Thermal evolution of aliphatic and aromatic moieties of asphaltenes from coals of different rank:possible implication to the molecular architecture of asphaltenes
2015-01-17AminuBayawaMuhammad
Aminu Bayawa Muhammad
Thermal evolution of aliphatic and aromatic moieties of asphaltenes from coals of different rank:possible implication to the molecular architecture of asphaltenes
Aminu Bayawa Muhammad1
The compositional and structural changes that coal asphaltenes undergo with increasing thermal maturation were investigated using solid-state13C NMR and Fourier transform infrared spectroscopy.The results show a gradual increase in the relative proportion of carbon content with a concomitant decrease in the hydrogen,sulphur and oxygen content.The amount of aromatic carbon also increases while the aliphatic carbon decreases with increasing maturity.Ester and carboxylic groups are particularly sensitive to maturation and decrease in relative abundance with increasing maturity while the amount of aromatic carbonyl groups increases.In general,the asphaltenes were observed to evolve towards a more thermally stable structure with increasing amounts of aromatic moieties and relatively lower amounts of aliphatic moieties.The results are in agreement with the ultimate dominance of the island molecular architecture(the Yen–Mullins model)in asphaltenes.
Asphaltenes·Coals·FTIR· Solid-state13C NMR·Thermal maturation
1 Background
Asphaltenes are complex mixture of carbonaceous macromolecular substances that have been associated with many problems including obstruction of reservoirs,plugging of wellsandpipelinesaswellasfoulingandstabilisationofoil–water emulsions in the petroleum industry(Galoppini 1994; Kokal and Sayegh 1995;Khadim and Sarbar 1999;Shedid andZekri2006;Maetal.2008).Althoughthetermismainly applied to the heaviest components of petroleum,its functionalsolubility-baseddefnitionallowsextensiontoinclude similar substances from coals and bitumens(Behar et al. 1984;William 1985;Solli and Leplat 1986)and compositionally the two are quite similar(Badre et al.2006).
The composition of asphaltenes has been a subject of many investigations(Bunger and Li 1981;Peters 1986; Sheu and Mullins 1995;Sheu 2002;Sabbah et al.2011; Mullins et al.2012;Muhammad and Abbott 2013;Wu et al.2013).In general asphaltenes macromolecules are considered to consist of aliphatic side-chains linked to aromatic moieties via C–C,C–O and C–S bonds(Yen 1974;Peng et al.1997).The aromatic moieties consist largely of condensed pericyclic sheets of 4 and 20 rings (Groenzin and Mullins 1999;Badre et al.2006)while the alkyl moieties have been shown to average at C3to C7in size(Calemma et al.1995),although homologues in range of C1to over C32have observed(del Rio et al.1995;Peng et al.1999;Muhammad and Abbott 2013).Furthermore, the aliphatic moieties have been found to consists of both acyclic(n-alkyl and iso-alkyl)and cyclic(hopanoids, steroids etc.)alkyl groups(Mojelsky et al.1992;Triflieff et al.1992;Peng et al.1999;Strausz et al.1999; Muhammad and Abbott 2013).A more complex structure in which the aromatic moieties in the asphaltenes are further interconnected by aliphatic bridges(i.e.the so called polymeric structure)has been subject of intense debate (Bunger and Li 1981;Hammami et al.1995;Speight 2004; Badre et al.2006;Strausz et al.2008;Mullins 2009).
The effect of thermal stress on the molecular composition of aliphatic moieties and stereochemistry of the alicyclic moieties has been reported(Muhammad and Abbott2013).However,the destructive effect of ruthenium oxide oxidation on aromatic moieties prohibits obtaining a complete picture of the thermal evolution of the asphaltenes.In this paper,NMR and FTIR were employed to reveal the changes undergone by the aromatic moieties,as well as aliphatic moieties,of the asphaltenes with possible consequence on the molecular architecture of these enigmatic complex substances.
2 Materials and methods
2.1 Samples
Four pulverised coal samples,namely North Sea Brent coals C04,C56,C69[with vitrinite refectance(R0)values of 0.40%,0.56%,0.69%,respectively]and C15(R0value of 1.50%)from Harvey Beaumont seam,County Durham, United Kingdom,were used in this investigation.The samplescover thethermalmaturationrangefromlatediagenesis (R0=0.2%–0.450%)throughcatagenesis(R0=0.6%–0.9%)into metagenesis(R0=0.9%–2.0%)stages of transformation of sedimentary organic matter.
2.2 Extraction of bitumen
Known weights of the coal samples were extracted for 72 h with DCM/MeOH mixture(93:7,v/v)using Soxhlet extraction method.The solvent was removed from the extracts by rotary evaporation(30°C,25 mmHg)followed by drying under nitrogen stream until a constant weight was obtained.
2.3 Precipitation of asphaltenes and elemental analysis
The coal bitumen,dissolved in 1 cm3of DCM,was treated with 40 cm3of n-hexane.The mixture was stirred for 2 h and allowed to equilibrate for 24 h.The asphaltenes were recovered by centrifugation(3500 rpm,15 min)and redissolved in 1 cm3DCM followed by re-precipitation with 40 cm3of n-hexane.After stirring for 30 min,the asphaltenes were recovered.This was repeated two more times.The dry asphaltenes were extracted with n-hexane for 10 days using the Soxhlet method to remove co-precipitated maltene(Alboudwarej et al.2002).After drying, carbon,hydrogen,nitrogen and sulphur composition of the asphaltenes were determined using a Carlo Erba 1108 Elemental Analyser.The oxygen content was obtained by difference.
2.4 FTIR measurements and curve-ftting of the spectra
About 1%(w/w)mixture of the asphaltenes in KBr powder(99.5%IR spectroscopic grade,Sigma-Aldrich) was prepared with an agate mortar and pestle.13 mm pellets were prepared from about 200 mg of the homogenised sample/KBr powder using a Specac 15 Ton Manual Hydraulic Press.
Thermo Nicolet Nexus 870 FTIR spectrometer(Thermo Nicolet Corp.),with DTGS KBR detector and XT-KBr beam splitter,was used to acquire the IR spectra in the mid-infrared region(400–4000 cm-1)by co-adding 70 scans at a resolution of 4 cm-1.
Prior to curve-ftting with GRAMS/AI(Thermo Scientifc,Inc.),each spectrum was:(i)normalised to the concentration of the asphaltene in the pellets,(ii)baseline corrected using multipoint option,and(iii)smoothened using Savitzky-Golay algorithm to reduce noise.Curveftting of relevant spectral regions and assignment of bands were done as described in the literature(Maddams 1980; Painter et al.1981;Yen et al.1984;Ibarra et al.1996). Model trend with best ft(from R2values)was adopted in plotsofareasofabsorption bandsagainstvitrinite refectance.
2.5 Solid-state13C NMR measurements
The Soxhlet extracted asphaltene samples were used without further preparation.The solid-state13C NMR spectra were obtained on a Varian VNMRS 400 spectrometer using a double-resonance(H–X)MAS probe equipped with a 4 mm rotor.All the spectra were obtained at 100.56 MHz with 700 scans.The rotor was spun at 12 kHz.A pulse repetition delay of 2 s and a cross polarization(CP)contact time of 0.50 ms were applied for all the experiments.Modulated(TPPM)decoupling was carried out at a nutation frequency of 76 kHz.Dipolar dephasing(DP)delay was set at 40.0 μs.Spectral referencing is with respect to external neat tetramethylsilane.
The data obtained was used to estimate(Eqs.1–5):the fractions of the aromatic carbon that is protonated(i.e. tertiary aromatic carbon)and nonprotonated(quaternary aromatic carbon,);fraction of the total carbon that is protonatedand nonprotonatedas well as the fraction of the total hydrogen present in the aromatic systems(Ha)(Wilson et al.1984;Wilson and Vassallo 1985).

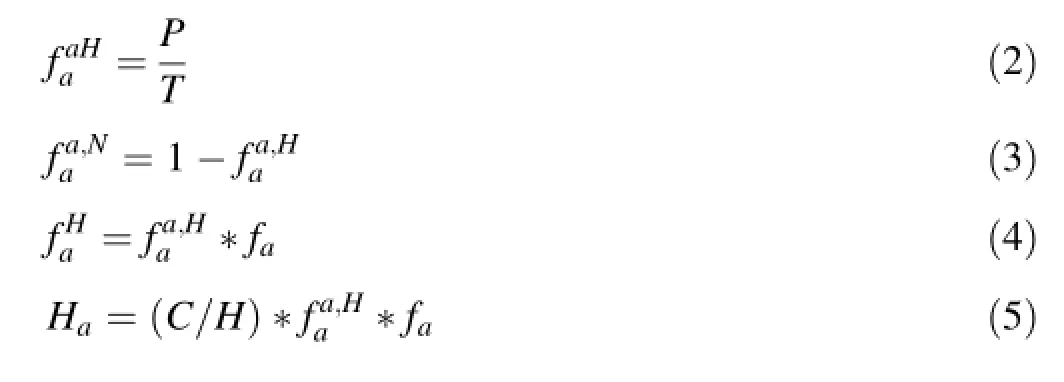
3 Results and discussion
3.1 Elemental composition
The percentage carbon content of the asphaltenes increases with increasing maturity of the coals of the coals(Fig.1a), while atomic O/C and S/C ratios decrease with increase in maturity(Fig.1).These are similar to observations of Rouxhet et al.(1980)in coals and kerogens.Unlike other elements,however,the relative nitrogen content(N/C ratio)increases with increasing maturity of the coals (Fig.1d).This might be due to two dynamic processes occurring during the diagenesis of the organic matter. Firstly,relative to carbon,the loss of oxygen and sulphur might occur more rapidly(Fig.1b,c)than nitrogen (Fig.1d)as suggested by the stiffness of the gradients of the respective plots of the element against maturity (Fig.1).Secondly,a signifcant proportion of the total nitrogen might be fxed in or transformed into functionalities (e.g.aromatic pyridinic and pyrrolic nitrogen(Baxby et al. 1994))that are relatively more stable than sulphur and oxygen functional groups.Therefore,although the absolute amounts of the nitrogen might be decreasing with increasing maturity,the overall observable effect is its relative enrichment in the residual organic matter with increasing maturity(Fig.1d).In general,however all the trends(Fig.1)appear to stabilise as higher maturity level is approached suggesting the transformation proceeds towards a more stable carbon-rich equilibrium structure.
3.2 Distribution and evolution of carbon in asphaltenes
The solid-state13C NMR spectra of the asphaltenes revealed two broad peaks between 0–60 and 100–150 ppm (Fig.2a)which are generally assigned to aliphatic and aromatic carbons,respectively(Weinberg et al.1983; Wilson and Vassallo 1985)although they can be deconvolved into many peaks assignable to different types of aromatic and aliphatic carbons,respectively(Dutta Majumdar et al.2013).The values of the aromaticity factor (fa)obtained(Table 1)are similar to reported carbonaromaticity of petroleum asphaltenes(Andrews et al.2011; Dutta Majumdar et al.2013)although much lower than those of coal asphaltenes(Andrews et al.2011)possibly due to the effect of thermal liquefaction of the coals(see Sect.3.4).In general,the aromaticity factor increases with increasing maturity(Table 1)suggesting aromatisation of aliphatic(naphthenic)structures with increasing maturity although dealkylation may also occur simultaneously (Rouxhet et al.1980;Buch et al.2003).However,the former is further supported by increase in both the fraction of aromatic hydrogen(Ha)and the fraction of the total carbon that is protonated(tertiary)aromatic carbonwith maturity(Table 1).

Fig.1 Plots of(a)%C,(b)O/C,(c)S/C,and(d)N/C against vitrinite refectance showing the evolution of the relative amounts of elements in the asphaltenes with increasing maturity
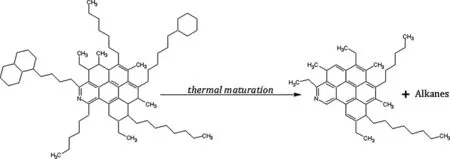
Fig.2 Molecular illustration showing evolution of aromatic and aliphatic moieties of asphaltene macromolecule with increase in thermal stress

Table 1 Structural parameters calculated from theasphaltenes’solid-state13C NMR spectra
Furthermore,the distribution of the aromatic carbon shows that over 50%of the carbon is in the form of nonprotonated(i.e.quaternary)carbon as refected byalthough itappearsto be independentofmaturity (Table 1).It should however be noted that the relatively high value ofof C04 asphaltene does not necessarily mean it has more condensed aromatic moieties than the more matured asphaltenes;rather,it may be a refection of positive contributions tofrom alkylated(therefore nonprotonated)carbons which are more abundant in less mature samples.Thus,as the alkyl groups are replaced with hydrogens following dealkylation with increasing maturity, the apparent proportion of the quaternary carbonfalls and aromatic hydrogen(Ha)rises(Table 1).In general, increase in the aromaticity of the asphaltene with increasing maturity is mainly due to aromatisation of aliphatic carbon and possibly increasing condensation of the aromatic structures.These observations are consistent with results obtained by Wilson and Vassallo(1985)in their study of distributions of carbon in coals with increasing thermal stress.
3.3 Thermal evolution of functional groups in the asphaltenes
The absorption bands obtained from curve-ftting of the asphaltenes IR spectra are assigned to various functional groups as outlined in Table 2.The O–H/N–H band of the asphaltenes is prominent irrespective of the maturity of the coals suggesting the functionalities are not signifcantly affected by increase in maturity until at much higher maturation levels(Rouxhet et al.1980)not covered in this study.
The total absorption due to the fve aliphatic C–H stretching vibrations (3000–2800 cm-1)indicative of aliphatic moieties decreases with increase in maturity (Fig.3a).Furthermore,the intensities of the two alkyl bands at 1455 and 1346 cm-1decrease with increasing maturity (Fig.3b)indicating dealkylation as the asphaltenes areexposed to increasing thermal stress.All these are in agreement with decrease in aliphatic carbon with increasing maturityasobservedfrom13CNMRresults(Sect.3.2).Thisisa furtherindicationthatbothdealkylationandaromatisationof aliphatic unitsoccur simultaneously asmaturity increasesas illustrated in Fig.2.However,the two other bands at 1424 and 1375 cm-1,also commonly assigned to aliphatic moietiesappeartobeindependentofmaturityofthecoalswithin the maturity region under investigation.

Table 2 List and assignment of infrared absorption bands obtained from the curve-ftting of the FTIR spectra of the asphaltenes from the coal samples
ThearomaticC–Hstretchingvibrations(3000–3100 cm-1) are barely detectable in the immature C04 asphaltenes but increase with increasing maturity with the strongest absorption observed in C15 asphaltene(Fig.3a).The evolution of the aromatic structures is further revealed by increase in the intensities of the nine bands between 900 and 700 cm-1with increase in maturity(Fig.3c,d).These bands are indicative of aromatic C–H out-of-plane deformations(Yen et al.1984; Ibarraetal.1996)fromdifferentenvironments(Table 2).Note however that although the bands at 838 and 723 cm-1are respectively commonly assigned to cyclic methylene(–CH2–) andC5+methyleneunits(Guilianoetal.1990),thefactthatthe intensities of the bands increase with increasing maturity (Fig.3d)suggesttheyaremorelikelytobeduetoaromaticC–H deformation in agreement with Ibarra et al.(1996).The differences in the slopes of the plots in Fig.3d suggest that the different aromatic C–H evolve at different rates during maturation.
The carbonyl (C=O) functionalities from ester (1768 cm-1)and carboxyl group(1698 cm-1)(Guiliano et al.1990;Ibarra et al.1996)decrease with increasing maturity while the highly conjugated carbonyl group of the quinone-type (1654 cm-1) increases with maturity (Fig.3e)in agreement with observations of Rouxhet et al. (1980)in coals.Lost of the ester and carboxyl groupsappear to precede loss of hydroxyl groups even in coals (Rouxhet et al.1980).This indicates both the quinone-type carbonyl group and hydroxyl group are thermally more stable than ester and carboxyl groups in asphaltenes.

Fig.3 The normalised areas of the absorption bands between 3000 and 700 cm-1,obtained from curve-ftting of the FTIR spectra of the asphaltenes, plotted against vitrinite refectance showing evolution of the different chemical functionalities with increasing maturity
Although the bands at 1607 and 1497 cm-1are commonly assigned to aromatic C=C stretching vibrations (Guiliano et al.1990;Ibarra et al.1996),in this study both bands decrease with increasing maturity(Fig.3f)and correlate positively with O/C ratio(R2>0.80;p<0.05) and thus might be due to oxygen functional groups possibly carboxylate ion(Nakanishi 1962).
Intensities of the six bands obtained from curve-ftting the spectral region between 1300 and 1000 cm-1decrease with increasing maturity(Fig.3g,h).Although these bands are quite diffcult to assign due to the many functional groups that absorb in the region(Socrates 1980;Painter et al.1981),the stiffness with which they decrease with increasing maturity suggests they might be due to variousC–O functionalgroupsfrom differentenvironments (Table 2).

Fig.4 Simplifed diagram illustrating thermal evolution of archipelago-type asphaltene molecules into island-type molecules with increasing thermal maturation
3.4 Possible implication to the molecular model of asphaltenes
The molecular structure of asphaltenes has been subject of intense investigations(Badre et al.2006;Sabbah et al. 2010,2011;Mullins et al.2012;Alvarez-Ramı´rez and Ruiz-Morales 2013).Consequently,it has now been shown that the dominant molecular architecture is the Yen–Mullins model,which consider asphaltenes to have the socalled island structure consisting of a condensed heteroaromatic core with alkyl side chains(Mullins et al. 2012).
The observed structural evolution of asphaltenes with increase in thermal maturation may have a fundamental implication in asphaltene chemistry.First,asphaltene molecules from relatively immature sedimentary organic matter may be dominated by aliphatic moieties relative to aromatic moieties.Second,even if thermally immature asphaltene contain relatively signifcant archipelago-type molecular architecture,at higher degree of thermal maturity most of the inter-aromatic aliphatic linkages,as well as aliphatic side chains(Buch et al.2003),would be lost to thermal dealkylation.This will ultimately generate complex,albeit more aromatic,molecular units dominated by island molecular architecture(the Yen–Mullins model)as illustrated in Fig.4 in agreement with the observed dominant molecular architecture of asphaltenes from petroleum and artifcially matured coal(Badre et al.2006; Sabbah et al.2011;Wu et al.2013).It worth noting that thermal liquefaction of coal could result in its artifcial thermal maturation and this could partly be the reason why coal asphaltenes have been reported to have higher relative carbon content and lower hydrogen content(and thus lower H/C atomic ratio)than the petroleum asphaltenes(Andrews et al.2011).This therefore buttresses the need to investigate thermally immature asphaltenes(e.g.extracted from virgin immature coal)and particularly to determine the relative signifcance of the archipelago structures in them.
4 Conclusion
Thermal maturity was observed to affect the composition of asphaltenes from coals of different ranks.With increasing thermal stress,asphaltenes were observed to evolve towards an equilibrium structure or composition in which aromatic moieties become dominant over aliphatic moieties as a result of increasing aromatisation and dealkylation.Distribution of alkyl moieties shifts towards increasing proportions of the lower molecular weight homologues with increasing thermal maturity.The thermal stress also results in loss of oxygen functionalities from ester and carboxyl groups while hydroxyl groups appear to be more stable.The results are in agreement with the ultimate dominance of the island structure(the Yen–Mullins model)in petroleum asphaltenes.
AcknowledgmentsThe author is grateful to Dr.Geoff Abbott, Newcastle University,for supervising the work,Dr.Claire Fialips formerly School of Civil Engineering&Geosciences,Newcastle University,UK for technical assistance and Dr.David Apperley and Mr.Fraser Markwell of Solid-state NMR Service at Durham University,UK for solid-state13C NMR analysis.The author would like to thank Petroleum Technology Development Fund(PTDF), Nigeria for Ph.D scholarship.
Alboudwarej H,Beck J,Svrcek WY,Yarranton HW,Akbarzadeh K (2002)Sensitivity of asphaltene properties to separation techniques.Energy Fuels 16(2):462–469
Alvarez-Ramı´rez F,Ruiz-Morales Y(2013)Island versus archipelago architecture for asphaltenes:polycyclic aromatic hydrocarbon dimer theoretical studies.Energy Fuels 27(4):1791–1808
Andrews AB,Edwards JC,Pomerantz AE,Mullins OC,Nordlund D, Norinaga K(2011)Comparison of coal-derived and petroleum asphaltenes by13C nuclear magnetic resonance,DEPT,and XRS.Energy Fuels 25(7):3068–3076
Badre S,Goncalvesa CC,Norinagab K,Gustavsona G,Mullins OC (2006)Molecular size and weight of asphaltene and asphaltene solubility fractions from coals,crude oils and bitumen.Fuel 85:1–11
Baxby M,Patience RL,Bartle KD(1994)The origin and diagenesis of sedimentary organic nitrogen.J Pet Geol 17(2):211–230
Behar F,Pelet R,Roucache J(1984)Geochemistry of asphaltenes. Org Geochem 6:587–595
Buch L,Groenzin H,Buenorostro-Gonzalez E,Andersen SI,Lira-Galeana C,Mullins C(2003)Effect of hydrotreatment on asphaltene fractions.Fuel 82:1075–1084
Bunger JW,Li NC(eds)(1981)Chemistry of Asphaltenes,Advances in chemistry series 195,American Chemical Society,Washington D.C,p 260
Calemma V,Iwanski P,Nali M,Scotti R,Montanari L(1995) Structural characterization of asphaltenes of different origins. Energy Fuels 9(2):225–230
del Rio JC,Martin F,Gonzalez-Vila FJ,Verdejo T(1995)Chemical structural investigation of asphaltenes and kerogens by pyrolysis-methylation.Org Geochem 23(11–12):1009–1022
Dutta Majumdar R,Gerken M,Mikula R,Hazendonk P(2013) Validation of the Yen-Mullins model of athabasca oil-sands asphaltenes using solution-state 1H NMR relaxation and 2D HSQC spectroscopy.Energy Fuels 27(11):6528–6537
Galoppini M(1994)Asphaltene deposition monitoring and removal treatments:an experience in ultra deep wells.In:SPE Paper 27622 p 10
Groenzin H,Mullins OC(1999)Asphaltene molecular size and structure.J Phys Chem A 103(50):11237–11245
Guiliano M,Mille G,Doumenq P,Kister J,Muller JF(1990)Study of various rank french demineralized coals and maceral concentrates:band assignment of FTIR spectra afetr resolution enhancement using fourier deconvolution.In:Charcosset H (ed)advanced methodologies in coal characterisation(coal science and technology).Elsevier,Amsterdam,pp 399–417
Hammami A,Chang-Yen D,Nighswander JA,Stange E(1995)An experimental study of the effect of paraffnic solvents on the onset and bulk precipitation of asphaltenes.Fuel Sci Technol Int 13(9):1167–1184
Ibarra JV,Mun˜oz E,Moliner R(1996)FTIR study of the evolution of coal structure during the coalifcation process.Org Geochem 24(6–7):725–735
Khadim M,Sarbar M(1999)Role of asphaltene and resin in oil feld emulsion.J Pet Sci Eng 23:213–221
Kokal SL,Sayegh SG (1995)Asphaltenes:the cholesterol of petroleum.In:SPE paper 29787.pp 169–81
Ma A,Zhang S,Zhang D(2008)Ruthenium-ion-catalyzed oxidation of asphaltenes of heavy oils in Lunnan and Tahe oilfelds in Tarim Basin,NW China.Org Geochem 39(11):1502–1511
Maddams WF(1980)The scope and limitations of curve ftting.Appl Spectrosc 34:245–267
Mojelsky TW,Ignasiak TM,Frakman Z,McIntyre DD,Lown EM, Montgomery DS,Strausz OP(1992)Structural features of Alberta oil sand bitumen and heavy oil asphaltenes.Energy Fuels 6(1):83–96
Muhammad AB,Abbott GD(2013)The thermal evolution of asphaltene-bound biomarkers from coals of different rank:a potential information resource during coal biodegradation.Int J Coal Geol 107:90–95
Mullins OC(2009)Rebuttal to Strausz et al.regarding time-resolved fuorescence depolarization of asphaltenes.Energy Fuels 23:2845–2854
Mullins OC,Sabbah H,Eyssautier J,Pomerantz AE,Barre´L, Andrews AB,Ruiz-Morales Y,MostowfF,McFarlane R,Goual L,Lepkowicz R,Cooper T,Orbulescu J,Leblanc RM,Edwards J,Zare RN(2012)Advances in asphaltene science and the yenmullins model.Energy Fuels 26(7):3986–4003
Nakanishi K(1962)Infrared absorption spectroscopy.Nankodo Co Ltd,Tokyo,p 233
PainterPC,SnyderRW,StarsinicM,ColemanMM,KuehnDW,Davis A(1981)ConcerningtheapplicationofFT-IRtothestudyofcoal: a critical assessment of band assignments and the application of spectral analysis programs.Appl Spectrosc 35:475–485
Peng P,Morales-Izquierdo A,Hogg A,Strausz OP(1997)Molecular structure of athabasca asphaltene:sulfde,Ether,and Ester Linkages.Energy Fuels 11(6):1171–1187
Peng P,Fu J,Sheng G,Morales-Izquierdo A,Lown EM,Strausz OP (1999)Ruthenium-ions-catalyzed oxidation of an immature asphaltene:structural features and biomarker distribution.Energy Fuels 13(2):266–277
Peters KE(1986)Guidelines for evaluating petroleum source rock using programmed pyrolysis.AAPG Bull 70:318–329
Rouxhet PG,Robin PL,Nicaise G(1980)Characterization of kerogens and their evolution by infrared spectroscopy.In: Durand B(ed)Kerogen:Insoluble organic matter from sedimentary rocks.Technip,Paris,pp 163–190
Sabbah H,Morrow AL,Pomerantz AE,Mullins OC,Tan X,Gray MR,Azyat K,Tykwinski RR,Zare RN(2010)Comparing laser desorption/laser ionization mass spectra of asphaltenes and model compounds.Energy Fuels 24(6):3589–3594
Sabbah H,Morrow AL,Pomerantz AE,Zare RN(2011)Evidence for island structures as the dominant architecture of asphaltenes. Energy Fuels 25(4):1597–1604
Shedid SA,Zekri AY(2006)Formation damage caused by simultaneous sulfur and asphaltene deposition.In:SPE paper 86553. pp 58–64
Sheu EY(2002)Petroleum asphaltene-propertiesm,characterization, and issues.Energy Fuels 16(1):74–82
Sheu EY,Mullins OC(eds)(1995)Asphaltenes:fundamentals and applications.Plenum Press,New York,p 245
Socrates G(1980)Infrared characteristic group frequencies:tables and charts,2nd edn.Wiley,Chichester,p 249
Solli H,Leplat P(1986)Pyrolysis-gas chromatography of asphaltenes and kerogens from source rocks and coals–a comparative structural study.Org Geochem 10(1–3):313–329
Speight JG(2004)Petroleum asphaltenes—Part 1:asphaltenes,resins andthestructureofpetroleum.OilGasSciTechnol59(5):467–477
Strausz OP,Mojelsky TW,Lown EM,Kowalewski I,Behar F(1999) Structural features of boscan and duri asphaltenes.Energy Fuels 13(2):228–247
Strausz OP,Safarik I,Lown EM,Morales-Izquierdo A(2008)A critique of asphaltene fuorescence decay and depolarizationbased claims about molecular weight and molecular architecture. Energy Fuels 22(2):1156–1166
Triflieff S,Sieskind O,Albrecht P(1992)Biological markers in petroleum asphaltenes:possible mode of incorporation.In: Moldowan JM,Albrecht P,Philp RP(eds)Biological markers in sedimentsand petroleum.Prentice Hall,New Jersey, pp 350–369
Weinberg VA,Yen TF,Murphy PDB,Gerstein BC(1983)Hypothetical average structures of two coal liquid asphaltenes from solid state 13 C nuclear magnetic resonance and 1 H nuclear magnetic resonance data.Carbon 21(2):149–156
William S(1985)Coal asphaltenes:a review.Fuel Process Technol 10(3):209–238
Wilson MA,Vassallo AM(1985)Developments in high-resolution solid-state13C NMR spectroscopy of coals.Org Geochem 8(5):299–312
Wilson MA,Pugmire RJ,Karas J,Alemany LB,Woolfenden WR, Grant DM,Given PH(1984)Carbon distribution in coal and coal macerals by cross-polarization magic angle spinning carbon-13 nuclear magnetic resonance spectroscopy.Anal Chem 56(933):43
Wu Q,Pomerantz AE,Mullins OC,Zare RN(2013)Laser-based mass spectrometric determination of aggregation numbers for petroleum-and coal-derived asphaltenes.Energy Fuels 28(1): 475–482
Yen TF(1974)Structure of petroleum asphaltene and its signifcance. Energy Sources 1:447–463
Yen TF,Wu WH,Chilingar GV(1984)A study of the structure of petroleum asphaltenes and related substances by infrared spectroscopy.Energy Sources Part A 7(3):203–235
Received:17 August 2014/Revised:7 January 2015/Accepted:21 January 2015/Published online:29 May 2015
©Science Press,Institute of Geochemistry,CAS and Springer-Verlag Berlin Heidelberg 2015
✉ Aminu Bayawa Muhammad
ambayawa@yahoo.co.uk
1Civil Engineering&Geosciences,Newcastle University, Newcastle upon Tyne,NE1 7RU,UK
杂志排行
Acta Geochimica的其它文章
- Sulfur determination by laser ablation high resolution magnetic sector ICP-MS applied to glasses,aphyric lavas, and micro-laminated sediments
- A large carbon pool in lake sediments over the arid/semiarid region,NW China
- Distributional characteristics and sources of elements in soil from typical area of Pearl River Delta economic zone,Guangdong Province,China
- Heavy metal(loid)pollution in mine wastes of a Carlin-type gold mine in southwestern Guizhou,China and its environmental impacts
- The controls on the composition of biodegraded oils in the Liuhua11-1 Oilfeld,Pearl River Mouth Basin,South China Sea
- Spatial pattern and distribution regularity of soil environmental quality in East China
