Infuence of Triarylamine and Indoline as Donor on Photovoltaic Performance of Dye-Sensitized Solar Cells Employing Cobalt Redox Shuttle
2015-01-13YueZhngZhihuiWngYujieHoQunpingWuMoLingSongXue
Yue ZhngZhi-hui WngYu-jie HoQun-ping WuMo LingSong Xue
a.Tianjin Key Laboratory of Control Theory&Applications in Complicated Systems,Tianjin University of Technology,Tianjin 300384,China
b.School of Chemistry&Chemical Engineering,Tianjin University of Technology,Tianjin 300384, China
Infuence of Triarylamine and Indoline as Donor on Photovoltaic Performance of Dye-Sensitized Solar Cells Employing Cobalt Redox Shuttle
Yue Zhanga,Zhi-hui Wangb,Yu-jie Haob,Quan-ping Wua,Mao Liangb,Song Xueb∗
a.Tianjin Key Laboratory of Control Theory&Applications in Complicated Systems,Tianjin University of Technology,Tianjin 300384,China
b.School of Chemistry&Chemical Engineering,Tianjin University of Technology,Tianjin 300384, China
Two organic dyesXS51andXS52derivated from triarylamine and indoline are synthesized for dye-sensitized solar cells(DSCs)employing cobalt and iodine redox shuttles.The efects of dye structure upon the photophysical,electro-chemical characteristics and cell performance are investigated.XS51with four hexyloxyl groups on triarylamine performs better steric hindrance and an improvement of photovoltage.XS52provides higher short-circuit photocurrent density due to the strong electron-donating capability of indoline unit.The results from the redox electrolyte on cell performances indicate that the synthesized dyes are more suitable for tris(1,10-phenanthroline)cobalt(II/III)redox couple than I−/I3−redox couple in assembling DSCs.Application ofXS52in the cobalt electrolyte yields a DSC with an overall power conversion efciency of 6.58%under AM 1.5(100 mW/cm2)irradiation.
Dye-sensitized solar cells,Indoline,Triarylamine,Photovoltaic,Cobalt redox shuttle
Recently,we have demonstrated that functionalizedindoline-based organic dyes possess high extinction coefcients apart from their impressive short-circuit photocurrent density(Jsc),beneft from the powerful electron-donating capability of the indoline unit [21].The bulky rigid groups(i.e.dipropylfuorene and hexapropyltruxene unit)on the dyes retard electron transfer from the conduction band of TiO2to the oxidized redox species in the electrolyte,which enables the attainment of high photovoltages.As a part of our systematic development of bulky organic dyes,we have designed and synthesized an organic dye with hexyloxyl group on indoline moiety. The long alky chains on the dye prefer to perform good steric hindrance for improvement of photovoltage.The alkoxy substituents on indoline moiety are expected to further enhance the electron-donating capability of dye donor for attainment of highJsc.The classical 3,4-ethylenedioxythiophene(EDOT)is used asπ-conjugated spacer for its broadening absorption spectra and good light harvest.For comparison,a triarylamine dye with the similar hexyloxyl group and conjugated bridging segment(π)is synthesized for understanding the relationship of dye structure and cell performance.Their molecular structures are shown in Fig.1.
II.MATERIAL AND METHODS
A.General synthetic procedure
The synthetic route forXS51andXS52is shown in Scheme 1 and 2.All the reactions were conducted under nitrogen atmosphere in oven dried glassware with magnetic stirring.Diethyl ether and THF were dried and distilled from sodium and benzophenone under nitrogen protection.Phosphorus oxychloride was freshly distilled before use.Dichloromethane was dried and freshly distilled from calcium hydride under nitrogen atmosphere.All other solvents and chemicals used in this work were analytical grade without further purifcation.Melting points of the samples were taken on an RY-1 melting point apparatus(Tianfen,China).1H NMR and13C NMR spectra were recorded on Bruker 400 instruments by using the residual signalsδ=7.26 and 77.0 ppm from CDCl3,δ=2.50 and 39.4 ppm from DMSO-d6.High resolution mass spectra were obtained with a Micromass GIQ-TOF mass spectrometer.
B.Detailed experimental procedures and characterization
1.Preparation of compound3
Tris(4-bromophenyl)amine1(1.16 g,2.4 mmol), 2-(2,5-bis(hexyloxy)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborlane2(1.94 g,4.8 mmol),K2CO3(827.5 mg, 5.9 mmol)and Pd(PPh3)4(49 mg,0.043 mmol)were added into a solution of 15 mL mixture of DME and water(3∶1).The mixture was heated to refux for 6 h.The reaction was quenched by the addition of water(20 mL)and extracted with CH2Cl2(2×20 mL). The combined extracts were dried over anhydrous Na2SO4and fltered.Solvent was removed by rotary evaporation in vacuo to give the crude product,which was purifed by column chromatograph packed with silica gel using petroleum ether/ethyl acetate(10∶1)as eluent to give a white solid(1.47 g,70%yield).1H NMR(400 MHz,CDCl3)∶δ(ppm)7.49(d,J=8.5 Hz, 4H),7.36(d,J=8.7 Hz,2H),7.14(d,J=8.5 Hz, 4H),7.07(d,J=8.7 Hz,2H),6.95(d,J=2.9 Hz,2H), 6.90(d,J=8.8 Hz,2H),6.84−6.81(m,2H),3.95(t,J=6.5 Hz,4H),3.89(t,J=6.4 Hz,4H),1.81−1.77(m, 4H),1.73−1.70(m,4H),1.50−1.45(m,4H),1.37−1.36 (m,12H),1.31−1.30(m,8H),0.94−0.91(m,6H), 0.89−0.88(m,6H).13C NMR(100 MHz,DMSO-d6)δ=153.4,150.2,146.9,146.0,133.3,132.1,131.3,130.4, 125.5,123.5,116.9,114.8,114.4,113.7,71.8,69.5,68.6, 59.1,31.5,29.4,25.8,22.6,14.1 ppm.
2.Preparation of compound4
2-(Tributylstannyl)-3,4-(ethelenedioxy)thiophene (0.52 g,1.2 mmol),3(842 mg,0.96 mmol),and Pd(PPh3)4(60 mg,0.054 mmol)were dissolved in toluene(20 mL),and the reaction was refuxed under N2for 24 h.After cooling down to room temperature, the mixture was poured into water and extracted with CH2Cl2(2×20 mL).The combined organic layers were washed 3 times with water,dried over anhydrous Na2SO4.After rotary evaporation of the solvent under reduced pressure,the residue was purifed on a silicagel column(petroleum ether∶ethyl acetate=10∶1 as eluent)to give a light green oil(325 mg,35%yield).1H NMR(400 MHz,CDCl3)∶δ(ppm)7.64(d,J=8.5 Hz, 2H),7.51−7.49(m,4H),7.21−7.18(m,6H),6.97(d,J=2.9 Hz,2H),6.93(d,J=8.8 Hz,2H),6.84−6.83 (m,2H),4.33−4.28(m,4H),3.99(t,J=6.4 Hz,4H), 3.93(t,J=6.3 Hz,4H),1.82−1.78(m,4H),1.74−1.71 (m,4H),1.49−1.47(m,4H),1.37−1.35(m,12H), 1.31−1.30(m,8H),0.93−0.90(m,12H).13C NMR (100 MHz,DMSO-d6)δ=151.2,149.9,147.7,146.9, 146.1,133.3,132.2,131.3,130.3,125.4,123.2,116.8, 114.6,113.9,112.6,95.1,69.2,65.9,65.1,31.5,29.4, 25.8,22.7,14.1 ppm.

Scheme 1 Synthetic route forXS51.
Scheme 2 Synthetic route forXS52.
3.Preparation of compound5
To a solution of compound4(514 mg,0.54 mmol) in anhydrous DMF(10 mL,127 mmol)at 0◦C was added POCl3(0.19 mL,2.1 mmol)dropwise and stirred for 1 h.Subsequently,the mixture was moved into room temperature for another 12 h.The mixture was poured into an ice-water with vigorous stirring,followed by neutralizing with 4 mol/L NaOH.The mixture was extracted with CH2Cl2(3×15 mL),dried over anhydrous Na2SO4.After rotary evaporation of the solvent under reduced pressure,the resulting solid was purifed by column chromatography on silica gel (petroleum ether∶ethyl acetate=5∶1 as eluent)to give a yellow solid(333.8 mg,64%yield).1H NMR(400 MHz,CDCl3)∶δ(ppm)9.92(s,1H),7.71(d,J=8.6 Hz,2H), 7.53−7.51(m,4H),7.21−7.17(m,6H),6.96−6.91(m, 4H),6.84−6.81(m,2H),4.43−4.39(m,4H),3.98(t,J=6.5 Hz,4H),3.93(t,J=6.4 Hz,4H),1.81−1.77(m, 4H),1.74−1.70(m,4H),1.48−1.46(m,4H),1.36−1.34 (m,12H),1.29−1.27(m,8H),0.92−0.91(m,12H).13C NMR(100 MHz,DMSO-d6)∶δ=179.4,148.6,148.3, 147.5,146.2,138.8,131.8,128.8,128.4,128.2,126.4, 124.2,123.3,117.0,115.5,112.5,109.5,67.9,64.2,31.0, 28.7,24.8,21.8,12.1 ppm.
4.Preparation of compoundXS51
To a mixture of compound5(114 mg,0.12 mmol) and cyanoacetic acid(50 mg,0.58 mmol)were added acetonitrile(8 mL),chloroform(4 mL)and piperidine (50µL).The solution was refuxed for 24 h.After cooling,the solvent was removed in vacuo.The pure product was obtained by silica gel chromatogrephy(CH2Cl2∶MeOH=10∶1 as eluent)as red powder (80.6 mg,65%yield).Mp>300◦C.IR(KBr)∶3428, 2930,2367,1632,1062 cm−1.1H NMR(400 MHz, DMSO-d6)∶δ(ppm)8.21(s,1H),7.70(d,J=8.8 Hz, 2H),7.54−7.52(m,4H),7.13−7.08(m,6H),7.00(d,J=8.8 Hz,2H),6.89−6.84(m,4H),4.50−4.42(m, 4H),3.94(t,J=6.4 Hz,4H),3.89(t,J=6.2 Hz,4H), 1.71−1.67(m,4H),1.63−1.59(m,4H),1.43−1.39(m, 4H),1.31−1.30(m,12H),1.24−1.22(m,8H),0.89−0.80 (m,12H).13C NMR(100 MHz,DMSO-d6)∶δ=164.6, 153.3,150.1,145.4,138.0,134.1,130.9,130.7,128.2, 125.1,124.4,122.6,117.9,116.5,115.1,114.5,108.7, 69.3,68.3,55.3,31.4,29.2,25.6,22.5,14.2 ppm. HRMS(ESI)calculated for C64H76N2O8S(M+H+)∶1033.5400,found 1033.5417.
5.Preparation of compound8
To a 100 mL two neck round-bottom fask were added compound6(2.00 g,4.63 mmol),compound7(0.88 g, 5.56 mmol),Pd(OAc)2(62 mg),t-BuOK(624 mg), P(t-Bu)3(0.6 mL)and toluene(30 mL).The reaction mixture was refuxed overnight under nitrogen.After cooling to room temperature,saturated NH4Cl was added and extracted with ethyl acetate(3×10 mL).The combined organic layers were washed with brine and then dried over anhydrous magnesium sulfate,fltered, and concentrated in vacuo to give the crude product, which were purifed by column chromatograph packed with silica gel using petroleum ether∶ethyl acetate(15∶1) as eluent to aford a light-yellow solid of compound8(1.37 g,58%yield).Mp∶87−88◦C;IR(KBr)∶3454,2933,2855,1597,1497,1461,1384,1262,1211, 1051 cm−1;1H NMR(400 MHz,CDCl3)∶δ(ppm)7.71 (d,J=8.6 Hz,2H),7.45(d,J=8.6 Hz,2H),7.30−7.18 (m,3H),7.10(d,J=3.0 Hz,1H),7.02(d,J=8.8 Hz, 1H),6.92(dd,J=8.8 Hz,J=3.0 Hz,1H),6.90−6.86(m, 1H),4.93−4.88(m,1H),4.08(t,J=6.5 Hz,2H),4.01(t,J=6.5 Hz,2H),3.98−3.96(m,1H),2.22−2.12(m,2H), 2.05−1.76(m,7H),1.72−1.38(m,13H),1.08−1.01(m, 6H);13C NMR(100 MHz,CDCl3)∶δ=153.6,150.4, 146.9,142.3,135.3,131.8,131.7,131.6,130.3,127.3, 124.8,119.4,118.3,117.0,114.6,114.5,113.6,109.2, 69.6,69.3,68.7,45.7,34.8,34.0,31.8,31.6,29.6,29.5, 25.9,25.9,24.6,22.7,22.7,14.2,14.2 ppm;HRMS(ESI) calculated for C35H46NO2(M+H+)∶512.3450,found∶512.3448.
6.Preparation of compound9
To a stirred solution of compound8(1.20 g, 2.34 mmol)in DMF(15 mL)was added dropwise a solution ofN-bromosuccinimide(NBS,438 mg,2.46 mmol) dissolved in dimethylformamide(DMF,5 mL)at room temperature.After the addition,the mixture was stirred overnight in dark.Ice water was added to terminate the reaction and the product was extracted with ethyl acetate.The combined organic layers were washed with brine water and dried with anhydrous MgSO4. The solvent was evaporated in vacuo.The crude product was purifed by column chromatograph packed with silica gel using petroleum ether∶ethyl acetate(15∶1)as eluent to aford a light-yellow oil of compound9(1.36 g, 98%yield).IR(KBr)∶3454,2931,2852,1595,1496, 1390,1262,1050 cm−1;1H NMR(400 MHz,CDCl3)∶δ(ppm)7.59(d,J=8.6 Hz,2H),7.31(d,J=8.6 Hz, 2H),7.25−7.24(m,1H),7.18(dd,J=8.8 Hz,J=3.0 Hz, 1H),6.97−6.93(m,3H),6.84(dd,J=8.8 Hz,J=3.0 Hz, 1H),4.85−4.81(m,1H),3.99(t,J=6.5 Hz,2H),3.92(t,J=6.5 Hz,2H),3.89−3.84(m,1H),2.10−2.03(m,2H), 1.86−1.68(m,7H),1.54−1.28(m,13H),0.98−0.90(m, 6H);13C NMR(100 MHz,CDCl3)∶δ=153.5,150.3, 146.4,141.6,137.5,131.8,131.6,130.3,129.8,127.6, 118.4,117.0,114.6,113.6,109.8,109.6,69.6,69.2,68.7, 45.4,34.8,34.0,31.6,31.5,31.4,29.7,29.4,29.4,25.8, 25,8,24.5,22.6,22.6,14.0,14.0 ppm;HRMS(ESI)calculated for C35H45BrNO2(M+H+)∶590.2634,found∶590.2632.
7.Preparation of compound10
To a 100 mL two-neck round-bottom fask were added Pd(PPh3)4(60 mg),compound9(1.15 g,1.95 mmol), 2-(tributylstannyl)-3,4-(ethelenedioxy)thiophene (1.26 g,2.92 mmol)and toluene(30 mL)subsequently. The mixture was refuxed overnight under nitrogen. The reaction mixture was quenched by saturated aqueous ammonium chloride solution and extracted with ethyl acetate(3×10 mL).The combined organic layers were washed with brine water and then dried over anhydrous magnesium sulfate,fltered,and concentrated in vacuo to give the crude product,which were purifed by column chromatograph packed with silica gel using petroleum ether∶ethyl acetate(15∶1 to 10∶1)as eluent to aford green-yellow oil of compound10(660 mg,52%yield).IR(KBr)∶3451,1729,1637, 1340,1287 cm−1;1H NMR(400 MHz,CDCl3)∶δ(ppm)7.52(d,J=8.6 Hz,2H),7.30(d,J=8.6 Hz, 2H),7.23−7.16(m,2H),6.95−6.93(m,3H),6.77(dd,J=8.8 Hz,J=3.0 Hz,1H),6.25(s,1H),4.90−4.88 (m,1H),4.45−4.44(m,2H),4.41−4.40(m,2H),3.99 (t,J=6.60 Hz,2H),3.95−3.90(m,3H),2.15−2.07 (m,2H),1.94−1.76(m,7H),1.45−1.30(m,13H), 0.98−0.90(m,6H);13C NMR(100 MHz,CDCl3)∶δ=167.7,167.7,148.2,143.4,132.3,132.3,130.9,130.9 128.8,128.6,121.3,120.1,117.5,113.4,109.8,109.5, 65.6,65.5,42.3,34.5,33.8,31.6,30.6,29.7,29.4,29.3, 25.8,22.6,19.2,19.0,14.0,13.7,13.7 ppm;HRMS (ESI)calculated for C41H50NO4S(M+H+)∶652.3461, found∶652.3459.
8.Preparation of compound11
POCl3(412 mg,2.69 mmol)was added dropwise to dry DMF(7 mL)at 0◦C.The reaction was stirred at 0◦C for 30 min and a solution of compound10(350 mg, 0.54 mmol)in dry DMF(5 mL)was added via a syringe. After the addition,the mixture was stirred for 4 h at room temperature.Ice water was added to terminate the reaction,and the product was extracted with ethyl acetate(3×10 mL).The combined organic layers were washed with brine and then dried over anhydrous magnesium sulfate,fltered,and concentrated in vacuo.The crude product was purifed by column chromatograph packed with silica gel using petroleum ether∶ethyl acetate(10∶1 to 5∶1)to give a yellow solid of aldehyde11(297 mg,81%yield).Mp∶109−110◦C;IR(KBr)∶3454, 2926,2360,1646,1445,1400,1084 cm−1;1H NMR (400 MHz,CDCl3)∶δ(ppm)9.90(s,1H),7.60−7.55 (m,4H),7.33(d,J=8.6 Hz,2H),7.06(d,J=8.8 Hz, 1H),6.96(d,J=3.0 Hz,1H),6.93(d,J=9.0 Hz,1H), 6.83(dd,J=8.8 Hz,J=3.0 Hz,1H),4.92−4.89(m, 1H),4.43−4.42(m,2H),4.40−4.39(m,2H),3.98(t,J=6.6 Hz,2H),3.93−3.88(m,3H),2.14−2.00(m,2H), 1.90−1.69(m,7H),1.43−1.28(m,13H),0.96−0.89(m, 6H);13C NMR(100 MHz,CDCl3)∶δ=179.0,153.5, 150.3,149.2,148.2,141.0,136.1,135.7,132.3,131.6, 131.2,130.3,127.0,123.4,122.2,119.1,117.0,114.6, 113.8,113.7,107.9,69.6,69.2,68.7,65.2,64.5,53.4, 45.3,35.0,33.8,31.6,31.5,29.4,29.4,26.9,25.8,25,8, 24.4,22.6,22.6,14.0 ppm;HRMS(ESI)calculated for C42H50NO5S(M+H+)∶680.3461,found∶680.3459.
9.Preparation of compoundXS52
To a stirred solution of compound11(270 mg, 0.4 mmol)and cyanoacetic acid(52 mg,0.6 mmol)in acetonitrile(10 mL)were added chloroform(6 mL) and piperidine(120µL,1.2 mmol).The reaction mixture was refuxed for 8 h.Additional cyanoacetic acid (34 mg,0.4 mmol)and piperdine(80µL,0.8 mmol) were added.The mixture was refuxed for additional 8 h and then acidifed with 1 mol/L hydrochloric acid aqueous solution(10 mL).The crude product was extracted with CH2Cl2(3×10 mL).The combined organic layers were washed with brine and then dried over anhydrous magnesium sulfate,fltered,and concentrated in vacuo to give the crude product.The residue was purifed by column chromatography packed with silica gel using CH2Cl2/CH3OH(20∶1 to 10∶1)as eluent to give red powder ofXS52(281 mg,94%yield). Mp∶176−178◦C;IR(KBr)∶3453,1636,1518,1403, 1249 cm−1;1H NMR(400 MHz,DMSO-d6)∶δppm 8.18(s,1H),7.58−7.54(m,4H),7.35(d,J=8.6 Hz, 2H),7.04(d,J=8.8 Hz,1H),7.00(d,J=9.0 Hz,1H), 6.88(d,J=3.0 Hz,1H),6.84(dd,J=8.8 Hz,J=3.0 Hz, 1H),5.02−4.94(m,1H),4.51−4.49(m,2H),4.45−4.40 (m,2H),4.00(t,J=6.85 Hz,2H),3.90−3.87(m,3H), 1.73−1.58(m,6H),1.37−1.23(m,16H),0.88−0.83(m, 6H);13C NMR(100 MHz,CDCl3)∶δ=153.4,150.3, 150.0,148.8,142.2,140.6,136.2,135.9,133.7,132.7, 131.4,130.4,127.8,123.7,121.9,119.4,117.3,117.0, 114.5,113.7,108.8,107.7,69.6,69.2,68.7,65.5,64.4, 45.1,35.2,33.6,31.9,31.6,31.5,30.3,29.7,29.4,29.3, 25.8,25,8,24.4,22.6,22.6,14.0,14.0 ppm;HRMS (ESI)calculated for C45H51N2O6S(M+H+)∶747.3468, found∶747.3466.
C.Optical and electrochemical measurements
The absorption spectra of the dyes were measured by HITACHI U-3310 spectrophotometer.Fluorescence measurements were carried out with a HITACHI F-4500 fuorescence spectrophotometer.Cyclic voltammetry (CV)measurements for the dye-sensitized flms were performed on a Zennium electrochemical workstation (ZAHNER),with sensitized electrodes as working electrode,Pt-wires as counter electrode,and an Ag/AgCl electrode as reference electrode with a scan rate of 50 mV/s.Tetrabutylammonium perchlorate(TBAP, 0.1 mol/L)and MeCN were used as supporting electrolyte and solvent,respectively.The measurements were calibrated with ferrocene as standard.
Charge densities at open circuit and intensity modulated photovoltage spectroscopy(IMVS)were performed on Zennium electrochemical workstation(ZAHNER,Germany),which includes a green light-emitting diode(LED,532 nm)and the corresponding control system.The intensity-modulated spectra were measured at room temperature with light intensity ranging from 5 W/m2to 75 W/m2,modulation frequency ranging from 0.1 Hz to 10 kHz,and modulation amplitude less than 5%of the light intensity.
D.Fabrication and characterization of DSCs
The TiO2paste(particle size,20 nm)was printed on a conducting glass(Nippon Sheet Glass,Hyogo, Japan,fuorine-doped SnO2over layer,sheet resistanceof 10 Ω/sq)using a screen printing technique.The flm was dried in air at 120◦C for 30 min and calcined at 500◦C for 30 min under fowing oxygen before cooling to room temperature.The heated electrodes were impregnated with a 0.05 mol/L titanium tetrachloride solution in a water-saturated desiccator at 70◦C for 30 min and fred again to give a ca.3µm thick mesoscopic TiO2flm.The TiO2electrode was stained by immersing it into a 0.5 mmol/L dye solution in a mixture of DCM/EtOH(volume ratio,1∶1) and kept at room temperature for 16 h to complete the sensitizer uptake.Then the sensitized electrodes were rinsed with dry ethanol and dried by a dry air fow.Pt catalyst was deposited on the FTO glass by coating with a drop of H2PtCl6solution(40 mmol/L in ethanol)with the heat treatment at 395◦C for 15 min to give photoanode.The sensitized electrode and Pt-counter electrode were assembled into a sandwich type cell by a 25µm-thick Surlyn(DuPont)hotmelt gasket and sealed up by heating.The DSCs had an active area of 0.16 cm2and electrolyte composed of 0.25 mol/L[Co(II)(phen)3](PF6)2,0.05 mol/L[Co(III) (phen)3](PF6)3,0.5 mol/L 4-tertpyridine(TBP)and 0.1 mol/L lithium bis-(trifuoromethanesulfonyl)imide (LiTFSI)in acetonitrile.For comparison,the iodine electrolyte composed of 0.6 mol/L 1,2-dimethyl-3-npropylimidazolium iodide(DMPImI),0.1 mol/L LiI, 0.05 mol/L I2,and 0.5 mol/L tertbutylpyridine in acetonitrile was tested as well.
The photocurrent-voltage(J-V)characteristics of the solar cells were carried out with Keithley 2400 digital source meter controlled by a computer and a standard AM1.5 solar simulator-Oriel 91160-1000(300 W)SOLAR SIMULATOR 2×2 BEAM.The light intensity was calibrated by an Oriel reference solar cell.A metal mask with an aperture area of 0.2 cm2was covered on a testing cell during all measurements.The action spectra of monochromatic incident photon-to-current conversion efciency(IPCE)for solar cells were performed by a commercial setup(QTest Station 2000 IPCE Measurement System,CROWNTECH,USA).
III.RESULTS AND DISCUSSION
A.UV-Vis absorption and electrochemical properties
The UV-Vis absorption spectra of dyesXS51andXS52are shown in Fig.2,and the photophysical properties are summarized in Table I.Both of the dyes have a relatively broad and strong absorption in the ultraviolet and visible region,and exhibit intramolecular charge-transfer transition(ICT)electron transition peaks located in the range of 400−600 nm.The major absorption peaks forXS51andXS52locate at 512 and 536 nm,respectively.In comparision withXS51,XS52shows a red-shifted absorption due to the powerful electron-donating capability of the indoline unit.
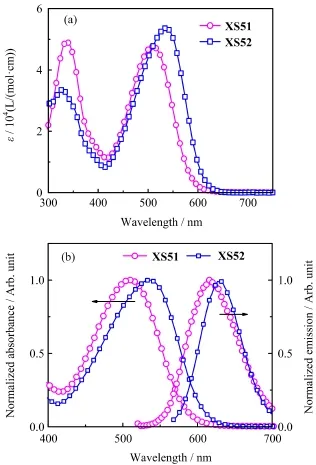
FIG.2(a)UV-Vis absorption spectra ofXS51andXS52in dichloromethane.(b)Normalized absorption and emission spectra ofXS51andXS52.
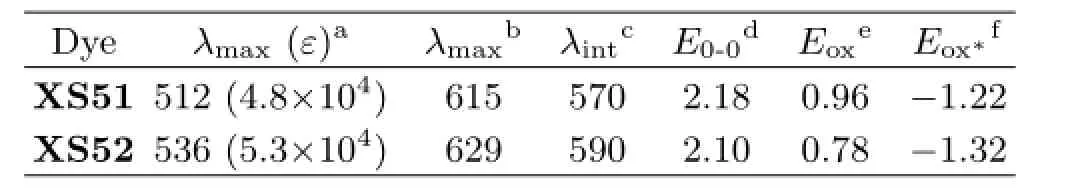
TABLE I Optical properties and electrochemical properties of the dyes(λin nm,εin L/(mol·cm),E0-0in eV,andEoxandEox∗in V).
The values of molar extinction coefcient,ε,at the maximum absorption wavelength forXS51andXS52are 4.8×104and 5.3×104L/(mol·cm),respectively.The high absorption coefcient indicates a good ability of light harvesting,which is desirable for a thin TiO2flm. When the two dyes are excited by visible light,they exhibit strong luminescence maxima at 550 nm to 700 nm(Fig.2(b)).
The absorption spectra ofXS51andXS52anchored on transparent mesoporous titania flms(3µm)are shown in Fig.3.The absorption maximum ofXS51lies at 432 nm,forXS52at 452 nm.In contrast to their absorption peaks in solution,both of them show hypsochromic shifts,which could be ascribed to a weaker electron-withdrawing capability of the carboxylate titanium assembly than that of carboxylic acid[22].
Electrochemical properties ofXS51andXS52are presented in Table I.The redox potentials of dyes are obtained by cyclic voltammetric(CV)curve with 0.1 mol/L tetrabutylammonium hexafuorophosphate in acetonitrile solution,and representative cyclic voltammograms are shown in Fig.4.The frst quasireversible one-electron oxidation wave is taken as the ground-state oxidation potentials(Eox).TheEoxvalues ofXS51andXS52are calculated to be 0.96 and 0.78 V (vs.NHE),respectively,which are more positive than the[Co(II/III)(1,10-phenanthroline)3]n+redox couple (0.62 Vvs.NHE)[23].It can be said that the driving force from the cobalt to the dyes is sufcient and the oxidized dyes will favorably accept electrons from Co(II)ions to be regenerated.
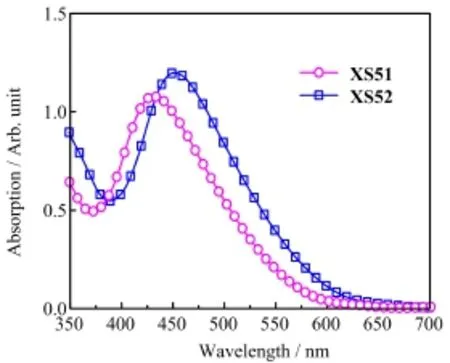
FIG.3 Absorption spectra of sensitized electrodes withXS51andXS52.

FIG.4 Cyclic voltammograms of theXS51-andXS52-loaded TiO2flms.

FIG.5 Incident photon-to-current conversion efciency spectra of DSCs.
The excited-state reduction potential(Eox∗)of the dyes are calculated from the ground-state oxidation potentials(Eox∗)and the 0-0 band gaps(E0-0),which is determined from the intersection of absorption and emission spectra.TheEox∗values ofXS51andXS52are calculated to be−1.22 and−1.32 Vvs.NHE,respectively.The more negative value ofXS52is originated from the stronger electron-donoring capability of indoline unit than that of triarylamine.Both are much more negative than the conduction band of TiO2at approximate−0.5 Vvs.NHE.Therefore,these dyes used as sensitizers will produce sufcient driving forces for electron injection from the excited dye molecules to the conduction band of TiO2electrode.
B.Photovoltaic performance of DSCs
The incident monochromatic photon-to-current conversion efciency(IPCE)was obtained with a sandwich cell employing cobalt and iodine electrolytes,respectively.IPCE spectra based onXS51andXS52can be seen from Fig.5.Both of the dyes show higher IPCE values in iodine electrolytes than in cobalt electrolytes. For example,the top IPCE value ofXS51is 80.6% for iodine electrolyte and 78.7%for cobalt electrolyte. A little lower IPCE values ofXS52were observed in iodine and cobalt electrolyte corresponding to those ofXS51.However,XS52shows obviously broader IPCE spectra thanXS51,which is in accordance with the preceding light absorption measurements.The broad IPCE spectrum is benefcial to high photocurrent density of DSCs.
J-Vcurves of the devices based onXS51andXS52in electrolytes of[Co(II)(phen)3](PF6)2are measured under AM 1.5 irradiation(100 mW/cm2),and the results are shown in Fig.6.The detailed photovoltaic parameters are summarized in Table II.A relatively thin titania flm(3µm)was applied so as to minimize the mass transport limitation of Co(II/III)(phen)3redox couple.As it can be seen from Table II,5.88%of so-lar energy to power conversion efciency(PCE)based onXS51is achieved with aJscof 10.5 mA/cm2,aVocof 860 mV,and a fll factor(FF)of 0.65.The cells based onXS52show an increasedJscof 11.5 mA/cm2and a decreasedVocof 830 mV.The improvement ofJsccompensates for a slight drop inVoc,contributing to an enhanced PCE of 6.58%.The observed results here suggest that indoline donor with strong electrondonoring capability induce the improvements ofJscin comparison with triaryamine donor,which is in agreement with the observed light absorption and IPCEs. The increasedVocobtained forXS51can be attributed to the sterically bulky four hexyloxyl groups on the dye, which prevent Co(III)ions at the vicinity of the TiO2from recombining at the titania/electrolyte interface.
We have also recorded theJ-Vcurves of the DSCs employing an iodine electrolyte consisting of 0.25 mol/L DMPImI,0.1 mol/L LiTFSI,0.05 mol/L I2,and 0.5 mol/L TBP in acetonitrile for comparison,and the detailed photovoltaic parameters are listed in Table II. A similar trend inJscandVoccan be found for the two dyes.The cells based onXS52employing iodine electrolyte show higherJscand enhanced FF,corresponding to an improved PCE in contrast to those of the cells based onXS51.On the other hand,for the same dye, the cobalt electrolyte DSCs evidently outperform the devices based on the conventional iodide/triiodide redox couple.For example,the cell based onXS52with cobalt electrolyte generates an improvedVoc(830 mVvs.740 mV)in comparison with the iodine electrolyte, resulting in an enhanced PCE.The efciency gain is primarily due to the signifcant increase inVoc,which compensates for a minor drop inJsc.These results indicate that the synthesizedXS51andXS52are more suitable for Co(II/III)tris(phen)redox couple than I−/I3−redox couple in assembling DSCs.

FIG.6J-Vcharacteristics of DSCs.
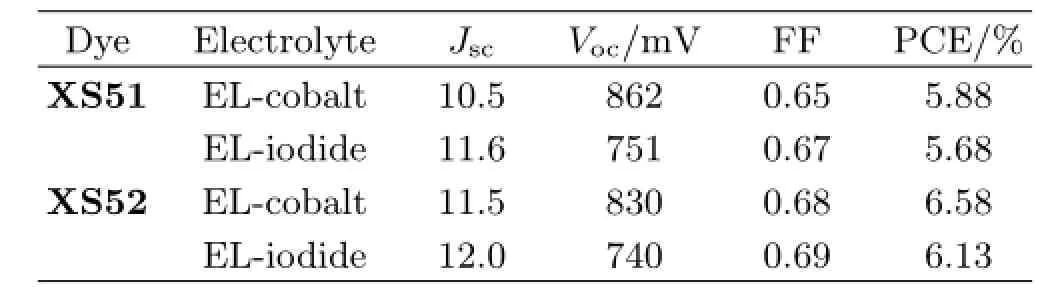
TABLE II Photovoltaic performance of DSCs employing cobalt or iodine electrolytes(Jscin mA/cm2).

FIG.7 Denpendence of open circuitVocon charge densityQfor DSCs.
C.Dependence of photovoltage on the conduction band movement and charge recombination
The respectable increase ofVocobtained by the delicate change in molecular structure is intriguing.To scrutinize the origin of the improvement inVoc,we discuss the infuence factors on the photovoltage.Generally,Vocof a DSC is dependent on the diference between the Fermi-level of TiO2(EF,n)and redox electrolyte(EF,redox),i.e.Voc=EF,redox−EF,n[24].As for a fxed redox electrolyte,EF,redoxwill not change obviously in DSCs.EF,nof TiO2can be expressed as
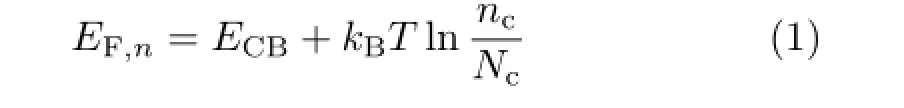
whereECBis the conduction band of TiO2,kBis the Boltzmann constant,Tis the temperature(293 K in this work),ncis the free electron density on TiO2andNcis the density of accessible states in the conduction band of TiO2[25].As shown in Eq.(1),EF,ndepends on the variation ofECBandnc.Correspondingly,whenEF,redoxis fxed,Vocis intimately correlated toECBandnc.The relative conduction band positions and electron lifetimes of DSCs based onXS51andXS52were investigated to clarify the variation ofVocin performance of cells.
Charge densities and electron lifetimes(τoc)at opencircuit were measured by charge extraction technique and by controlled intensity modulated photovoltage spectroscopy(IMVS),respectively.Figure 7 shows the relationship betweenVocand the extracted charge density(Q)at open circuit.The curves for the DSCs based onXS51andXS52are roughly parallel to each other. At a fxedQ,XS52cells,both with cobalt and iodineelectrolytes,show higherVocthan their counterparts, which means that indoline moiety is preferable to triarylamine unit because it can lead more negative shift of the conduction band edge position of TiO2.As seen in Eq.(1),a more negative shift ofECBwill provide a possibility ofVocimprovement.
Figure 8 shows the dependence of electron lifetime on charge density at open circuit.Typical data are illustrated in Table III.At the sameQ,the electron lifetime ofXS51based DSCs is much longer than that ofXS52,indicating that charge recombination between electrons in TiO2flm and Co(III)ions in the electrolyte are signifcantly retarded by the four hexyloxyl groups on triarylamine moiety.Since the diferent shift ofECBis observed in Fig.7,XS52based DSCs might provide higherVocthan those ofXS51.On the other hand,XS51based DSCs show longer electron lifetime than those ofXS52.In combination withECBand electron lifetime,the improvement inVocis mainly ascribed to the retarded charge recombination rather than the different positions of the conduction band edge of TiO2. These results indicate that indoline dye is benefcial to more negative shift ofECB,but the bulky substituent on the dye is not enough for retardation of interfacial charge recombination in DSCs.The triarylamine dye with four hexyloxyl groups shows an improvement of photovoltage through full steric hindrance,which prevents back-recombination and prolongs the electron lifetime in the semiconductor.
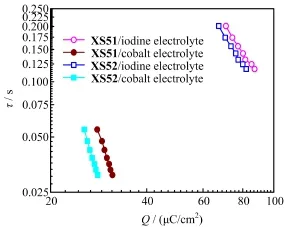
FIG.8 Dependence of electron lifetimeτon charge densityQ.
IV.CONCLUSION
We have developed two new dyesXS51andXS52based on triarylamine and indoline,respectively,for dye-sensitized solar cells(DSCs).The indoline dyeXS52shows a red-shifted absorption and higher molar extinction coefcient than the triarylamine dye.High short-circuit photocurrent density is obtained forXS52due to the strong electron-donoring capability of indoline unit.The two hexyloxyl groups on indoline dye are not enough for retardation of charge recombination at the titania/electrolyte interface in DSCs,which leads to a little lower open-circuit voltage in comparison withXS51.The photovoltaic performances of the Co(II/III)tris(phen)redox couple are superior to those of I−/I3−redox couple for thin-flm DSCs sensitized byXS51-XS52,indicating that rational design of sterically bulky organic dyes is needed for further development of high-efciency iodine-free devices.
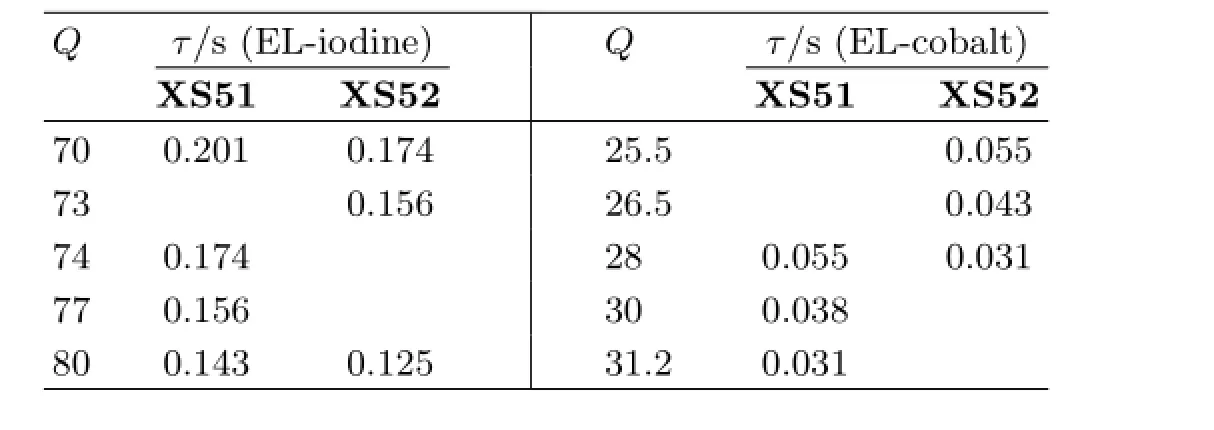
TABLE III Electron lifetime data under diferent change densities(QinµC/cm2).
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.21376179,No.21103123) and the Massive Open Online Courses Project in Tianjin University of Technology.
[1]A.Yella,H.W.Lee,H.N.Tsao,C.Yi,A.K.Chandiran,M.K.Nazeeruddin,E.W.G.Diau,C.Y.Yeh, S.M.Zakeeruddin,and M.Gr¨atzel,Science334,629 (2011).
[2]A.Hagfeldt,G.Boschloo,L.C.Sun,L.Kloo,and H. Pettersson,Chem.Rev.110,6595(2010).
[3]M.Liang and J.Chen,Chem.Soc.Rev.42,3453 (2013).
[4]G.Boschloo and A.Hagfeldt,Acc.Chem.Res.42,1819 (2009).
[5]B.C.O’Regan,I.L´opez-Duarte,M.V.Mart´ınez-D´ıaz, A.Forneli,J.Albero,A.Morandeira,E.Palomares,T. Torres,and J.R.Durrant,J.Am.Chem.Soc.130,2906 (2008).
[6]T.Marinado,K.Nonomura,J.Nissfolk,M.K.Karlsson,D.P.Hagberg,L.C.Sun,S.Mori,and A.Hagfeldt, Langmuir26,2592(2010).
[7]M.Planells,L.Pelleja,J.N.Cliford,M.Pastore,D. Angelis,F.L´opez,N.Marder,and S.R.Palomares, Energy Environ.Sci.4,1820(2011).
[8]A.Hagfeldt and M.Gr¨atzel,Chem.Rev.95,49(1995).
[9]H.Nusbaumer,S.M.Zakeeruddin,J.E.Moser,and M. Gr¨atzel,Chem.Eur.J.9,3756(2003).
[10]S.M.Feldt,E.A.Gibson,E.Gabrielsson,L.C.Sun, G.Boschloo,and A.Hagfeldt,J.Am.Chem.Soc.132, 16714(2010).
[11]S.M.Feldt,G.Wang,G.Boschloo,and A.Hagfeldt,J. Phys.Chem.C115,21500(2011).
[12]H.N.Tian and L.C.Sun,J.Mater.Chem.21,10592 (2011).
[13]B.C.O’Regan and M.Gr¨atzel,Nature353,737(1991).
[14]U.Bach,D.Lupo,P.Comte,J.E.Moser,F.Weiss¨ortel, J.Salbeck,H.Spreitzer,and M.Gratzel,Nature395, 583(1998).
[15]M.M.Lee,J.Teuscher,T.Miyasaka,T.N.Murakami, and H.J.Snaith,Science338,643(2012).
[16]J.H.Yum,E.Baranof,F.Kessler,T.Moehl,S.Ahmad,T.Bessho,A.Marchioro,E.Ghadiri,J.E.Moser, C.Yi,M.K.Nazeeruddin,and M.Gr¨atzel,Nat.Commun.3,631(2012).
[17]Y.Bai,J.Zhang,D.Zhou,Y.Wang,M.Zhang,and P. Wang,J.Am.Chem.Soc.133,11442(2011).
[18]H.Nusbaumer,J.E.Moser,S.M.Zakeeruddin,M.K. Nazeeruddin,and M.Gr¨atzel,J.Phys.Chem.B105, 10461(2001).
[19]H.X.Wang,P.G.Nicholson,L.Peter,S.M.Zakeeruddin,and M.Gr¨atzel,J.Phys.Chem.C114,14300 (2010).
[20]Y.Liu,J.R.Jennings,Y.Huang,Q.Wang,S.M. Zakeeruddin,and M.Gr¨atzel,J.Phys.Chem.C115, 18847(2011).
[21]G.Li,M.Liang,Z.Sun,H.Wang,L.N.Wang,Z. Wang,and S.Xue,Chem.Mater.25,1713(2013).
[22]D.Zhou,N.Cai,H.Long,M.Zhang,Y.Wang,and P. Wang,J.Phys.Chem.C115,3163(2011).
[23]A.Yella,R.Humphry-Baker,B.Curchod,N.Astani, J.Teuscher,and L.Polander,Chem.Mater.25,2733 (2013).
[24]A.Usami,S.Seki,Y.Mita,H.Kobayashi,H.Miyashiro, and N.Terada,Sol.Energy Mater.Sol.Cells93,840 (2009).
[25]D.Zhou,Q.Yu,N.Cai,Y.Bai,Y.Wang,and P.Wang, Energy Environ.Sci.4,2030(2011).
I.INTRODUCTION
10.1063/1674-0068/28/cjcp1409155
∗Author to whom correspondence should be addressed.E-mail:xuesong@ustc.edu.cn
(Dated:Received on September 16,2014;Accepted on January 21,2015)
Dye-sensitized solar cells(DSCs),as a new type of photovoltaic technology,have been considered to be a credible alternative to conventional inorganic siliconbased solar cells because of their ease of fabrication, high efciency,and cost-efectiveness[1,2].During the last two decades,many diferent photosensitizers,including metal complexes,porphyrins,phthalocyanines and metal-free organic dyes,have been designed and applied into DSCs[2].Among them,triarylamine organic dye-sensitized solar cells employing widely used iodine(I−/I3−)electrolytes have reached power conversion efciencies as high as 10%−11%[3],comparable to those of Ru complexes.Nevertheless,iodine redox couple also shows some drawbacks,which limit further improvement of DSCs’performance,such as the relatively high overpotential that has led to a noticeable energy loss[4],the larger charge recombination rate at the titania/electrolyte interface[5-7],the light harvesting loss[8,9],and the corrosiveness of I−/I3−redox couple [10,11].To achieve a breakthrough in developing highly efcient DSCs,alternative redox couples,including metal complexes,hole conductors,halogens and pseudohalogens and some redox active organic compounds[12] have been explored for DSCs to avoid the above problems.During the past few years,researchers have made astonishing progress by moving from a multi-electron iodide/tri-iodide redox couple to one-electron outersphere redox couples,such as cobalt complexes[1,13-15].A combination of zinc porphyrin dye(YD2-o-C8) and organic dye(Y123)showed an excellent efciency of 12.3%employing tris(2,2′-bipyridine)cobalt(II/III)redox couple[1].The cobalt(II/III)complex with a tridentate ligand 6-(1H-pyrazol-1-yl)-2,2′-bipyridine(bpypz)yielded a power conversion efciency of over 10%at 100 mW/cm2in combination with Y123[16].Wanget al.explored tris(1,10-phenanthroline)cobalt(II/III) redox shuttles in conjunction with the triphenylaminebased organic dyes,displaying power conversion efciency of 9.4%[17].A remarkable advantage of cobalt redox shuttle over I−/I3−redox couple is that very high open-circuit voltage(Voc)can be achieved without reducing short circuit photocurrent or fll factor[3,10]. However,the slow mass transport of cobalt redox shuttle and increased recombination of injected electrons with the oxidized redox species in the electrolyte may lead to poorly performing devices with low photovoltages and photocurrents[9,18-20].To overcome the disadvantages of cobalt redox shuttle,a relatively thin TiO2flm and high extinction coefcient photosensitizers with steric properties are warranted for high efciency of the dye-sensitized solar cells.
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- High Performance of Enhanced Mode Field Efect Transistor and Ultraviolet Sensor Based on ZnO Nanosheet
- Quantitative Moisture Measurement with a Cavity Ring-down Spectrometer using Telecom Diode Lasers
- Accurate Measurement of Raman Depolarization Ratio in Gaseous CO2
- Methanol Adsorption on TiO2Film Studied by Sum Frequency Generation Vibrational Spectroscopy
- Ultraviolet Source Assisted Enhancement of Attosecond Pulse
- Decay Dynamics ofN,N-Dimethylthioacetamide in S3(ππ∗)State
