Antimicrobial Expanded Polytetrafuoroethylene Film Prepared byγ-ray Radiation Induced Grafting of Poly(acrylic acid)
2015-01-13YunlongWngMozhenWngQichoWuXioZhouXuewuGe
Yun-long WngMo-zhen WngQi-cho WuXio ZhouXue-wu Ge
a.CAS Key Laboratory of Soft Matter Chemistry,Department of Polymer Science and Engineering, University of Science and Technology of China,Hefei 230026,China
b.Guangdong Tianan New Material Co.,Ltd.,Foshan 528000,China
Antimicrobial Expanded Polytetrafuoroethylene Film Prepared byγ-ray Radiation Induced Grafting of Poly(acrylic acid)
Yun-long Wanga,Mo-zhen Wanga∗,Qi-chao Wub,Xiao Zhoub,Xue-wu Gea
a.CAS Key Laboratory of Soft Matter Chemistry,Department of Polymer Science and Engineering, University of Science and Technology of China,Hefei 230026,China
b.Guangdong Tianan New Material Co.,Ltd.,Foshan 528000,China
The simultaneousγ-ray-radiation-induced grafting polymerization of acrylic acid on expanded polytetrafuoroethylene(ePTFE)flm was investigated.It was found that the degree of grafting(DG)of poly(acrylic acid)(PAA)can be controlled by the monomer concentration, absorbed dose,and dose rate under an optimal inhibitor concentration of[Fe2+]=18 mmol/L. SEM observation showed that the macroporous structure in ePTFE flms would be covered gradually with the increase of the DG of PAA.The prepared ePTFE-g-PAA flm was immersed in a neutral silver nitrate solution to fabricate an ePTFE-g-PAA/Ag hybrid flm after the addition of NaBH4as a reduction agent of Ag+to Ag atom.SEM,XRD,and XPS results proved that Ag nanoparticles with a size of several tens of nanometers to 100 nanometers werein situimmobilized on ePTFE flm.The loading capacity of Ag nanoparticles could be tuned by the DG of PAA,and determined by thermal gravimetric analysis.The quantitative antibacterial activity of the obtained ePTFE-g-PAA/Ag hybrid flms was measured using counting plate method.It can kill all theEscherichia coliin the suspension in 1 h. Moreover,this excellent antibacterial activity can last at least for 4 h.This work provides a facile and practical way to make ePTFE meet the demanding antimicrobial requirement in more and more practical application areas.
Expanded polytetrafuoroethylene flm,Radiation grafting,Poly(acrylic acid), Silver nanoparticles,Antibacterial property
I.INTRODUCTION
The expanded polytetrafuoroethylene(ePTFE)flms are obtained by rapidly stretching the polytetrafuoroethylene(PTFE)flm in one more directions at 327◦C,then quenching to room temperature to fx the macroporous structure formed during the stretching[1]. ePTFE flms are widely used in many areas,not only as water-proof and wind-proof cloth materials[2],fltration flms[3],and advanced dielectric materials[4, 5],but also as synthetic blood vessels[6],patches for soft tissue regeneration[7]and surgical sutures[8]for its low toxicity and perfect stability[9,10].In spite of these excellent properties,ePTFE still has some problems in practice use.For example,in the application of clothing and water fltration,the antimicrobial property of ePTFE flms is urgently needed for the high demand of water quality and human health care[11].Silver nanoparticles have been proven to have excellent antimicrobial activity so as to be loaded in many kinds of materials as antibacterial agents[12−14].Commonly, silver ions are frst absorbed in the matrix,and thenin situreduced and aggregated into silver nanoparticles [15].However,it is impossible for the direct absorption of silver ions or silver nanoparticles on ePTFE flms for its extreme hydrophobic property.Therefore,modifcations on ePTFE flms should be studied to improve the hydrophilicity so that antimicrobial agents,such as silver nanoparticles,can be loaded in ePTFE flm to meet the needs in practical applications.
γ-Ray induced graft copolymerization has been widely applied to surface modifcation of various polymer materials[16−18].In our previous work [19],poly(acrylic acid)(PAA)was grafted onto poly(ethylene terephthalate)flms,and silver nanoparticles were loaded onto the grafted flm successfully.The prepared hybrid flm performed excellent antibacterial property againstEscherichia coli(E.coli).The modifcation of PTFE was generally carried out by chemical etching[20],electron and ion beams irradiation[21,22], and plasma modifcation[23].However,few work refers to the application ofγ-ray radiation on the modifcation of PTFE in literatures because PTFE has been believed to be a typical radiation-degradable polymer,although Tabata group confrmed the radiation induced crosslin-
In this work,we frst studied the conditions for the graft copolymerization of acrylic acid(AA)on ePTFE flm under the radiation ofγ-ray.Then,Ag nanoparticles werein situloaded in the grafted PTFE flm.Further,the bactericidal activity of the ePTFE-g-PAA/Ag hybrid flm was evaluated by the efciency of killingE.coli.The antibacterial efcacy(ABE)of the hybrid flm can reach as high as 100%,which meets the antibacterial requirement for the practical use of ePTFE flms.
II.EXPERIMENTS
A.Materials
ePTFE flms were provided by Xinxiang Xinxingfenghua Membrane Co.,Ltd.(Henan,China). Chemical-grade acrylic acid(Sinopharm Chemical Reagents Co.,Ltd.)was purifed by vacuum distillation and stored at−20◦C before use.Analytical grade reagents including ferrous sulfate hydrate (FeSO4·7H2O),sliver nitrate(AgNO3),sodium borohydride(NaBH4),and aqueous ammonia(NH3·H2O, 25%−28%)were all purchased from Sinopharm Chemical Reagents Co.,China,and used without further purifcation.Distilled water was used in all the experiments.Mueller-Hinton(MH)Medium was purchased from Xiya Reagent(Chengdu,China).E.coliDH5αwas used in the antibacterial tests.
B.Preparation of ePTFE-g-PAA flm byγ-ray radiation induced grafting polymerization
ePTFE flm with a size of 3 cm×3 cm was washed with acetone,and then dried in a vacuum oven overnight at 50◦C.The dried flm was immersed in an aqueous solution of AA containing a certain amount of FeSO4.Then the system was exposed to the radiation of60Coγ-ray(55 kCi,located in University of Science and Technology)at a dose rate of 10 Gy/min for a certain time after being bubbled with nitrogen for 10 min at a speed of three bubbles per second to expel the dissolved oxygen.The grafted flms were all washed with water in a Soxhlet extractor for 24 h,to remove homopolymer and unreacted monomers after the grafting polymerization.
C.In situloading Ag nanoparticles on the ePTFE-g-PAA flm
The prepared ePTFE-g-PAA flm was immersed into a mixture of 100 mL of water and 0.5 mL of NH3·H2O. The system was ultrasonicated for 5 min and continuously agitated at room temperature on a shaking bed (Jingda,WHY2)for another 2 h.Then the flm was taken out and washed with water for several times till the pH value of the washing water reached 7.0.Later, the ePTFE-g-PAA flm was placed in a beaker containing 20 mL of distilled water.An aqueous solution of AgNO3(0.5 mol/L,10 mL)was added under magnetic stirring.The system was agitated at room temperature on a shaking bed for 2 h to reach the absorption equilibrium of Ag+ions on the ePTFE-g-PAA flm. The aqueous solution of NaBH4(0.1 mol/L,10 mL) was then added into the solution under magnetic stirring.The reaction was carried out for 3 h on a bed shaker(200 r/min).Then,the flm was cleaned with water ultrasonically for half an hour for 3 times,to remove the residual reduction agent.Finally,the obtained ePTFE-g-PAA/Ag flm was dried in a vacuum oven overnight at 50◦C.
D.Characterization
The degree of grafting(DG)of PAA on the ePTFE flm is defned by the following Eq.(1)∶

wherem0andmgare the weight of the original ePTFE flm and the grafted flm respectively.The morphologies of the flms were observed with SEM(JEOL JSM-6700F,5.0 kV).X-ray difraction(XRD)was recorded on MXP 18 AHF X-ray difractometer(MAC Science Co.,Ltd)with monochromatized Cu Kαradiation(λ=1.54056˚A).X-ray photoelectron spectroscopy (XPS)was recorded with a VG ESCALAB MKII XPS, using a Mg Kαline(1486.6 eV)as the excitation source under high vacuum(5 nPa).Thermal gravimetric analysis(TGA)was carried out under a nitrogen atmosphere at a rate of 10◦C/min with a Shimadzu DTG-60H thermal analyzer.
E.Antibacterial activity tests
E.coliwas cultivated in MH medium at 37◦C.All glassware,samples and medium used were sterilized in an autoclave at 121◦C for 20 min before experiment. The bacteria were centrifugally separated from the liquid medium at a speed of 3000 r/min for 10 min.Then the collected cells were washed twice with a sterile phosphate bufer solution(PBS),and redispersed in PBS at a concentration of 107cells/mL.One piece of flm with a size of 1 cm×1 cm was immersed into the dispersion and kept at 37◦C on a bed shaker at a speed of 200 r/min. After 1 and 4 h,1 mL of bacterial culture was sampled respectively,and diluted ten times with PBS serially. Then 0.1 mL of the diluted sample was spread on MHagar plates.After incubation of the plates at 37◦C for 12 h,the number of colony-forming units(CFU)was counted manually.The mean CFU per milliliter was expressed as the result of multiplying the counted CFU by the dilution factor.ABE of the specimen was calculated according to
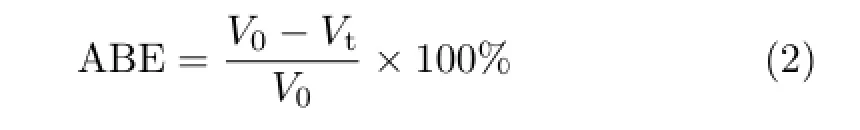
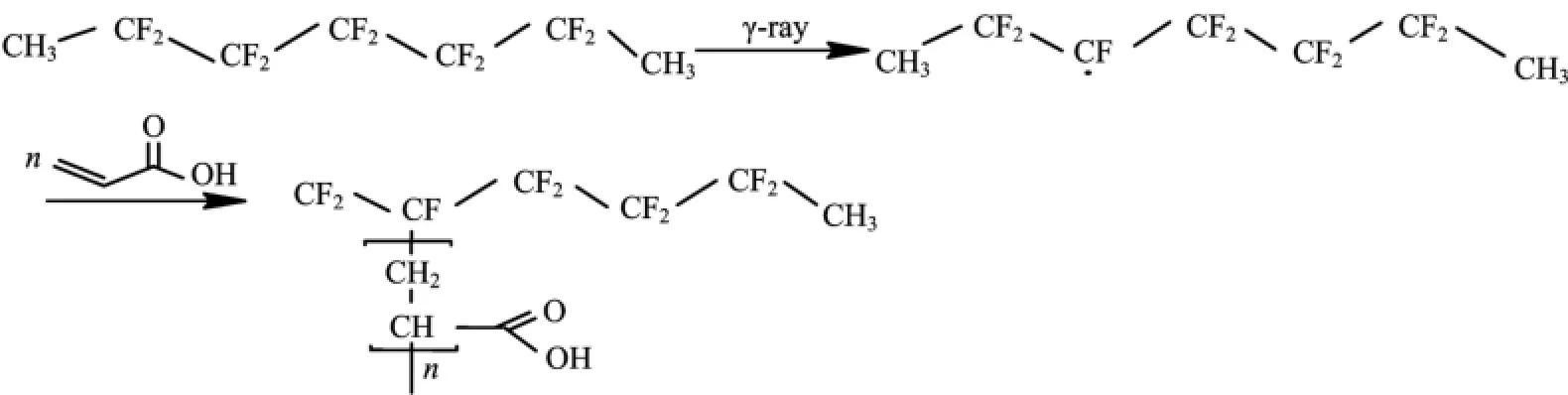
Scheme 1 The main mechanism of simultaneousγ-ray radiation induced grafting of PAA on PTFE.
whereV0is the number of CFU in pure PBS bufer solution,andVtis the number of CFU in the test solution.
III.RESULTS AND DISCUSSION
A.γ-Ray radiation induced graft polymerization of AA on ePTFE flm
The main mechanism of simultaneousγ-ray-induced grafting of PAA on ePTFE flm are illustrated in Scheme 1 according to previous electron spin resonance studies on free radicals trapped in PTFE irradiated byγ-ray at low temperature or room temperature[26].
Considering the balance of homopolymerization and grafting polymerization of AA monomers and the radiation-induced degradation of PTFE,we frst tried the grafting polymerization of AA at a low dose rate (10.4 Gy/min)and absorbed dose(10 kGy),a moderate monomer concentration(15.6wt%),and diferent inhibitor concentrations([Fe2+]).The results are listed in Table I(run 1−5).When[Fe2+]is lower than 1.8 mmol/L,AA monomers will mainly homopolymerize into a gel,rather than graft polymerize on PTFE flm.Once[Fe2+]exceeds 1.8 mmol/L,the homopolymerization of AA could be efectively suppressed.The DG of PAA on ePTFE flm starts to increase with[Fe2+] until[Fe2+]reaches 18.0 mmol/L.At this point,the DG of PAA is up to 11.8%.However,it should be noted that Fe2+will not only deactivate homopolymerization but also restraint propagation of the grafting chains.Thus, when[Fe2+]is over 18.0 mmol/L,the DG of PAA decreases rapidly to about 5.0%.Evidently,there is an optimal inhibitor concentration for the grafting of PAA on ePTFE flm.So,[Fe2+]is fxed to 18.0 mmol/L during all the following research in this work.

TABLE I The DG of PAA on ePTFE flms under diferent irradiation conditions.Absorbed dose rate in Gy/min, absorbed dose in kGy,[Fe2+]in mmol/L,AA in wt%.
The efect of absorbed dose on the morphological changes of the ePTFE flms are displayed in Fig 1.The original ePTFE flm(Fig.1(a))shows a macroporous structure constructed by interlaced PTFE fbers.When the absorbed dose is lower than 10 kGy,some deposits can be seen in Fig.1(b).With the continuous increase of the absorbed dose,the deposits on the fbers become more and more,and gradually block in Fig.1(c)and(d). Finally,the deposits fll up all the pores in the flm when the absorbed dose is as high as 20 kGy.This morphological change is in accordance with the corresponding DG of PAA that grows with the absorbed dose,as listed in Table I(run 3 and 7−9),indicating that the deposits should be the grafted PAA.The FTIR spectra of the ePTFE-g-PAA flm with a DG of 11.8%(run 3)and 20.3%(run 9)are shown in Fig.2(b),with a comparison with that of the original ePTFE flm in Fig.2(a). Besides the characteristic absorption peaks of PTFE,i.e.,502 cm−1(CF2wagging),555 cm−1(CF2defor-mation),636 cm−1(CF2rocking),1155 cm−1(νasC−F), and 1215 cm−1(νsC−F)[27],new absorption peaks contributed by AA structural units at 1719 cm−1(νC=O),~2900 cm−1(νC−H),and 1457 cm−1(δC−H)appear. The intensities of these bands also become stronger as the DG of PAA increases.All the above results verify that PAA can be successfully grafted on ePTFE flm initiated byγ-ray irradiation at the appropriate conditions.
The DG of PAA also depends on the dose rate under a constant absorbed dose.As listed in Table I(run 3 and 10−12),the DG of PAA decreases rapidly from 21%to 2.5%when the dose rate increases from 5.6 Gy/min to 41.6 Gy/min.This phenomenon was caused by two reasons.First,the radical concentration will increase with the dose rate so that the possibility of radical termination rises.Secondly,the high degree of gelation in the system at high dose rate results in a high viscosity of the grafting system,which makes the difusion of monomers difcult so as to inhibit the grafting reactions.
It is also found that excessive monomer concentration may not be good for the grafting of PAA,as shown in Table I(run 3 and 13−18).The DG of PAA commonly increases with AA concentration.However,the gelation induced by the crosslinking of the PAA chains in the solution also becomes more and more serious with the increase of AA concentration.When the AA concentration rises up to 41.6%,the whole system becomes a gel and the ePTFE flm even cannot be separated.
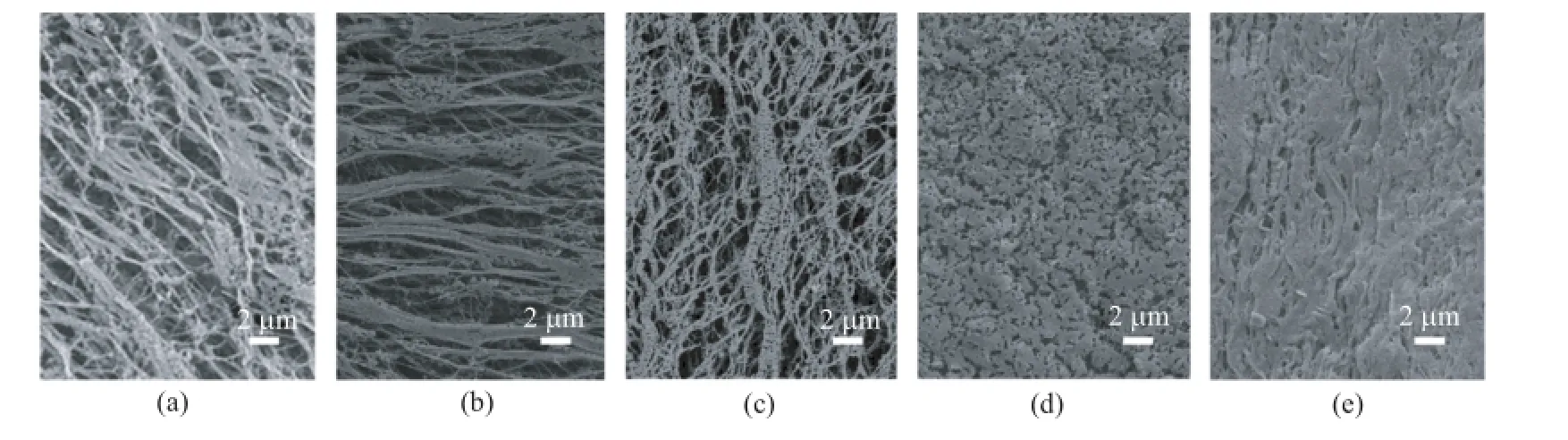
FIG.1 SEM images of the ePTFE flm after irradiated byγ-ray at diferent absorbed dose(a)0 kGy,(b)5 kGy(run 7), (c)10 kGy(run 3),(d)15 kGy(run 8),and(e)20 kGy(run 9).
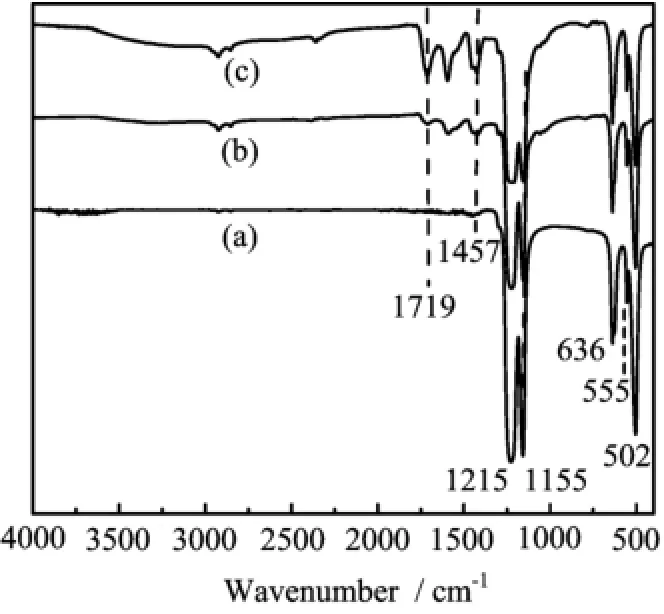
FIG.2 FTIR spectra of(a)original ePTFE flm, ePTFE-g-PAA flm with a DG of(b)11.8%(run 3),and (c)20.3%(run 9).
B.Loading silver nanoparticles onto ePTFE-g-PAA flms
Compared with the raw ePTFE flm,ePTFE-g-PAA flm has an improved hydrophilicity due to the grafted hydrophilic carboxyl groups.The aqueous solution of AgNO3can permeate into the ePTFE-g-PAA flm. When pH equals to 7.0,Ag+ions can be adsorbed on the grafted PAA chains through strong electrostatic interaction between Ag+and COO−ions.At the time, the absorbed Ag+ions can be reduced into silver atom by the addition of NaBH4.The produced silver atoms furtherin situaggregate into silver nanoparticles to form ePTFE-g-PAA/Ag hybrid flm.Since silver ions are immobilized by COO−ions,the as-prepared silver nanoparticles will also be fxed and distributed along the PAA chains so that the loading capacity of silver nanoparticles can be tunable by the DG of PAA.Figure 3(a)is a typical surface morphology of the prepared ePTFE-g-PAA/Ag hybrid flm.It can be clearly seen that particles with a size of several tens of nanometers to 100 nanometer are absorbed on the flm.Figure 3(b)shows the XRD pattern of the corresponding hybrid flm in Fig.3(a).Three peaks located at 2θvalues of 38.26◦,44.34◦,and 64.57◦correspond to(111),(200),and(220)refection of fcc structure of metallic silver respectively[28].This demonstrates that the produced nanoparticles are the crystals of silver 3c (JCPDS card No.04-0783).The full width at half maximum of the peak at 2θof 38.26◦is 0.561.So according to Scherer equation[29],the size of the crystalline silver nanoparticles can be calculated as 15 nm,which is much less than the observed particle size in Fig.3(a).This result indicates that the loaded Ag nanoparticles are the aggregates,rather than individual nano-Ag grain.
The chemical state of the silver nanoparticles loaded on the ePTFE-g-PAA flm can also be verifed by XPS analysis,as shown in Fig.4(a).The peaks of 368.3 eV (3d5/2)and 374.1 eV(3d3/2)indicate that the metallicstate silver(Ag0)atoms are formed.The loading capacity of Ag nanoparticles can be determined by TGA analysis,as shown in Fig.4(b).Two distinct weight losses corresponding to decomposition of PAA and PTFE could be detected at 240−450 and 470−650◦C,respectively.There is a 14.4%of residual weight fraction at above 650◦C,which corresponds to the load capacity of silver nanoparticles.Meanwhile,the 9.2%of weight loss of PAA in the hybrid flm equals to a 10.8%of PAA in the pure ePTFE-g-PAA flm,which is very close to the DG measured by the weighing method,i.e.11.8%.

FIG.3(a)SEM image and(b)XRD spectrum of ePTFE-g-PAA/Ag hybrid flm prepared from run 9 in Table I.The inset in(a)is the SEM image with high magnifcation.
C.Antibacterial activity of ePTFE-g-PAA/Ag hybrid flms
The quantitative antibacterial activity of the prepared ePTFE-g-PAA/Ag hybrid flms was measured using counting plate method.The numbers of viableE.colicells in the suspensions in contact with the different sample flms are listed in Table II,as well as shown in Fig.5.For the original ePTFE flm,the ABE is only 45.3%after 4 h.Grafting of PAA on ePTFE flm could make a little improvement on the flm,i.e, the ABE rises to 63.6%.However,ePTFE-g-PAA/Ag hybrid flms show very efective antibacterial activity againstE.colino matter what the DG of PAA is. Both of them can kill all the bacteria in the suspension in 1 h,i.e.,the ABE is 100%.Moreover,this excellent antibacterial activity can last at least for 4 h.

FIG.4(a)XPS spectrum and(b)TGA curve of ePTFE-g-PAA/Ag hybrid flm prepared from run 3.

TABLE II Antibacterial efect of ePTFE,ePTFE-g-PAA, and ePTFE-g-PAA/Ag hybrid flms againstE.coli.
IV.CONCLUSION
In this work,the grafting polymerization of AA has been successfully conducted on ePTFE flm using simultaneous γ-ray-radiation-induced grafting method under the appropriate conditions including an optimal inhibitor concentration[Fe2+],low dose rate,low absorbed dose,and moderate monomer concentration. The DG of PAA can be controlled by the monomer concentration,absorbed dose,and dose rate.The pores in ePTFE flms could be flled with the increase of theDG of PAA.SEM,XRD,and XPS results prove that the grafted PAA chains can immobilize silver ions in a neutral silver nitrate solution,and furtherin situfx the produced Ag nanoparticles on ePTFE flm in the presence of reduction agent,NaBH4.The loading capacity of Ag nanoparticles could be tuned by the DG of PAA,and determined by TGA.The prepared ePTFE-g-PAA/Ag hybrid flm exhibits excellent and lasting antibacterial activity.It can kill all theE.coliin the suspension in one hour,i.e.,the ABE is 100%.Moreover,this excellent antibacterial activity can last at least for 4 h.This technique provides a facile strategy for the preparation of efective antibacterial ePTFE flm,which has great signifcance on expanding the practical applications of ePTFE in the feld of antibactericidal and biomedical materials.

FIG.5 The antibacterial efect againstE.coliafter 4 h of(a)original ePTFE flm,(b)ePTFE-g-PAA flm with a DG of 20.3%,(c)ePTFE-g-PAA/Ag hydrid flm with a DG of 11.8%,(d)ePTFE-g-PAA/Ag hydrid flm with a DG of 20.3%.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.51103143,No.51173175, and No.51473152),the Fundamental Research Funds for the Central Universities(No.WK2060200012),and the Foshan Scientifc and Technological Innovation Team Project(No.2013IT100041).
[1]R.W.Gore,US Patent US3953566,(1976).
[2]A.Mukhopadhyay and V.K.Midha,J.Ind.Text.37, 225(2008).
[3]B.D.Mccloskey,H.B.Park,H.Ju,B.W.Rowe,D.J. Miller,and B.D.Freeman,J.Membr.Sci.413/414, 82(2012).
[4]S.Zhukov,S.Fedosov,and H.von Seggern,J.Phys.D44,105501(2011).
[5]H.von Seggern,S.Zhukov,and S.N.Fedosov,IEEE T.Dielect.El.In.17,1056(2010).
[6]G.D.Vaughan,K.L.Mattox D.V.Feliciano,A.C.Jr. Beall,and M.E.Debakey,J.Trauma.19,403(1979).
[7]S.A.Jovanovic,H.Spiekermann,and E.J.Richter,Int. J.Oral.Max.Impl.7,233(1991).
[8]A.Chandler-Temple,E.Wentrup-Byrne,A.K.Whittaker,and L.Grøndahl,J.Appl.Polym.Sci.117,3331 (2010).
[9]C.Lassus,Aesthetic.Plast.Surg.15,167(1991).
[10]R.Bleichrodt,R.Simmermacher,B.van der Lei,and J.Schakenraad,Surg.Gynecol.Obstet.176,18(1993).
[11]N.Aumsuwan,S.Heinhorst,and M.W.Urban, Biomacromolecules8,713(2007).
[12]H.Koga,T.Kitaoka,and H.Wariishi,J.Mater.Chem.19,2135(2009).
[13]P.Bober,J.Liu,K.S.Mikkonen,P.Ihalainen,M.Pesonen,C.Plumed-Ferrer,A.von Wright,T.Lindfors,C. L.Xu,and R.M.Latonen,Biomacromolecules15,3655 (2014).
[14]L.Y.Shen,B.L.Wang,J.L.Wang,J.H.Fu,C.Picart, and J.Ji,ACS Appl.Mater.Inter.4,4476(2012).
[15]U.Konwar,N.Karak,and M.Mandal,Prog.Org.Coat.68,265(2010).
[16]A.Bhattacharya,Prog.Polym.Sci.25,371(2000).
[17]R.L.Clough,Nucl.Instrum.Meth.B185,8(2001).
[18]B.Gupta,S.Mishra,and S.Saxena,Radiat.Phys. Chem.77,553(2008).
[19]X.Ping,M.Z.Wang,and X.W.Ge,Radiat.Phys. Chem.80,567(2011).
[20]S.R.Kim,J.Appl.Polym.Sci.77,1913(2000).
[21]K.Lunkwitz,U.Lappan,and D.Lehmann,Radiat. Phys.Chem.57,373(2000).
[22]S.K.Koh,S.C.Park,S.R.Kim,W.K.Choi,H.J. Jung,and K.D.Pae,J.Appl.Polym.Sci.64,1913 (1997).
[23]S.R.Kim,J.Appl.Polym.Sci.77,1913(2000).
[24]Y.Yamada,T.Yamada,S.Tasaka,and N.Inagaki, Macromolecules29,4331(1996).
[25]Y.Tabata,SolidStateReactioninRadiation Chemistry,Taniguchi Conference,Sapporo,Japan, (1992).
[26]Y.Tabata,A.Oshima,T.Kazunobu,and T.Seguchi, Radiat.Phys.Chem.48,563(1996).
[27]M.Kobayashi,M.Sakashita,T.Adachi,and M. Kobayashi,Macromolecules28,316(1995).
[28]L.Z.Jiang,Z.P.Wu,D.Z.Wu,W.T.Yang,and R. G.Jin,Nanotechnology18,185603(2007).
[29]G.D.Preston,Acta.Crystallogr.10,389(1957).
10.1063/1674-0068/28/cjcp1410180king of PTFE under a special irradiation condition,i.e., irradiation around 613 K in inert gas atmosphere or under vacuum[24,25].
∗Author to whom correspondence should be addressed.E-mail:pstwmz@ustc.edu.cn,Tel.:+86-551-63600843
(Dated:Received on October 9,2014;Accepted on November 25,2014)
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- High Performance of Enhanced Mode Field Efect Transistor and Ultraviolet Sensor Based on ZnO Nanosheet
- Quantitative Moisture Measurement with a Cavity Ring-down Spectrometer using Telecom Diode Lasers
- Accurate Measurement of Raman Depolarization Ratio in Gaseous CO2
- Methanol Adsorption on TiO2Film Studied by Sum Frequency Generation Vibrational Spectroscopy
- Ultraviolet Source Assisted Enhancement of Attosecond Pulse
- Decay Dynamics ofN,N-Dimethylthioacetamide in S3(ππ∗)State
