Synthesis of Hierarchically Porous CaFe2O4/Carbon Fiber Hybrids and Microwave Induced Catalytic Activity
2015-01-13YiSongChaoKongJiaLi
Yi SongChao KongJia Li
School of Material Science and Engineering,University of Jinan,Jinan 250022,China
Synthesis of Hierarchically Porous CaFe2O4/Carbon Fiber Hybrids and Microwave Induced Catalytic Activity
Yi Song,Chao Kong,Jia Li∗
School of Material Science and Engineering,University of Jinan,Jinan 250022,China
Hierarchically porous CaFe2O4/carbon fber hybrids with enhanced microwave induced catalytic activity for the degradation of methyl violet(MV)from water were synthesized from kapok by a novel two-step process coupling pore-fabricating and nanoparticles assembling. The as-prepared samples exhibited characteristic hollow fber morphology,CaFe2O4nanoparticles dispersed uniformly on the surface of hollow carbon fbers(HCF).The efects of various factors such as CaFe2O4loading,microwave power,catalyst doses,initial concentration of MV solution and pH value on the microwave induced degradation of MV over CaFe2O4/HCF were evaluated.It was found that the microwave induced degradation of MV over CaFe2O4/HCF had high reaction rate and short process time.The kinetic study indicated that the degradation of MV over CaFe2O4/HCF followed pseudo-frst-order kinetics model.The high catalytic activity of CaFe2O4/HCF was facilitated by the synergistic relationship between microwave induced catalytic reaction and adsorption characteristics.Key words:CaFe2O4,Microwave,Catalytic activity,Kapok,Degradation
I.INTRODUCTION
Large numbers of pigments and dyes are often used for industrial applications.There are approximately 104various industrial pigments and dyes,and annual production of over 7×105tons[1−3].Consequently, it is inevitable that pigments and dyes will appear in wastewater and spill into the environment without being efectively treated.Though there is a very small amount of dye in water(10−20 mg/L),color of efuents is highly visible[4].
Some methods have been used for the removal of pollutants from wastewater,such as chemical oxidations, adsorption[5,6],biological decoloration[7],ultrasonic degradation[8],photocatalytic degradation[9,10]and the advanced oxidation process with photo-Fenton reaction[11].In recent years,microwave induced catalytic degradation(MICD)technologies[12,13]merge as a useful and efective method to mineralize organic pollutants into non-toxic substances[14].Microwave can be applied alone[15],combined with oxidants,integrated with photochemical process and Fenton process,and coupled with microwave-absorbing materials. As known,some materials including porous carbon[16, 17],metal oxidation[18]and ferromagnetic materials [19,20]are widely used as microwave-absorbing materials.
Hierarchically porous catalysts containing both interconnected macroporous and mesoporous structure exhibit enhanced catalytic activity due to combining the accessible mass transport pathway of macroporous tunnel with high surface area.A large variety of natural materials,such as bacteria[21],diatoms[22],butterfy wings[23]and egg membranes[24]have been used to synthesize hierarchically porous materials.Notably, the cellulose-based substance becomes ma jor concern in synthesizing biomorphic materials due to its low cost, reproducibility and stable chemical composition mainly containing C and O.
In this work,hierarchically porous CaFe2O4/HCF hybrids were synthesized using kapok as biotemplate.The microwave-enhanced catalytic activity of CaFe2O4/HCF hybrids on the degradation of methyl violet(MV)was investigated.The infuences of processing parameters on the resulting products,and the microstructure characteristic relating to catalytic activity were evaluated in detail.
II.EXPERIMENTS
A.Catalyst preparation and characterization
All the chemicals used in this study,such as Ca(NO3)2·6H2O,Fe(NO3)3·3H2O,hydrochloric acid, sodium hydroxide,and MV,were of analytical grade. Kapoks were purchased from the local market.After being pre-oxidation treated at 200◦C for 2 h,kapok was impregnated with aqueous solution of analytical
Scanning electron microscopy(SEM,FEI QUANTA FEG250,USA)were equiped with energy-dispersive X-ray spectrometry(EDS).The samples were identifed by X-ray difractometry(XRD,Huber difractometer, Rimsting,Germany)pattern(Cu-Kαradiation source RUC300,λ=1.5418˚A,voltage 40 kV,current 160 mA, Rigaku,Japan).BET surface area was measured by nitrogen adsorption-desorption technique at 77 K using a physisorption analyzer(ASAP2020M+C,Micrometrics,GA,USA).Brunauer-Emmett-Teller and Barrett-Joyner-Halenda(BET/BJH)methods were used to determine specifc surface area and porosity.pHS-3C pH meter(Shanghai Leici Company,China)was used to determine acidities of the MV solutions.The UV continuous spectrum was obtained by UVC3200(Daojin, Japan).The magnetization measurements were carried out by MicroMag 2900 AGM(alternating gradient magnetometer,Princeton Measurements Corporation, USA).
B.Microwave assisted catalytic oxidation of MV
A 50 mL MV solution(5 mg/L)with pH close to 7.0 and a certain amount of catalyst were added in a beaker.The G8023ESL-V8 microwave oven(Guangdong Galanz Company,China)with microwave frequency of 2.45 GHz and output power of 800 W was used to irradiate this suspension for a certain time. Then the suspension was cooled for 5 min in the water bath and separated using a centrifuge.The UV-Vis absorption spectra of parathion solutions were recorded on Cary-50 UV-Vis spectrometer(Varian Company,USA). The absorbance was determined at the maximum absorbance wavelength(580 nm)of MV.Then degradation raterwas obtained by the following equation∶

whereC0is the initial concentration of MV andCtis the concentration of MV at timet.
III.RESULTS AND DISCUSSION
A.Characterization of CaFe2O4/HCF
The structure and components of the as-prepared CaFe2O4/HCF samples were characterized by XRD analysis.Figure 1 shows the typical XRD pattern of the as-prepared samples.The main characteristic difraction peaks of CaFe2O4(JCPDS card No.08-0100)were observed at 2θvalues of approximately 19.1◦,25.4◦, 33.6◦,35.6◦,40.4◦,42.8◦,49.8◦,55.3◦,60.6◦,61.4◦, 72.0◦,which could be assigned to(120),(220),(040), (201),(131),(311),(401),(260),(600),(161),(322) difraction planes,respectively.It is worth noting that the difraction baseline of XRD pattern was very broad, which could be attributed to the presence of amorphous carbon.Moreover,a small amount of Ca2Fe2O5(JCPDS card No.11-0675)was observed.The average crystallite sizeDof the CaFe2O4grain was 4.6 nm calculated by the Scherrer equation∶
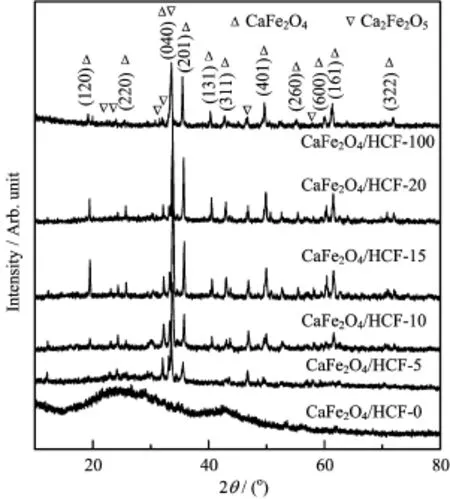
FIG.1 XRD pattern of the as-prepared samples.

whereKis a constant equal to 0.89,λis the X-ray wavelength equal to 0.154 nm,βis the full width at half maxima of the most intense peak,andθis the half difraction angle.
Figure 2(a)and(b)show the SEM images of raw kapok.It was found that raw kapok was characterized by the interwoven networks consisting of hollow fbers. The average diameter of hollow fbers was about 18µm (Fig.2(b)).For the synthesized sample,the hollow fber morphology of kapok was retained(Fig.2(c)−(e)).The diameters of hollow tubes ranged from 10µm to 15µm which were smaller than those of raw kapok due to the shrinkage caused by high temperature treatment.Tiny particles were found on the surface of HCF(Fig.2(c)). As revealed in Fig.2(d),CaFe2O4particles on the surface of HCF increased and distributed non-uniformly due to increasing concentration of iron nitrate solution and calcium nitrate solution.When the concentrationof iron nitrate solution and calcium nitrate solution increased again,carbon was almost burned as shown in Fig.2(e).The typical EDS of particles(Fig.2(f))indicated that they were composed of Ca,Fe,and O.According to the above XRD result,we assumed that these particles were CaFe2O4and Ca2Fe2O5.
The nitrogen adsorption-desorption isotherm and corresponding pore size distributions of synthesized CaFe2O4/HCF-10 sample are shown in Fig.3.According to the IUPAC classifcation scheme,the isotherm (Fig.3(a))belonged to the group of type IV,indicating that CaFe2O4/HCF was mesoporous material.The BET specifc surface area,average pore size,and pore volume of the CaFe2O4/HCF were measured to be 85.49 m2/g,5.3 nm,and 0.114 cm3/g,respectively.
As a promising technique for the recycling of the catalyst,magnetic separation could avoid the loss of catalyst.The magnetization measurements were carried out at room temperature in air.As shown in Fig.4, the hysteresis loops of the as-prepared CaFe2O4/HCF-10 catalyst indicated that it was the ferromagnetic material.The saturation magnetization(Ms)of the CaFe2O4/HCF-10 catalyst was calculated to be 16.92646 emu/g,which was enough to recover the catalyst from dye solution[25−27].
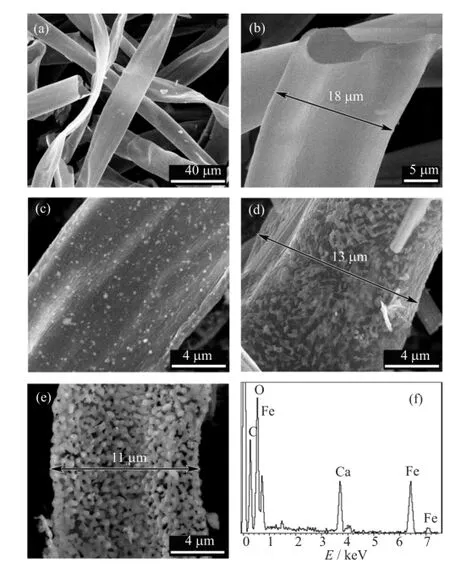
FIG.2 SEM images of Kapok template(a,b)and CAFE2O4/HCF-10(c,d,e).(f)EDS of CAFE2O4/HCF-10.
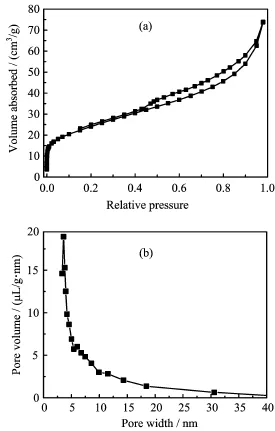
FIG.3(a)Nitrogen adsorption/desorption isotherms and (b)corresponding BJH pore diameter distribution of asprepared CaFe2O4/HCF-10 sample.
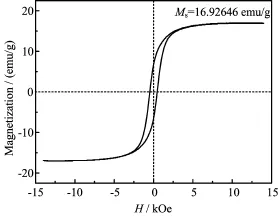
FIG.4 Magnetic hysteresis loops of porous CaFe2O4/HCF-10 sample at room temperature.
B.Changes in the UV-Vis absorption spectrum of MV
The UV-Vis absorption spectra of MV at diferent microwave irradiation time(t)are shown in Fig.5.As could be seen from the spectra,before the treatment, the UV-Vis spectrum of MV was characterized by one main band in the visible region,with its maximum absorption at 580 nm,and two bands in the ultraviolet region located at 246 and 300 nm.The band at 246 nm was ascribed to the benzene ring in the dye molecule, and the bands at 300 and 580 nm were attributed to the absorption of chromophore containing NH2group.It was obvious that these bands steadily decreased with increasingt.Whentwas 6 min,all bands became fat, which indicated that the MV was completely mineralized.
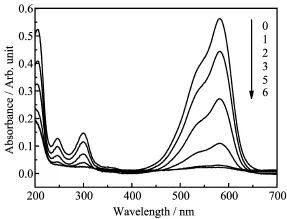
FIG.5 UV-Vis absorption of Aldrich HA during microwave degradation at diferent timet(0-6 min).

FIG.6 Efect of CaFe2O4loading on degradation of MV. (a)Without catalyst,(b)CaFe2O4/HCF-0,(c)CaFe2O4/ HCF-100,(d)CaFe2O4/HCF-20,(e)CaFe2O4/HCF-15, (f)CaFe2O4/HCF-5,(g)CaFe2O4/HCF-10.
C.Efect of loading on MV degradation
CaFe2O4loading on HCF usually played an important role in decolorization process of MV.Figure 6 shows that degradation of MV hardly occurs in the absence of CaFe2O4/HCF under 800 W microwave irradiation.From Fig.6,it could be seen that the extent of degradation increased with increasing CaFe2O4loading from 0wt%to 10wt%,and then degradation rate dropped to some extent at ratio of 10wt%−100% CaFe2O4.The optimum loading of CaFe2O4considering degradation rate was 10%,because that the number of“hot spots”on the surface of CaFe2O4/HCF increased with increased catalyst loading,which could enhance oxidation of MV.When CaFe2O4loading increased from 10wt%to 20wt%,catalyst surface area may be reduced owing to agglomeration of the particles [28].In addition,it could be seen that degradation rate of CaFe2O4/HCF was higher than that of HCF alone (49.99%)and CaFe2O4alone(56.67%).

FIG.7 Efect of microwave powers(a)and kinetics(b)on degradation rate of MV.5 mg/L MV,50 mL MV,0.3 g/L CaFe2O4/HCF-10.
D.Efect of microwave power and initial concentration of MV on the degradation of MV
Three levels of microwave power were used to investigate the efect of microwave power on the degradation of MV(Fig.7).It was found that the removal ratio of MV increased and the decolorization process became faster with the microwave power increasing(Fig.7(a)), because that higher microwave power could form more“hot spots”on the surface of CaFe2O4/HCF and produce more·OH radical in the aqueous solution.Hence, in subsequent experiments,800 W was chosen because of the best degradation rate.
Pseudo frst-order kinetic model[29]was used to investigate the efect of microwave power on the degradation of MV∶

whereC0is the initial concentration of MV,Ctis the concentration of MV at eachtandkappis the apparent rate constant.The−ln(C/C0)versustare plotted andshown in Fig.7(b).It could be seen that the relationship between−ln(C/C0)and t for each concentration was approximately linear.Table I shows the rate constants and linear correlation coefcients(R2).TheR2values obtained from pseudo-second-order model were close to unity,indicating that the degradation of MV ftted this model well.It is worth noting that the rate constant increased with increasing microwave powers.The highest rate constant was obtained as the microwave power was 800 W.
The efect of initial concentration of MV on the degradation of MV is shown in Fig.8.It could be seen that degradation rate of MV was the highest as the initial concentration of MV was 5 mg/L.This was because that the amount of MV molecules around the limited CaFe2O4/HCF catalyst increased with increasing dye concentration.In other words,the catalytic reactions were limited by the concentration of·OH radicals. Hence,the degradation rate decreased with increasingC0.

FIG.8 Efects of and initial concentration on degradation rate.50 mL MV,0.3 g/L CaFe2O4/HCF-10,800 W.

TABLE I Kinetic parameters from-ln(C/C0)vs.tplots for diferent microwave powers.
E.Efects of catalyst doses and pH on the degradation of MV
The efect of catalyst doses on the degradation of MV was investigated under microwave power 800 W. As shown in Fig.9(a),the degradation rate of MV increased with increasing catalyst dose.The degradation rate of three catalyst doses(5,10,and 15 mg)were 65.2%,88%,and 96%,respectively whentwas 6 min. The experiments demonstrated that degradation rate of MV increased with increasing catalyst dose.This was due to the fact that the number of“hot spots”on the surface of CaFe2O4/HCF increased with increase of catalyst doses under microwave irradiation.In addition,the increasing CaFe2O4/HCF could produce more·OH radicals.All of factors show clearly the increase of degradation capacity of CaFe2O4/HCF with catalyst doses rising.
The initial pH value was one of the most important parameter on decolorization process because the wastewater containing dye usually had a wide range of pH values.The change of pH could infuence the surface charge of CaFe2O4/HCF sample,and thus affected the reaction speed of degradation in the end. It could be seen from Fig.9(b)that the degradation rates of MV at pH=3,7,and 11 were 83.5%,84.5%, and 100%,respectively after 6 min microwave radiation. The CaFe2O4/HCF sample had the highest degradation rate at pH=11.0,which indicated that the degradation of MV was more efective in alkaline conditions.The efect of pH value might be explained from the viewpoint of adsorption.MV was readily to be adsorbed throughπ-πstacking and ionic interaction due to the fact that MV was an alkaline and cationic triphenylmethane dyes[30].In the acidic conditions(pH<4.0), the positively charged surface of catalyst resulted in repulsion between CaFe2O4/HCF and MV,so the adsorption occurred only through theπ-πstacking which led to a lower degradation efciency.With the pH increas-ing,the increasing negative charges were benefcial to the interaction between CaFe2O4/HCF and cationic dye MV.In addition,the production of·OH radicals needed the alkaline conditions(pH>7.0),which assisted in the MICD.Based on the above reasons,CaFe2O4/HCF exhibited higher catalytic activity at pH=11.0.

FIG.9 Efects of catalyst doses(a)and pH(b)on degradation rate.50 mL MV,0.3 g/L CaFe2O4/HCF-10,800 W.

FIG.10 Illustration of degradation of MV on the surface of CaFe2O4/HCF.
F.Possible degradation mechanism
Although the investigation on the microwave degradation in the presence of CaFe2O4/carbon fbers hybrids has not been reported too much yet,three ways to degrade MV may be deduced based on our studies (Fig.10).First,the microwave irradiation can induce a rotation and a migration violently for the motion of polar molecules in CaFe2O4/HCF as microwave absorbent under microwave irradiation,leading to a fast increase of the solution temperature due to friction.Also,with increasing of collision numbers between polar molecules, the violent motion of polar substances can transform microwave energy into the“hot spots”that would reach over 1200◦C[31].Then,not only the chromophore containing NH2group of MV can be degraded rapidly but also the benzene ring of it can be destroyed completely due to the function of thermal efect.Second,H2O, OH−,and OH groups in the structure of CaFe2O4can be transformed into·OH in aqueous solution due to such high temperature,which can non-selectively attack organic contaminants[32]and convert them into H2O, CO2,and inorganic salt.The existence of·OH results in accelerating the degradation rate of MV.In addition, there is synergy between CaFe2O4and microwave.Due to such high temperature,the electrophilic oxygen ions (O2−,O−and O2−)that come from the lattice oxygen on CaFe2O4ferrite show high catalytic activity in MICD and can be donated to participate in the degradation of MV[33,34].In this work,there may be one or more mechanisms of degradation.Therefore,the asprepared CaFe2O4/HCF may be considered to be good catalyst in MICD.
IV.CONCLUSION
In this work,the biomorphic CaFe2O4/HCF with high microwave induced catalytic activity for degradation of MV was prepared by using kapok as template.Hierarchically porous CaFe2O4/HCF hybrid catalysts exhibited hollow carbon fbers loaded with CaFe2O4nanoparticles.The catalytic performances of synthesized samples for the degradation of MV under microwave irradiation were investigated.It was demonstrated that the degradation process of MV over CaFe2O4/HCF catalyst was a fast process.Under the conditions of 50 mg/L and 50 mL MV solution,0.3 g/L catalyst dose and microwave power 800 W,completely degradation of MV could be obtained after 6 min microwave irradiation.The microwave induced degradation of MV ftted pseudo frst-order kinetic model.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.51172095 and No.61102006).
[1]E.Y.Ozmen,M.Sezgin,A.Yilmaz,and M.Yilmaz, Bioresour.Technol.99,526(2008).
[2]M.Chivukula,J.T.Spadaro,and V.Renganathan, Biochemistry34,7765(1995).
[3]C.I.Pearce,J.R.Lloyd,and J.T.Guthrie,Dyes Pigments58,179(2003).
[4]K.T.Chung and S.E.Stevens,Environ.Toxicol.Chem.12,2121(1993).
[5]L.S.Zhang,J.S.Lian,L.X.Wang,J.Jiang,Z.R. Duan,and L.J.Zhao,Chem.Eng.J.241,384(2014).
[6]K.Mohanty,J.T.Naidu,B.C.Meikap,and M.N. Biswas,Ind.Eng.Chem.Res.45,5165(2006).
[7]J.J.Jones and J.O.Falkinham,Antimicrob.Agents Chemother.47,2323(2003).
[8]E.Villaroel,J.Silva-Agredo,C.Petrier,G.Taborda, and R.A.Torres-Palma,Ultrason.Sonochem.21,1763 (2014).
[9]L.J.Deng,Y.Z.Gu,W.X.Xu,and Z.Y.Ma,Chin. J.Chem.Phys.27,321(2014).
[10]C.Li and W.D.Wang,Chin.J.Chem.Phys.22,423 (2009).
[11]R.Colombo,T.C.R.Ferreira,S.A.Alves,R.L. Carneiro,and M.R.V.Lanza,J.Environ.Manage.118,32(2013).
[12]K.C.Li,R.G.Xing,S.Liu,Y.K.Qin,X.T.Meng, and P.C.Li,Int.J.Biol.Macromol.51,767(2012).
[13]N.Remya and J.G.Lin,Chem.Eng.J.166,797(2011).
[14]P.Klan and M.Vavrik,J.Photochem.Photobiol.177, 24(2006).
[15]G.Cravotto,A.Binello,S.Di Carlo,L.Orio,Z.L.Wu, and B.Ondruschka,Environ.Sci.Pollut.Res.17,674 (2010).
[16]L.L.Bo,X.Quan,S.Chen,H.M.Zhao,and Y.Z. Zhao,Water.Res.40,3061(2006).
[17]G.S.Zhang,J.H.Qu,H.J.Liu,A.T.Cooper,and R. C.Wu,Chemosphere68,1058(2007).
[18]T.L.Lai,C.C.Lee,G.L.Huang,Y.Y.Shu,and C. B.Wang,Appl.Catal.B78,151(2008).
[19]L.Zhang,M.M.Su,and X.J.Guo,Sep.Purif.Technol.62,458(2008).
[20]L.Zhang,X.J.Guo,F.Yan,M.M.Su,and Y.Li, Water Sci.Technol.60,2563(2009).
[21]H.Zhou,T.X.Fan,and D.Zhang,Chem.Mater.19, 2144(2007).
[22]N.L.Rosi,C.S.Thaxton,and C.A.Mirkin,Angew. Chem.Int.Ed.43,5500(2004).
[23]J.Y.Huang,X.D.Wang,and Z.L.Wang,Nano.Lett.6,2325(2006).
[24]D.Yang,L.Qi,and J.Ma,Adv.Mater.14,1543(2002).
[25]Y.M.Ren,Q.Dong,J.Feng,J.Ma,Q.Wen,and M. L.Zhang,J.Colloid.Interface.Sci.382,90(2012).
[26]M.Shahid,J.L.Liu,Z.Ali,I.Shakir,M.F.Warsi, R.Parveen,and M.Nadeem,Mater.Chem.Phys.139, 566(2013).
[27]A.A.Farghali,M.H.Khedr,and A.F.Moustafa, Mater.Technol.23,104(2008).
[28]M.P.Titus,V.Garc¨oa-Molina,M.A.Banos,J. Gimenez,and S.Esplugas,Appl.Catal.B47,219 (2004).
[29]Z.H.Zhang,J.Jiatieli,D.N.Liu,F.Y.Yu,S.Xue, W.Gao,Y.Y.Li,and D.D.Dionysiou,Chem.Eng.J.231,84(2013).
[30]C.Z.Li,Y.H.Dong,J.J.Yang,Y.Y.Li,and C.C. Huang,J.Mol.Liq.196,348(2014).
[31]H.M.Kingston and L.B.Jassie,Introduction to Microwave Sample Preparation,Washington:American Chemical Society,(1998).
[32]J.Wang,W.Sun,Z.H.Zhang,Z.Jiang,X.F.Wang, R.Xu,R.H.Li,and X.D.Zhang,J.Colloid Interface Sci.320,202(2008).
[33]T.L.Lai,Y.L.Lai,and C.C.Lee,Catal.Today.131, 105(2008).
[34]A.Biela´nski and J.Haber,Catal.Rev.Sci.Eng.19,1 (1979).
10.1063/1674-0068/28/cjcp1407125grade of iron nitrate and calcium nitrate aqueous mixture at room temperature,and then dried at 120◦C for 4 h,hydrolysis and condensation reactions occurred. The white hybrid material was then heated in a tube furnace at 800◦C for 1 h under a constant fow of N2at 0.2 L/min.The as-prepared samples were obtained with diferent CaFe2O4loading on HCF of 0wt%, 5wt%,10wt%,15wt%,20wt%,100wt%,designated as CaFe2O4/HCF-0,CaFe2O4/HCF-5,CaFe2O4/HCF-10, CaFe2O4/HCF-15,CaFe2O4/HCF-20,CaFe2O4/HCF-100.
∗Author to whom correspondence should be addressed.E-mail:mse-lij@ujn.edu.cn,Tel.:+86-13953185430
(Dated:Received on July 29,2014;Accepted on December 22,2014)
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- High Performance of Enhanced Mode Field Efect Transistor and Ultraviolet Sensor Based on ZnO Nanosheet
- Quantitative Moisture Measurement with a Cavity Ring-down Spectrometer using Telecom Diode Lasers
- Accurate Measurement of Raman Depolarization Ratio in Gaseous CO2
- Methanol Adsorption on TiO2Film Studied by Sum Frequency Generation Vibrational Spectroscopy
- Ultraviolet Source Assisted Enhancement of Attosecond Pulse
- Decay Dynamics ofN,N-Dimethylthioacetamide in S3(ππ∗)State
