Aromatic Compounds Production from Sorbitol by Aqueous Catalytic Reforming
2015-01-13JinTnTiejunWngJinxingLongQiZhngLonglongMYingXuGunyiChen
Jin TnTie-jun WngJin-xing LongQi ZhngLong-long MYing XuGun-yi Chen
a
.School of Environmental Science and Engineering,Tianjin University,Tianjin 300072,China
b.Key Laboratory of Renewable Energy,Guangzhou Institute of Energy Conversion,Chinese Academy
of Sciences,Guangzhou 510640,China
Aromatic Compounds Production from Sorbitol by Aqueous Catalytic Reforming
Jin Tana,b,Tie-jun Wangb,Jin-xing Longb,Qi Zhangb,Long-long Maa,b∗,Ying Xub,Guan-yi Chena
a
.School of Environmental Science and Engineering,Tianjin University,Tianjin 300072,China
b.Key Laboratory of Renewable Energy,Guangzhou Institute of Energy Conversion,Chinese Academy
of Sciences,Guangzhou 510640,China
The rules on regulating aromatic compounds production was investigated by aqueous catalytic reforming of sorbitol.It was found that aromatics,ketones,furans,organic acids were main compounds in organic phase.The obvious efect of metal content showed that the highest carbon selectivity of aromatics was 34.36%when 3wt%Ni content was loaded on HZSM-5 zeolite modifed by MCM-41.However,it was decreased only to 4.82%when Ni content was improved to 20wt%.Meanwhile,diferent reaction parameters also displayed important impacts on carbon selectivity.It was improved with the increase of temperature, while it was decreased as liquid hourly space velocity and hydrogen pressure was increased. The results showed that appropriate higher temperature,longer contact time and lower hydrogen pressure were in favor of aromatics information,which suggested a feasible process to solve energy crisis.
Aromatic compound,Sorbitol,Aqueous catalytic reforming,Composite catalyst
I.INTRODUCTION
Energy crisis has become a serious problem for all the countries in the world.Recently,much more attention has been focused on the gasoline component additives which are mainly composed of benzene,toluene and xylenes[1,2].However,these aromatic compounds are usually derived from the sharply depleting fossil fuel. Therefore,it is urgent to exploit a new route for producing aromatic compounds from renewable biomass to substitute those originated from fossil fuels.
Usually,substantial eforts have been concentrated on three main technical routes∶(i)biomass pyrolysis,especially catalytic fast pyrolysis(CFP),under high temperature above 873 K[3-7].Although aromatic compounds could be produced from biomass over bifunctional catalysts directly,a large amount of coke would be inevitably formed during the pyrolysis process,resulting in the durative deactivation of catalysts[5].(ii) Gasifcation synthesis[8].Carbon oxide and hydrogen are main components of synthesis gas,and then aromatic products are synthesized by Fischer-Tropsch synthesis.However,there are no aromatic products during Fischer-Tropsch synthesis at low temperature and the yield of aromatic compounds are about only 6% even at high temperature[9].Moreover,coke is also main byproduct.(iii)Aqueous phase catalytic reforming[10,11].Converting biomass into aromatic compounds by aqueous phase catalytic reforming has attracted more and more attentions due to its two obvious advantages.It is easy to obtain fnal rough aromatic products distributed in organic phase,which will be separated from aqueous phase automatically.Furthermore,lower energy consumption is the second superiority of aqueous phase catalytic reforming compared with pyrolysis and gasifcation synthesis.Many kinds of platform chemicals have been selected as feedstock to produce aromatic compounds through aqueous phase catalysis,for example glycerol could be converted into alkyl-aromatic over HZSM-5 combined with Pd/ZnO at 673 K under 2 MPa hydrogen pressures[7].Furan compounds were also chosen as raw material to investigate the aromatic formative mechanism only using HZSM-5 zeolite at diferent temperature ranges[4].High concentration of sorbitol(60wt%)was converted into hydrophobic substances frstly over Pt-Re/C catalyst,and then aromatics was produced through ZSM-5 zeolite [12].
Although a great deal of efort has been made on the aromatic formation by aqueous phase catalytic reforming,diferent kinds of precious metal are usually adopted to achieve better results.Moreover,undesired coke also inevitably appeared during reaction under harsh conditions.These would cause much difculty in the application for industrialization.But adding meso-
II.MATERIALS AND METHODS
A.Materials
Sorbitol(analytical reagent)was purchased from Aladdin reagent company,Shanghai,China.HZSM-5 and MCM-41 were purchased from catalyst plant of Naikai University.Nickel nitrate(analytical reagent) was bought from Fuchen chemical reagents factory, Tianjin,China.
B.Catalyst preparation
The composite catalysts used in this work were synthesized by an incipient wetness impregnation method. Nickel nitrate solution and composite zeolites(HZSM-5∶MCM-41 weight ratio was 3∶2)were mixed together. This mixture was then dried overnight at 393 K and calcinated at 773 K for 4 h in air.After cooling down to room temperature,catalyst powders were compressed by a bead machine and selected by 40−60 mesh sieves. The Ni content of catalysts ranged from 1%to 20%, respectively.
C.Catalyst characterization
The crystalline structure of catalysts were characterized by X-ray difraction(XRD)(X Pert Pro MPD with Cu Kα(λ=0.154 nm)radiation,Philip)operated at 40 kV and 100 mA.Scanning angle(2θ)ranged from 5◦to 80◦.
Brunauer-Emmett-Teller(BET)surface area,external surface area,pore volume of catalysts were determined by nitrogen adsorption at 77 K using a QUADRASORB SI analyzer equipped with QuadraWin software system.All samples were degassed at 573 K for 8 h before adsorption measurement.After measurement,surface areas were calculated by the BET method and mesoporous pore volumes were calculated with the Barret-Joyner-Halenda(BJH)model.Micropore volumes were calculated with theT-plot method.
D.Experimental setups and procedures
Catalytic performance was conducted in a stainlesssteel tubular fow reactor.Hydrogen gas moved in a down fow direction with sorbitol solution,and both of them were heated by an electric heating furnace.The catalysts of 4.0 g were flled in reactor and silica wools were also flled at the both ends of catalysts.Prior to catalytic performances test,catalysts were reducedin situby a fow of H2(30 mL/min)at 773 K for 4 h and then cooled down to reaction temperature.After that,H2pressure,LHSV,and GHSV were adjusted to the designed conditions and then sorbitol solution was pumped into tubular to start reaction after being preheated at a certain temperature.Liquid products were accumulated in a gas-liquid separator and drained periodically into a collecting container.The analysis of oil products was performed on an Agilent GC-7890A gas chromatograph(HP innowax capillary column 19091N-133N,30 m×250µm×0.25µm)equipped with a mass spectrometer(5975C)using 99.995%of He as carrier gas.The conversion of feedstock was detected by HPLC (Waters 2695).Total organic carbon in aqueous phase was tested by TOC(Elementar in German Vario TOC). Sorbitol conversionXand carbon selectivitySof aqueous phase were calculated according to the following equations∶

wherexin,xout,andxtotalare sorbitol in,sorbitol out, and total carbon aqueous.
III.RESULTS AND DISCUSSION
A.XRD characterization of catalysts
XRD patterns of composite catalysts with diferent Ni loadings are shown in Fig.1.XRD characteristic peaks at 2θof 37.5◦,43.4◦,and 63.2◦were found over the catalysts,which can be attributed to the[111], [200],and[220]crystal faces of nickel oxide,respectively.Meanwhile,the peak intensity was increased sharply as Ni content was improved gradually,and it showed the highest peak intensity of catalyst when the Ni loading was increased to 20wt%.It indicated that inoculating crystals were easy to gather and form more regular nickel oxide crystals absorbed on the surface of composite zeolites.
B.BET characterization
Table I lists the physicochemical properties of the composite catalysts before reaction.The BET surface area of pure composite zeolites was found to be527.52 m2/g.When Ni component was loaded,the surface area of composite catalysts was decreased,and it was decreased to 318.02 m2/g when Ni content was up to 20wt%.The average pore diameter data(Fig.2) showed that composite catalysts had two typical pore structures(micropore and mesoporous).Nevertheless, the average mesoporous diameter was decreased obviously after diferent Ni content was loaded,which resulted in the new formative pores by metal flling action. This result showed that the infuence of metal content on mesoporous diameter was much greater than that of micropore.
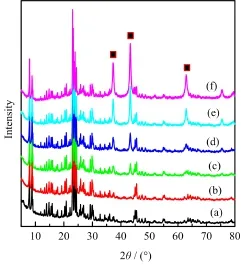
FIG.1 XRD patterns of diferent Ni content on composite zeolites.(a)0wt%Ni,(b)1wt%Ni,(c)3wt%Ni,(d)5wt%Ni, (e)10wt%Ni,and(f)20wt%Ni.

TABLE I Physicochemical properties of composite catalysts before reaction including surface areaAin m2/g and volumeVin mL/g.
C.The efect of diferent parameters on aromatics variation
1.The efect of Ni content
The efect of Ni content on oil components distribution was conducted in the range of 1wt%−20wt%.Sorbitol conversion and total carbon selectivity in aqueous phase are shown in Fig.3.It could be clearly seen from Fig.3 that sorbitol conversion nearly reached a maximum value of 100%over diferent Ni content catalysts. However,the total carbon selectivity in aqueous was 60.99%when Ni content was 1wt%.This result indicated that oxygen species in sorbitol molecules could not be fully removed under such reaction conditions. Majority products were hydrophilic existing in aqueous phase.But the carbon selectivity was the lowest and only reached 20.23%when 20wt%Ni content catalyst was used,implying that more Ni content absorbed on the zeolites was in favor of hydrodeoxygenation reaction to form hydrophobic compounds.In fact,thosehydrophilic chemicals,like propanal,acid,ester,ketone [13],glycerol[7]and furans[4],could yield aromatics at diferent selectivity.It revealed that hydrophilic compounds were intermediate chemicals when sorbitol was catalyzed into aromatics.
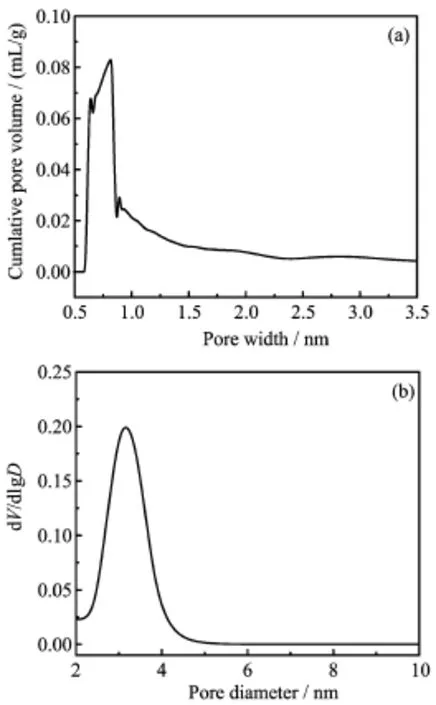
FIG.2 Pore diameter distribution of composite catalysts. (a)Micropore and(b)mesoporous.

FIG.3 Sorbitol conversion and carbon selectivity in aqueous phase after aqueous catalysis reforming.Reaction conditions:H2pressure=4.0 MPa,T=553 K,LHSV=1.5 h-1, GHSV=1000 h-1,and sorbitol:20 wt%.
Table II lists the carbon selectivity of main components in oil phase.The result of catalysis over 1%Ni content catalyst was not shown here due to poor oil yield,which was unable to be analyzed.The highest carbon selectivity of aromatics was 34.36%when 3wt%Ni content catalyst was used.But it was decreased to 4.82%when Ni content was up to 20wt%. Obviously,3wt%Ni content catalyst showed a wonderful performance on aromatics production.The synergic action between metal and composite zeolites could play a vital role in the aromatization.Higher metal content catalyst would lead to bonds cracking easily,resulting in low carbon compounds formation.Therefore,we chose 3wt%Ni content as optimum load and investigated the regulation of temperature,LHSV,GHSV and hydrogen pressure on aromatics production next.
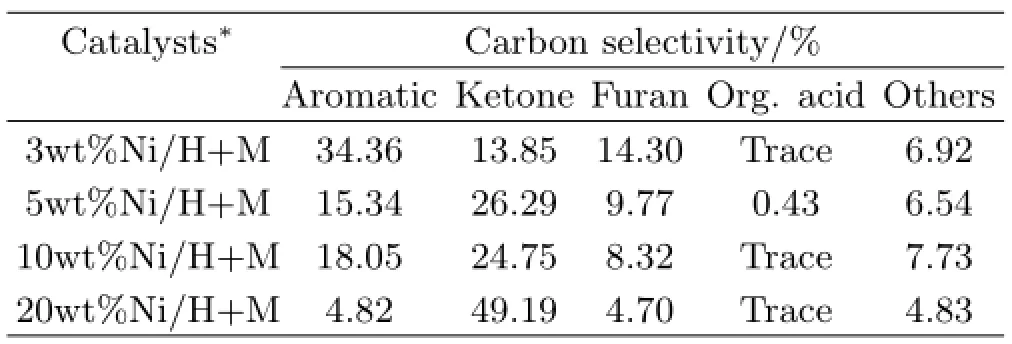
TABLE II Carbon selectivity of diferent oil components after reaction over various catalysts.
2.The efect of temperature
The efect of reaction temperature on aromatics production was conducted at the range of 513 K to 593 K. As shown in Fig.4,the activity of catalyst showed lower performance at 513 K,and sorbitol conversion was 77.38%.Although sorbitol conversion reached a maximal value of 100%at 533 K,there was nearly no oil appearing in fnal liquid phase,which was homogeneous.78.31%and 65.96%carbon derived from feedstock was found in the aqueous phase at 513 and 533 K, respectively.This result suggested that lower temperature was not in favor of hydrodeoxygenation reaction. Majority C−O bonds were main structure of aqueous compounds.However,the carbon selectivity of aqueous phase was decreased to 13.46%as temperature was improved to 593 K.Meanwhile,the carbon selectivity of aromatics was increased from 27.28%to 41.86%(Table III).These results implied that hydrodeoxygenation and aromatization reactions were easier to occur at higher temperatures.But coke formation was inevitable over catalysts under harsh conditions,indicating the cracking of sorbitol by pyrolysis[10,15].Furthermore,C−C bonds cleavage activity was strengthened under high temperature over metal catalyst,resulting in light hydrocarbon production[16].

FIG.4 Sorbitol conversion and carbon selectivity in aqueous phase under diferent temperature.Reaction conditions:H2pressure=4.0 MPa,LHSV=1.5 h-1,GHSV=500 h-1,sorbitol=20%.

TABLE III Efect of temperature on carbon selectivity of oil components.
3.The efect of LHSV
Various LHSV conditions were adopted to investigate the efect of residence time of feedstock with catalyst to aromatics production.As shown in Fig.5,sorbitol conversion always kept a maximal value of 100%when LHSV was at the range of 0.75 h−1to 3.00 h−1.But it was decreased to 29.77%sharply at 6.00 h−1.The change of carbon selectivity in aqueous phase was correspondent with that of LHSV.Carbon selectivity was increased from 1.62%to 74.94%obviously when LHSV was improved from 0.75 h−1to 6.00 h−1.These results indicated that perfect conversion could be achieved at longer contact time of feedstock with catalyst.Meanwhile,the carbons in feedstock could be better shifted from aqueous phase to oil phase.The results in Table IV further showed that residence time was a key factor not only in hydrodeoxygenation reaction,but also in aromatization reactions.For example,the carbon selectivity of aromatics reached the highest value of 65.65% when LHSV was at 0.75 h−1.But it was decreased to 9.34%drastically when LHSV was only reduced to 2.25 h−1.Although sorbitol was nearly converted com-pletely when LHSV was at 3.0 h−1,the fnal products formed a homogeneous aqueous solution,let alone aromatics production.These hydrophilic substances need to be further reacted to remove oxygen atoms from self-structure to form aromatics and other hydrophobic compounds by dehydrogenation,decarbonylation and aromatization reactions[10,17,18].
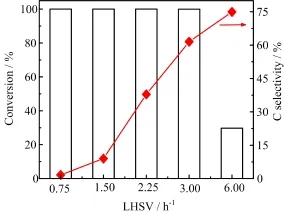
FIG.5 Efect of LHSV on sorbitol conversion and carbon selectivity in aqueous phase.Reaction conditions:H2pressure=4.0 MPa,T=553 K,GHSV=50 h-1,sorbitol=20%.

TABLE IV Efect of LHSV on carbon selectivity of oil products.LHSV in h-1.
4.The efect of GHSV
Figure 6 shows the efect of GHSV on sorbitol conversion and aqueous carbon selectivity.There was little change on sorbitol conversion under various GHSV ranging from 500 h−1to 4000 h−1,and reached the maximum value of 100%.However,carbon selectivity in aqueous was increased from 17.96%to 35.89%as GHSV was improved from 500 h−1to 4000 h−1.These results indicated that GHSV of hydrogen also played a vital role in hydrodeoxygenation reaction as Ni content,temperature and LHSV.In fact,sorbitol could be frst converted into hydrophilic substances over Ni catalysts without hydrogen.And then,these intermediate compounds were further converted into aromatics and hydrocarbon compounds in the presence of hydrogen and catalysts simultaneously.Majority of intermediate compounds could not be further converted completely when hydrogen few fast through catalyst mixed with feedstock.There was no enough time for hydrogen to adsorb on the metal surface to engage in hydrodeoxygenation and aromatization reactions.Our investigation results shown in Table V confrmed this opinion, which was in accordance with that of olazar[19].It was obviously found that the carbon selectivity of aromatics was at the lowest value of 3.40%when GHSV was at 4000 h−1.
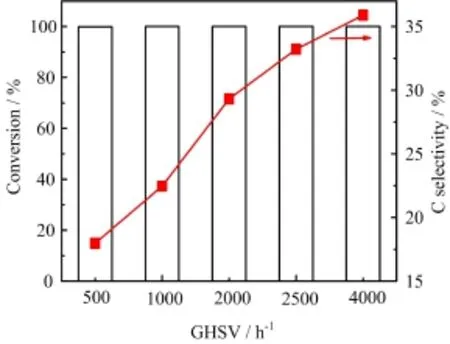
FIG.6 Efect of GHSV to sorbitol conversion and carbon selectivity in aqueous phase.Reaction conditions:H2pressure=4.0 MPa,T=553 K,LHSV=2.25 h-1,sorbitol=20%.
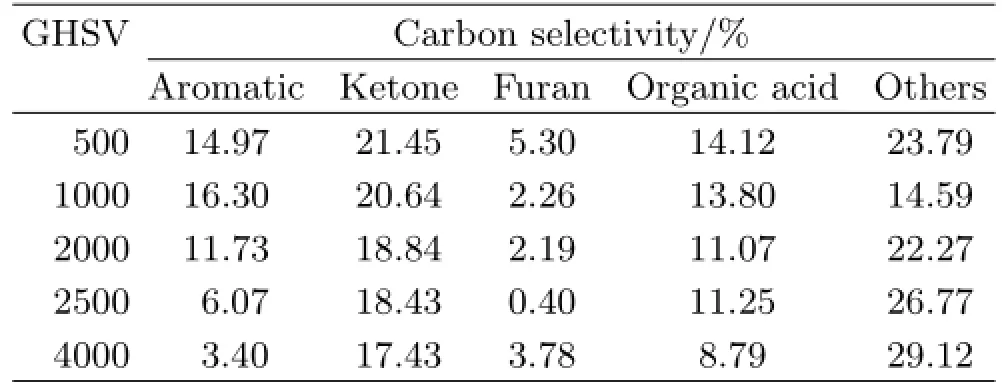
TABLE V Efect of GHSV on carbon selectivity of oil products.GHSV in h-1.
5.The efect of hydrogen pressure
The results of hydrogen pressure efect on catalytic performance are listed in Fig.7.Sorbitol conversion reached a perfect value of 100%under various hydrogen pressures.However,carbon selectivity in aqueous was increased from 17.77%to 42.39%when hydrogen pressure was improved from 1.0 MPa to 5.0 MPa.This result indicated that majority carbon remained in hydrophilic compounds,and higher pressure could cause inhibition to hydrodeoxygenation and aromatization reactions.Hydrogen pressure could be used to adjust the relative rates of C−C versus C−O bond cleavage.Improving system pressure increased the hydrogen concentration and most likely resulted in a decrease in the rate of dehydrogenation[17,21].
The results in Table VI were in accordance with this opinion.Carbon selectivity of aromatics was decreased from 46.02%to 4.48%when hydrogen pressure was im-proved from 1.0 MPa to 5.0 MPa.The dehydrogenation was inhibited heavily at higher hydrogen pressure,resulting in the decrease of aromatics.

FIG.7 Sorbitol conversion and carbon selectivity in aqueous phase varied with hydrogen pressure.Reaction conditions:T=553 K,LHSV=2.25 h-1,GHSV=2500 h-1,sorbitol=20%.

TABLE VI Efect of hydrogen pressure on carbon selectivity of oil products.
IV.CONCLUSION
The regulation of aromatic compounds production were proposed through aqueous catalytic reforming of sorbitol.Results showed that the highest carbon selectivity of aromatics was 34.36%over 3wt%Ni loading dosage catalyst.Meanwhile,higher temperature, longer contact time and lower pressure are in favor of aromatics formation.The optimized result of aromatic compounds can be obtained at 593 K,1.0 MPa H2,and 0.75 h−1of LHSV.Therefore,the results presented in this work suggest a strategy for developing a feasible process to produce aromatic compounds for sorbitol derived from biomass.
V.ACKNOWLEDGMENTS
This work was supported by the National Key Basic Research and Development Plan(No.2012CB215304), he Key Laboratory of Renewable Energy Foundation (No.y407j51001),the National Natural Science Foundation of China(No.51376185 and No.51106108),the National High-Tech Research and Development Programme(No.2012AA101806).
[1]T.R.Carlson,G.A.Tompsett,W.C.Conner,and G. W.Huber,Top.Catal.52,241(2003).
[2]Y.T.Cheng,J.Jae,J.Shi,W.Fan,and G.W.Huber, Angew.Chem.Int.Edit.51,1387(2012).
[3]K.Y.Chen,M.Tamura,Z.Yuan,Y.Nakagawa,and K.Tomishige,ChemSusChem6,613(2013).
[4]Y.T.Cheng and G.W.Huber,ACS Catal.1,611 (2011).
[5]J.W.Diehl and F.P.Di Sanzo,J.Chromatogr.A1080, 157(2005).
[6]R.A.Dagle,J.A.Lizarazo-Adarme,V.L.Dagle,M. J.Gray,J.F.White,D.L.King,and D.R.Palo,Fuel Process.Technol.123,65(2014).
[7]T.Q.Hoang,X.L.Zhu,T.Danuthai,L.L.Lobban,D. E.Resasco,and R.G.Mallinson,Energ.Fuel.24,3804 (2010).
[8]S.Ilias and A.Bhan,J.Catal.311,6(2014).
[9]T.Juganaru,C.Ionescu,D.Bombos,M.Bombos,and V.Matei,Rev.Chim.58,698(2007).
[10]J.Jae,R.Coolman,T.J.Mountziaris,and G.W.Huber,Chem.Eng.Sci.108,33(2014).
[11]J.Jae,G.A.Tompsett,A.J.Foster,K.D.Hammond, S.M.Auerbach,R.F.Lobo,and G.W.Huber,J.Catal.279,257(2011).
[12]E.L.Kunkes,D.A.Simonetti,R.M.West,J.C. Serrano-Ruiz,C.A.G¨artner,and J.A.Dumesic,Science17,417(2008).
[13]X.L.Zhu,L.L.Lobban,R.G.Mallinson,and D.E. Resasco,J.Catal.271,88(2010).
[14]Q.Zhang,T.Jiang,B.Li,T.J.Wang,X.H.Zhang,Q. Zhang,and L.L.Ma,ChemCatChem4,1084(2012).
[15]T.R.Carlson,J.Jae,Y.C.Lin,G.A.Tompsett,and G.W.Huber,J.Catal.270,110(2010).
[16]L.Vilcocq,A.Cabiac,C.Especel,S.Lacombe,and D. Duprez,Catal.Today189,117(2012).
[17]N.Li and G.W.Huber,J.Catal.270,48(2010).
[18]C.A.Mullen,A.A.Boateng,D.J.Mihalcik,and N. M.Goldberg.Energ.Fuel25,5444(2011).
[19]M.Olazar,R.Aguado,J.Bilbao,and A.Barona,Aiche J.46,1025(2000).
[20]B.M.Moreno,N.Li,J.Lee,G.W.Huber,and M.T. Klein,RSC Adv.3,23769(2013).
10.1063/1674-0068/28/cjcp1408131pores into HZSM-5 catalyst was able to overcome difusion limitation and char formation[13].Furthermore, in our previous investigation,the Ni/HZSM-5 catalyst modifed by MCM-41 showed exciting performance on the C5-C6 alkanes production from sorbitol[14].Therefore,in this work,cheaper metal Ni and composite zeolites were adopted in this reaction.The efect of reaction parameters such as reaction temperature,LHSV (liquid hourly space velocity),GHSV(gas hourly space velocity),hydrogen pressure as well as diferent metal content on composite zeolites were investigated in detail to illustrate the rule of aromatic compounds production.
∗Author to whom correspondence should be addressed.E-mail:mall@ms.giec.ac.cn,Tel.:+86-20-87048614,FAX:+86-20-87057751
(Dated:Received on August 11,2014;Accepted on October 20,2014)
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- High Performance of Enhanced Mode Field Efect Transistor and Ultraviolet Sensor Based on ZnO Nanosheet
- Quantitative Moisture Measurement with a Cavity Ring-down Spectrometer using Telecom Diode Lasers
- Accurate Measurement of Raman Depolarization Ratio in Gaseous CO2
- Methanol Adsorption on TiO2Film Studied by Sum Frequency Generation Vibrational Spectroscopy
- Ultraviolet Source Assisted Enhancement of Attosecond Pulse
- Decay Dynamics ofN,N-Dimethylthioacetamide in S3(ππ∗)State
