Observation of Excitedνs(NO2)and Relaxation Process of HNS in Solution by CARS Technique
2015-01-13GenChuMinShuiYunfeiSongToXuYuqiuGuYnqingYng
Gen-i ChuMin ShuiYun-fei SongTo XuYu-qiu GuYn-qing Yng
a.Science and technology on Plasma Physics Laboratory,Research Center of Laser Fusion,China Academy of Engineering Physics,Mianyang 621900,China
b.Department of Physics,Harbin Institute of Technology,Harbin 150001,China c.Institute of Chemical Materials,China Academy of Engineering Physics,Mianyang 621900,China
Observation of Excitedνs(NO2)and Relaxation Process of HNS in Solution by CARS Technique
Gen-bai Chua,Min Shuia,Yun-fei Songb,Tao Xuc∗,Yu-qiu Gua∗,Yan-qiang Yangb
a.Science and technology on Plasma Physics Laboratory,Research Center of Laser Fusion,China Academy of Engineering Physics,Mianyang 621900,China
b.Department of Physics,Harbin Institute of Technology,Harbin 150001,China c.Institute of Chemical Materials,China Academy of Engineering Physics,Mianyang 621900,China
Investigation on vibrational excitation and relaxation process will provide important information for a better understanding of ultrafast dynamic response of energetic materials. Using sub-ps time-resolved coherent anti-Stokes Raman scattering(CARS)experiments,we directly observe excitation of vibrational modeνs(NO2)and its relaxation process of ground state HNS(2,2′,4,4′,6,6′-hexanitrostillbenein)in solution.The results show thatνs(NO2)at 1385 cm−1has been excited and relaxation time of 0.38 and 8.5 ps is obtained.The possible quantum beat frequencies are also discussed via fs-CARS experiments.The original results provide an insight into ultrafast process of energetic materials.
2,2′,4,4′,6,6′-Hexanitrostillbene,Vibrational mode,Coherent anti-Stokes Raman scattering
I.INTRODUCTION
It is an ultrafast dynamic response of energetic material molecules to a number of diferent ignition processes,such as photons,sparks or shocks[1,2].Yet on the molecular level,it is very little known about how the ignition energy from an impact couples into molecules. Excited states played a crucial role in the energy conversion of energetic materials and have been extensively investigated[2−6].The local heating efect called“hot spot”is previously thought to be induced by fast nonradiative transition of excited states,which also accelerate the chain reactions of energetic materials.Recently, smaller and colder hot spot has been created by longwavelength infrared radiation(28−30 THz),providing direct evidence in violation of“hot spot”via fast nonradiative transition of excited states[7].In addition,the fragmentation of 2,2′,4,4′,6,6′-hexanitrostillbene(HNS, Fig.1)has been observed at irradiation of long wavelength of 1024 nm[8].The phenomena make researchers rethink about how the initiation energy couples into molecules.In this sense,vibrational relaxation of initially excited mode and the fow of vibrational energy into low frequency continuum of energetic materials should be performed in the UV or infrared range[9].It is noted that ultraviolet laser may excite energetic material molecules to excited electronic states,which will decompose on electronic excited potential surface[2].

FIG.1 Geometrical structure of HNS molecule.
In the UV or infrared range,fundamental ultrafast motions including molecular vibrations and rotations characterize the chemical bonding and determine the reactions dynamics[10].The timescales for these motions are typically 100 fs for vibrations and 100 ps for rotations,respectively.Vibrationally hot ground electronic states have been recognized as an important factor in the energy transfer process of photochromic molecules.And the rotational dynamics accessed on S0or excited electronic potential surface may be quenched by intermolecular steric hindrance with the increase of nitro group in energetic materials[11].In diferent kinds of ultrafast pump-probe techniques,sub-ps or fs time-resolved coherent anti-Stokes Raman scattering(CARS)technique is a powerful tool to study highfrequency vibrational modes with high spectral resolution,and it has been widely employed in liquid phase and gas phase materials[10,12−16].The functional NO2group of HNS studied by CARS technique is very signifcant[4].
Nevertheless,due to the nonresonant part of susceptibility,the disadvantage of CARS technique limits de-
In this work,sub-ps and fs-CARS experiments are utilized to observe excitation of vibration and its relaxation process of HNS in solution.The results show thatνs(NO2)at 1385 cm−1has been excited and the relaxation time of 0.38 and 8.5 ps is obtained by subps-CARS.The possible quantum beat frequencies are also discussed via fs-CARS experiments.The original results provide an insight into the ultrafast process of HNS.

FIG.2 Experimental setup of the CARS technique.BS:beam splitter,L:lens.
II.EXPERIMENTS
The schematic diagram of CARS experimental setup [23]is shown in Fig.2 and the measurements are performed at room temperature.The laser pulses are generated from a regenerative amplifer(1 kHz,Spectra-Physics,Spitfre)with center wavelength of 800 nm, single pulse energy of 500µJ,pulse duration of 130 fs, and they are split into three parts.In sub-ps-CARS experiments,the frst two are stretched by a grating, pass through a slit of 3 mm width and then are compressed by another grating,after which,pulse duration of 500 fs and spectral resolution of 3.6 nm(47 cm−1) are obtained.In fs-CARS experiments,no slit or grating is used,where pulse duration of 130 fs and spectral resolution of 10 nm(150 cm−1)are obtained.The two pulses are used as the pump and probe pulses,respectively.The third one is collimated on 1 mm thick H2O to produce a white-light continuum(WLC),which is used as the Stokes pulse.Two delay lines are employed, where the Stokes pulse defnes an arbitrary temporal zero point.In this way,diferent delay time between the pump and Stokes pulse and that between the probe and Stokes pulse can be achieved.A folded Box-CARS confguration with properly chosen angles(less than 2◦) between the beams,determined by the phase matching condition,is used.The CARS signal is collected by a silica fber,dispersed in a spectrometer(Bruker Optics 500 IS/SM)and detected by a CCD detector(Andor DU440-BU2).
The sample is frst subjected to two time-coincident pump and Stokes pulses.If the frequency diference of the two pulses matches the frequency of a specifc vibrational mode,this mode will be excited coherently. The temporal evolution of the coherent vibrations is probed by the third time-delayed probe pulse,giving rise to a CARS signal.The signal intensity is recorded as a function of the time delay between the probe pulse and the simultaneous pump and Stokes pulses.In this work,we use a WLC for the Stokes pulse,which has an ultra-broadband spectral profle ranging from 400 nm to 1100 nm.In order to suppress the excitation of other modes and the generation of the degenerate four-wave mixing process,the WLC is spectrally fltered using a flter to cut of the components of wavelength less than 830 nm.Due to the chirp characteristics of the ultra-broadband WLC,no complicated laser system is required for the wavelength tuning of the Stokes pulse. The pump,Stokes,and probe pulses are focused on the samples to a diameter of about 300µm using a lens with a focal length of 300 mm.The length of the spatial overlap of all the three laser beams is about 10 mm and can cover well the thickness of the samples(1 mm).The single pulse energies at the sample are 1−10µJ for the pump and probe pulses,and 1µJ for the Stokes pulse, respectively.The polarization of the three pulses is set to be parallel to each other.The temporal overlap between the pump and Stokes pulses is properly adjusted to accomplish selective excitation of appointed vibrational modes.
The low-frequency vibration modes ranging up to 1000 cm−1are submerged in a fast intense nonresonant part due to the electronic system.In sub-ps-CARS experiments,the CARS at one time delay are selected for clarifcation,in which only the high frequency vibration modes are shown in the work.The sub-ps resolved spectra are obtained with the typical step of 50 fs and maximum time delay of 10 ps,covering the overall relaxation process.In the fs resolved spectra,a typical step of 25 fs and maximum time delay of 5 ps are adopted.
HNS(with a purity>99.5%)used in the experiments is synthesized at Institute of Chemical Materials, China Academy of Engineering Physics.The synthesis of HNS is a two-step reaction,frst oxidative cou-pling of trinitrotoluene(TNT),and then dehydration and recrystallization.In the experiments,HNS powder is dissolved into CH3CN with the concentration of 2 mmol/L(HNS in(CH3)2SO(DMSO)with concentration of 1 mmol/L,the results are shown in supplementary material).The Raman spectrum of HNS in solid state is shown in Fig.3(a).The geometrical structure and frequency calculations are performed at the B3LYP/6-311G(d,p)level by using Gaussian 09 suite of programs[24],and the calculated Raman spectrum result is shown in Fig.3(b).

FIG.3(a)Experimental and(b)calculated Raman spectra of HNS.

TABLE I Vibrational mode and IR frequency(cm-1)of experimental and calculated HNS as well as CH3CN.
III.RESULTS AND DISCUSSION
The strong peaks at 1342 and 1359 cm−1in Raman spectrum of HNS are consistent with 1371 and 1390 cm−1in calculated Raman spectrum in Table I, respectively.Both of them are assigned toνs(NO2)at diferent positions in HNS molecule,as shown in Fig.1, respectively.The corresponding vibrations are also observed in FTIR spectrum with high intensity[20].But the relatively low concentration of HNS in CH3CN leads to nearly no observable intensity of HNS Raman signal in Fig.S1(supplementary material).By utilization of coherent excitation at wavelength of 800−1100 nm and non-background detection,CARS signal is 104−106times sensitive than that of Raman spectrum and solvent efect is greatly reduced[17].Since CARS is a superior tool for obtaining spectra of certain solutes in solution,observation of HNS in CH3CN by CARS technique is feasible.
In order to illuminate the vibrations of HNS in CH3CN,sub-ps-CARS at time delay of 1 ps are selected for clarifcation and shown in Fig.4.The peak at 1385 cm−1is attributed to signal of HNS.The two peaks of 1342 and 1359 cm−1in Raman spectrum could not be distinguished in CARS at spectral resolution of 47 cm−1.Nevertheless,theδs(CH3)mode of CH3CN at 1374 cm−1in Fig.S1 may infuence the observation of theνs(NO2)of HNS.Thus,we carefully check CH3CN CARS with and without HNS at diferent time delays, identify the infuence is neglectable and safely assume that the peak at 1385 cm−1is from HNS.In addition, a vibration at 1385 cm−1for HNS in DMSO is also observed in Fig.S2(supplementary material),further confrming 1385 cm−1band arises from HNS.The vibrational relaxation is extracted as a function of peak in-tensityvs.time delay,and it lasts for~6 ps in Fig.4(b). The signal shows expected exponential decays for the high density of coherent excited normal modes in the ground state of this system.The diference of the vibration relaxation from response function fulflls the Eq.(1) as follows∶

FIG.4(a)CARS of HNS in CH3CN at time delay of 1 ps and(b)relaxation process ofνs(NO2)as well as ftting curves.A,B,Cshow the ftting curves referring to the three coefcients in Eq.(1).

whereIs(t),A(B,C),T1(T2),∗,andG(t)refer to the dynamic CARS signal,coefcient,relaxation time,convolution,and response function,respectively.The different components of three parts are shown in Fig.4(b). The decay timesT1andT2of 0.38±0.05 and 8.5±1 ps are extracted from the best ft of the curve,respectively.
After initial excitation at 800 nm or longer wavelength,HNS molecule populates a virtual state or vibrationally excited state.Such state may be unobservable.By the coherent pump and Stokes pulses with frequency diference matches,a high frequency vibrationalνs(NO2)mode at 1385 cm−1could be excited and observed.This vibration corresponds to the symmetric vibration of crucial NO2group in energetic materials. The vibration can provide energy to the molecule,break bonds by overcoming a reaction coordinate barrier,and lead to diferent chemistry(e.g.NO2release).This point is in good agreement with slow heating efect on energetic materials.It is noted that the initially vibrational excitation decays through pathways that involve the excitation of one or more vibrations of the solute or solvent,and one or more excitations of the low frequency continuum of the medium[9,25].The continuum is composed of the instantaneous normal modes in a liquid.While in a mixed crystal sample,the low frequency continuum is composed of the well-defned acoustic and optical phonons of the crystal.The fragmentation of HNS at irradiation of 1024 nm may result from this particular energy transfer from initially excited vibration to low frequency phonons in solid sample[8].The smaller and colder hot spot created by long-wavelength infrared radiation may also provide evidence for this explanation[7].In this sense,the insight into vibrational excitation will provide important information for a better understanding of thermal,shockinduced and photon-induced chemistry of energetic materials[26].
The vibrational relaxation pathways of high excited C≡N stretching modes have been studied by ultrafast back-electron transfer[27].The time scale of the frst excited vibrational state is typically 0.8 ps for intramolecular vibrational redistribution(IVR)and 20 ps for intermolecular vibrational energy relaxation(VER), respectively.The latter is close to the vibrational cooling dynamics of azobenzene in vibrationally hot ground electronic state which has been determined to be on a time scale of ca.20 ps via time-resolved IR spectroscopy with femtosecond time resolution[28].In this paper,we obtain relaxation times of 0.38 and 8.5 ps,

FIG.5(a)Time evolution of wavenumber 1350 cm-1and (b)Fourier transform of frequency slices at 1350 cm-1of HNS in CH3CN.
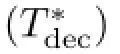

It is difcult to distinguish the contribution of the two processes.Terfor IVR process ofνs(NO2)is longer than 0.38 ps,in agreement with IVR rate of 0.6 ps forβ-carotene[29]and 0.8 ps for C≡N stretching modes [27].The solute-solvent interaction has little efect on IVR process[29].The timescale of 8.5 ps for VER process ofνs(NO2)is much shorter than 20 ps for C≡N stretching modes[27]and azobenzene[28].It is uncertain that such process may be accelerated by strong steric hindrance of HNS.Further efort should be made to more explicit and accurate investigation on low or high excited vibrational states of energetic materials.
In fs-CARS experiments,diferent vibrational modes could be excited simultaneously at one pulse wavelength because of large spectral bandwidth of fs laser pulses. The quantum beat phenomena of two excited vibrations may be observed in the experiments.We analyze the fs-CARS signals at wavenumber of 1350 cm−1starting from time delay of 500 fs in Fig.5,to avoid intense nonresonant contribution.These beats frequencies aredetermined by Fourier transform,following subtraction of the exponentially decaying backbond associated with thiswCARSsignal.In comparison with the known Raman active frequency of the ground state HNS,it is straightforward using the dispersed time resolved CARS to assign all of the observed beat frequencies to pairs of known vibrational modes.Two major beat frequencies at 29 and 52 cm−1are evident from this spectrally integrated signal.Four Raman active modes are known to be present in this region with frequencies of 1294,1342, 1359 and 1530 cm−1,thus 29 cm−1beat is assigned to coherence of 1342 and 1359 cm−1.The 52 cm−1beat is readily assigned to coherence of 1342 and 1294 cm−1vibrational mode.
IV.CONCLUSION
In this work,sub-ps and fs CARS experiments have been performed to observe the excitation of vibrational mode and its relaxation process of HNS in solution. Sub-ps CARS experiments have better spectral resolution and we directly observe the excitation of vibrational modeνs(NO2)at 1385 cm−1by this method. By changing the time delay between pump and probe beams,two relaxation times of 0.38 and 8.5 ps are obtained for the vibrational mode.The relaxation times are related with the intramolecular vibrational redistribution and intermolecular vibrational energy relaxation,respectively.The results are favorable with the fragmentation of HNS at irradiation of 1024 nm and colder hot spot created by long-wavelength infrared radiation.In fs CARS experiments,diferent vibrational modes could be excited simultaneously at one pulse wavelength.We observe the oscillation compound by changing pump-probe time delay,which could be attributed to beat frequencies.Two major beat frequencies at 29 and 52 cm−1are assigned to coherence of 1342−1359 cm−1and 1342−1294 cm−1vibrational mode,respectively.The original results provide an insight to the ultrafast response of HNS and a better understanding of thermal,shock-induced and photoninduced chemistry of energetic materials.
Supplementary material:Raman spectra of HNS in CH3CN,sub-ps CARS of HNS in DMSO,and fs-CARS of CH3CN are shown.
V.ACKNOWLEDGMENTS
This work is supported by the Science Foundation of China Academy of Engineering Physics (No.2011A0302013).
[1]S.Odiot,J.Phys.Colloq.48,225(1987).
[2]C.Rajchenbach,G.Jonusauskas,and C.Rulliere,J. Phys.IV5,365(1995).
[3]A.Bhattacharya and E.R.Bernstein,J.Phys.Chem. A115,4135(2011).
[4]A.Bhattacharya,Y.Guo,and E.R.Bernstein,J. Chem.Phys.136,024321(2012).
[5]Z.Yu and E.R.Bernstein,J.Chem.Phys.137,114303 (2012).
[6]Z.Yu and E.R.Bernstein,J.Phys.Chem.A117,1756 (2013).
[7]M.W.Chen,S.You,K.S.Suslick,and D.D.Dlott, Appl.Phys.Lett.104,061907(2014).
[8]Y.Sun,T.Xu,Y.J.Shu,X.Wang,Z.Q.Zhao,and Y. Q.Gu,Asian J.Chem.25,4247(2013).
[9]V.M.Kenkre,A.Tokmakof,and M.D.Fayer,J.Chem. Phys.101,10618(1994).
[10]T.C.W.Kiefer,M.Heid,A.Materny,M.Schmitt,T. Siebert,and A.Vierheilig,Proc.SPIE 4749(2002).
[11]N.Tamai and H.Miyasaka,Chem.Rev.100,1875 (2000).
[12]M.Schmitt,G.Knopp,A.Materny,and W.Kiefer, Chem.Phys.Lett.270,9(1997).
[13]O.Rubner,M.Schmitt,G.Knopp,A.Materny,W. Kiefer,and V.Engel,J.Phys.Chem.A102,9734 (1998).
[14]M.Schmitt,G.Knopp,A.Materny,and W.Kiefer,J. Phys.Chem.A102,4059(1998).
[15]C.L.Evans and X.S.Xie,Annu.Rev.Anal.Chem.1, 883(2008).
[16]A.Materny,J.Konradi,V.Namboodiri,M.Namboodiri,A.Scaria,V.K.Vaidyan,and V.S.Jayakumar, AIP Conf.Proc.43(2008).
[17]W.M.Tolles,J.R.Mcdonald,and A.B.Harvey,Appl. Spectro.31,253(1977).
[18]A.A.Zenin and S.V.Finjakov,Combustion,Explosion, and Shock Waves45,559(2009).
[19]J.S.Lee,C.K.Hsu,and C.L.Chang,Thermochimica Acta392,173(2002).
[20]G.d.Silva,K.Iha,A.M.Cardoso,E.C.Mattos,and R. d.C.L.Dutra,J.Aero.Techno.Manag.2,41(2010).
[21]B.C.Pein,Y.Sun,and D.D.Dlott,J.Phys.Chem.A117,6066(2013).
[22]G.B.Chu,Y.Xiong,J.Yi,K.M.Cheng,T.Xu, J.T.Xin,and Y.Q.Gu,RSC Advances(in press), DOI:10.1039/c4ra08305a.
[23]X.Du,M.Zhang,Q.Meng,Y.Song,X.He,Y.Yang, and J.Han,Opt.Exp.18,22937(2010).
[24]H.Okamoto and K.Yoshihara,Chem.Phys.Lett.177, 568(1991).
[25]H.Fujisaki and J.E.Straub,Proc.Natl.Acad.Sci.102,6726(2005).
[26]I.V.Adamovich,S.O.Marcheret,J.W.Rich,and C. E.Treanor,AIAA J.33,1064(1995).
[27]M.S.Lynch,K.M.Slenkamp,and M.Khalil,J.Chem. Phys.136,241101(2012).
[28]P.Hamm,S.M.Ohline,and W.Zinth,J.Chem.Phys.106,519(1997).
[29]K.Y.Hiromi Okamoto,Chem.Phys.Lett.177,568 (1991).
10.1063/1674-0068/28/cjcp1409153tection~1%for aqueous solution or opaque material [17].It is noted that the energetic material HNS is hardly dissolved into solution and its crystal is light yellow,which limits the utilization of CARS technique for trace HNS or other explosive study.The evolution of vibrational characteristics of liquid energetic materials such as CH3NO2,C6H5NO2etc.have been observed[2,18−21].The evolution of excited state of HNS has also been observed by the ultrafast absorption experiments[22].The thermal decomposition of HNS has been analyzed by Fourier transform infrared spectroscopy(FTIR)and diferential scanning calorimetry (DSC)[20].The continuing efort will be performed on HNS with sub-ps and fs-CARS technique.
∗Authors to whom correspondence should be addressed.E-mail:xuzhtao@126.com,yqgu@caep.ac.cn
(Dated:Received on September 14,2014;Accepted on October 23,2014)
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- High Performance of Enhanced Mode Field Efect Transistor and Ultraviolet Sensor Based on ZnO Nanosheet
- Quantitative Moisture Measurement with a Cavity Ring-down Spectrometer using Telecom Diode Lasers
- Accurate Measurement of Raman Depolarization Ratio in Gaseous CO2
- Methanol Adsorption on TiO2Film Studied by Sum Frequency Generation Vibrational Spectroscopy
- Ultraviolet Source Assisted Enhancement of Attosecond Pulse
- Decay Dynamics ofN,N-Dimethylthioacetamide in S3(ππ∗)State
