R,et al.Electrical wound-healing assay for cells in vitro[J].Proceedings of the National Academy of Sciences of the United States of America,2004,101(6):1554-1559.
[40]Wang L,Zhu J,Deng C,et al.An automatic and quantitative on-chip cell migration assay using self-assembled monolayers combined with real-time cellular impedance sensing[J].Lab on a Chip,2008,8(6):872-878.
[41]Stolwijk J A,Hartmann C,Balani P,et al.Impedance analysis of adherent cells after in situ electroporation: non-invasive monitoring during intracellular manipulations[J].Biosensors and Bioelectronics,2011,26(12): 4720-4727.
[42]Giaever I,Keese C R.Micromotion of mammalian cells measured electrically[J].Proceedings of the National A-cademy of Sciences,1991,88(17):7896-7900.
[43]Newbold C,Richardson R,Huang C Q,et al.An in vitro model for investigating impedance changes with cell growth and electricalstimulation:implications for cochlear implants[J].Journal of neural engineering, 2004,1(4):218-227.
[44]Xiao C,Lachance B,Sunahara G,et al.Assessment of cytotoxicity using electric cell-substrate impedance sensing:concentration and time response function approach[J].Analytical chemistry,2002,74(22):5748-5753.
[45]Xiao C,Luong J H T.Assessment of cytotoxicity by emerging impedance spectroscopy[J].Toxicology and applied pharmacology,2005,206(2):102-112.
[46]Peper J K,Schuster H,Löffler M W,et al.An impedance-based cytotoxicity assay for real-time and label-free assessment of T-cell-mediated killing of adherent cells[J].Journal of immunological methods,2014, 405:192-198.
[47]Ruemenapp C,Remm M,Wolf B,et al.Improved method for impedance measurements of mammalian cells[J]. Biosensors and Bioelectronics,2009,24(9):2915-2919.
[48]Yin H,Wang F L,Wang A L,et al.Bioelectrical Impedance Assay to Monitor Changes in Aspirin-Treated Human Colon Cancer HT-29 Cell Shape during Apoptosis[J].Analytical letters,2007,40(1):85-94.
[49]Pradhan R,Mandal M,Mitra A,et al.Monitoring cellular activities of cancer cells using impedance sensing de-
vices[J].Sensors and Actuators B:Chemical,2014,193: 478-483.
[50]Pradhan R,Rajput S,Mandal M,et al.Frequency dependent impedimetric cytotoxic evaluation of anticancer drug on breast cancer cell[J].Biosensors and Bioelectronics, 2014,55:44-50.
[51]Yeon J H,Park J K.Cytotoxicity test based on electrochemical impedance measurement of HepG2 cultured in microfabricated cell chip[J].Analytical biochemistry, 2005,341(2):308-315.
[52]Yu C,Zhu Z,Wang L,et al.A new disposable electrode for electrochemical study of leukemia K562 cells and anticancer drug sensitivity test[J].Biosensors and Bioelectronics,2014,53:142-147.
[53]Valdés L,Gueimonde M,Ruas-Madiedo P.Monitoring in real time the cytotoxic effect of Clostridium difficile upon the intestinal epithelial cell line HT29[J].Journal of microbiological methods,2015,119:66-73.
[54]Opp D,Wafula B,Lim J,et al.Use of electric cell–substrate impedance sensing to assess in vitro cytotoxicity [J].Biosensors and Bioelectronics,2009,24(8):2625-2629.
[55]Louis K S,Siegel A C.Cell viability analysis using trypan blue:manual and automated methods[M].Mammalian Cell Viability:Humana Press,2011.7-12.
[56]Elengoe A,Hamdan S.Evaluation of Hyperthermia Effect on Cell Viability using Crystal Violet Staining,LDH and Trypan Blue Assays[J].Advancesin Environmental Biology,2014,8(3):744-747.
[57]Foldbjerg R,Dang D A,Autrup H.Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line,A549[J].Archives of toxicology,2011,85(7): 743-750.
[58]Nikzad S,Baradaran M,Nasiri P.Dose-response modeling using MTT assay:a short review[J].Life Science Journal,2014,11(9s):432-437.
[59]Han X,Gelein R,Corson N,et al.Validation of an LDH assay for assessing nanoparticle toxicity[J].Toxicology, 2011,287(1):99-104.
[60]Boncler M,Rózalski M,Krajewska U,et al.Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells[J].Journal of pharmacological and toxicological methods,2014,69(1):9-16.
[61]Jo H Y,Kim Y,Park H W,et al.The Unreliability of MTT Assayin theCytotoxicTestofPrimaryCultured Glioblastoma Cells[J].Experimental neurobiology,2015, 24(3):235-245.
[62]Van Engeland M,Ramaekers F C S,Schutte B,et al.A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture[J]. Cytometry,1996,24(2):131-139.
[63]Di Palma S,Collins N,Faulkes C,et al.Chromogenic in situ hybridisation (CISH)should be an accepted method in the routine diagnostic evaluation of HER2 status in breast cancer[J].Journal of clinical pathology,2007,60 (9):1067-1068.
[64]Suresh S.Biomechanics and biophysics of cancer cells [J].ActaMaterialia,2007,55(12):3989-4014.
[65]Han A,Yang L,Frazier A B.Quantification of the heterogeneity in breast cancer cell lines using whole-cell impedance spectroscopy[J].Clinical Cancer Research, 2007,13(1):139-143.
[66]Kang G,Yoo S K,Kim H I,et al.Differentiation Between Normal and Cancerous Cells at the Single Cell Level Using 3-D Electrode Electrical Impedance Spectroscopy [J].Sensors Journal,IEEE,2012,12(5):1084-1089.
[67]Kang G,Kim Y,Moon H,et al.Discrimination between the human prostate normal cell and cancer cell by using a novel electrical impedance spectroscopy controlling the cross-sectional area of a microfluidic channel[J]. Biomicrofluidics,2013,7(4):044126.
[68]Chandra S,Barola N,Bahadur D.Impedimetric biosensor for early detection of cervical cancer[J].Chemical Communications,2011,47(40):11258-11260.
[69]Yang L,Arias L R,Lane T S,et al.Real-time electrical impedance-based measurement to distinguish oral cancer cells and non-cancer oral epithelial cells[J].Analytical and bioanalytical chemistry,2011,399(5):1823-1833.
[70]Solly K,Wang X,Xu X,et al.Application of real-time cell electronic sensing (RT-CES)technology to cellbased assays[J].Assay and drug development technologies,2004,2(4):363-372.
[71]Chiang Y,Jang L S,Tsai S L,et al.Impedance Analysis of Single Melanoma Cells in Microfluidic Devices[J]. Electroanalysis,2014,26(10):2129-2137.
The application process of impedance sensing in cell detection
Hu Qing-qing,Cui Yao,Zhang Fan*,He Pin-gang*
(School of Chemistry and Molecular Engineering,East China Normal University,Shanghai 200241,China)
Electric Cell-substrate Impedance Sensing is the combination of cell culture and impedance technology. Relevant cells cultured on working electrode are subjected to an alternating electric field,when cells attach and spread on this electrode,current signals of the whole system are collected to perceive cells state.With advantages like real-time,continuous,non-invasive,and label-free,impedance technology has been widely used in cell properties determination,cancer cells detection and anticancer drug screening etc.In this paper,we briefly review the recent application process of impedance in cell detection,involving the design of the detection system,the impedance monitoring of the physiological state of cells,the application of impedance in cytotoxicity and drug test, the differentiation of normal cells and cancer cells by impedance.
impedance;cell detection;review
国家自然科学基金资助项目(21405049);上海市教育委员会科研创新项目(13zz032)
*通信联系人,E-mail:fzhang@chem.ecnu.edu.cn;pghe@chem.ecnu.edu.cn
