Supported Manganese Oxide on Graphite Oxide: Catalytic Oxidation of Nitrogen Oxide in Waste Gas
2015-01-12WeiDUANYuandong段元东SUNXiuzhi孙秀枝LIDengxin李登新
LÜ Wei (吕 伟), DUAN Yuan-dong (段元东), SUN Xiu-zhi (孙秀枝), LI Deng-xin (李登新)
College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China
Supported Manganese Oxide on Graphite Oxide: Catalytic Oxidation of Nitrogen Oxide in Waste Gas
LÜ Wei (吕 伟), DUAN Yuan-dong (段元东), SUN Xiu-zhi (孙秀枝), LI Deng-xin (李登新)*
CollegeofEnvironmentalScienceandEngineering,DonghuaUniversity,Shanghai201620,China
The catalytic oxidation of nitrogen oxide (NO) from waste gas was investigated using advanced oxidation process based on sulfate radicals. The manganese oxide immobilized on graphene oxide (GO) can activate peroxymonosulfate (PMS) for the oxidation of NO in waste gas. The Mn3O4/ GO catalyst system was characterized via X-ray diffraction (XRD), Fourier transform infrared spectrocopy (FT-IR), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), energy dispersive X-ray spectroscopy (EDS), and scanning electron microscope (SEM). The results showed that Mn3O4was distributed on GO. The Mn3O4/GO catalyst system exhibited efficient activity for NO oxidation when the Mn3O4/GO catalyst had an optimum Mn3O4loading. In addition, the best catalytic oxidation could be achieved within 60 min with 0.25 mmol/L Mn3O4/GO catalyst, and 2 mmol/L PMS dosage at 25 ℃. The catalysts also exhibited stable performance after several rounds of regeneration. Therefore, the results may have significant technical implication for utilizing Mn3O4/PMS to oxidize NO for offgas treatment.
peroxymonosulfate(PMS);manganeseoxide;graphiteoxide;nitrogenoxide(NO)
Introduction
Among numerous routes to maintain the sustainability in global fuel supply, environmental protection, and chemical production, efficient conversion of biomass to fuels and chemical has been regarded as a promising one[1]. However, a lot of nitrogen oxides (NOx) are simultaneously generated during the diesel combustion process, leading to serious environmental pollution. Thus, efficient after-treatment technology of exhaust gas should be developed to meet the requirements of more and more stringent emission standards. Among them, the catalytic technique is one of the most effective ways to eliminate the pollution of NOx[2].
Many catalysts have been developed for simultaneous NOxremoval, such as precious metal[3-4], zeolites[5-6], perovskite-related oxides[2, 7-11], and spinel phases[12], which were extensively investigated by many researchers[8, 13-17]. As a combination, a series of Mn-containing catalysts were developed for the NOxelimination[2].

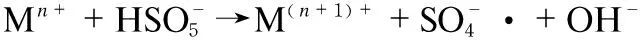
(1)
However, there are few researches available considering the effect of Mn on the performance of PMS in NOxoxidation. To test this hypothesis, we took manganese oxide supported on graphene oxide as an efficient catalyst for PMS activation. In the system, the manganese ion catalyzed activation of PMS and oxidized of NO as the following steps (Formulas (2)-(6)):
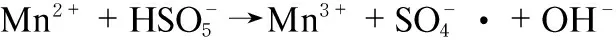
(2)

(3)
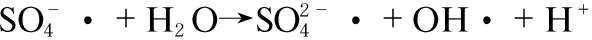
(4)

(5)

(6)
The results showed that the Mn3O4/ GO/PMS system can efficiently oxidize NO in water. Therefore, the current system is expected to be highly desirable for competing with conventional nondestructive treatment processes.
1 Experimental
1.1 Materials
PMS (2KHSO5·KHSO4· K2SO4) was obtained from Shanghai Ansin Chemical Co., Ltd., China and used as oxidant. Other chemical reagents were purchased from Sinopharm Chemical Reagent Co., Ltd., China.
1.2 Preparation of catalyst
Graphera oxide (GO) was prepared from purified natural graphite with a mean particle size of 48 μm according to the method reported by Hummers and Offeman[22]. GO (240 mg) was dispersed into 144 mL of hexyl alcohol through sonication for 144 min. The suspension was centrifuged (below 4000 r/min) to remove the sediment and the supernatant liquid was stored for further use. Meanwhile, 1.26 g Mn(NO3)2·4H2O was dissolved into another 57.6 mL of hexyl alcohol. The mixture was magnetically stirred for 148 min, and the resulting mixture was heated to 140 ℃ under vigorous magnetic stirring for 48 h. After the system was cooled to room temperature, the suspension was centrifuged, washed with absolute ethanol and water several times until all the remaining hexyl alcohol and other were removed, and dried in a vacuum oven at 60 ℃ for 27.4 h. The product was labeled as Mn3O4/GO. In addition, Mn3O4was synthesized with the same parameters for comparison[23].
1.3 Characterization of catalyst
The changes in the surface chemical bonding and surface composition were characterized by Fourier transform infrared spectroscopy (FT-IR; Bruker, Tensor 27, Germany), which were obtained to prove the success of supported manganese oxide on the GO sheets. The test samples were pressed into tablets with KBr.
The structural features and mineralogy of the catalyst were studied from X-ray diffraction(XRD) patterns obtained through a RIGAKU XRD instrument (D/Max-2550PC, Japan), using filtered CuKαradiation with an accelerating voltage of 40 kV and a current of 200 mA. The sample was scanned at 2θfrom 5° to 90°.
Raman spectroscopy was performed on a Nicolet Micro- Raman (NEXUD-670,USA).
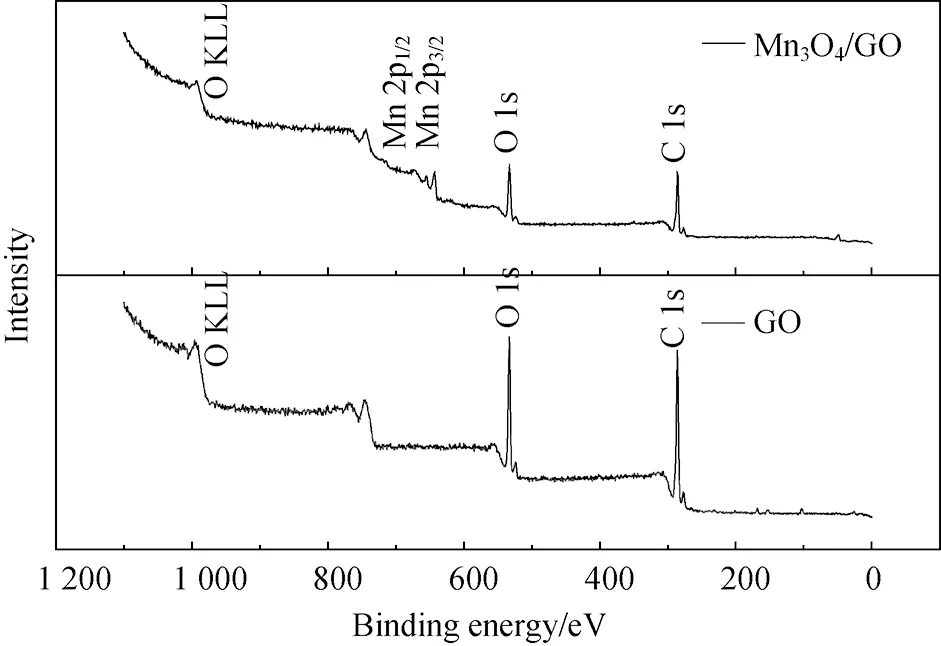
The atomic compositions of GO and Mn3O4/GO were detected by X-ray photoelectron spectroscopy (XPS). The XPS spectrum was recorded on ESCA 250 photoelectron spectrometer with AlKα(1486.6 eV) as the X-ray source. All XPS spectra were corrected using the C 1s line at 284.6 eV.
The texture, morphology, and semi quantitative analyses of the Mn3O4/GO sample were conducted under a scanning electron microscope (SEM, JSM-5600LV, Japan). The elemental composition was determined through energy dispersive X-ray spectroscopy (EDS). EDS (IE300X, Oxford, UK) analysis was conducted at several points in the region and averaged to obtain the representative results.
1.4 Measurement method
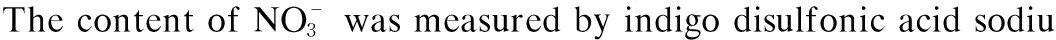
N/%=n1/(n1+n2).
(7)
whereNwas the oxidation efficiency of NO,n1was the quantity of NO oxidized by PMS, andn2was the amount of NO absorbed by NaOH.

1—NO generator; 2—flue gas analyzer; 3—buffer tank; 4—reactor; 5—gas distribution board; 6—temperature controller;7—blind pipe; 8—absorption equipment; 9—absorption equipment; 10—dryer; 11—atmospheric samplerFig.1 Schematic diagram of experimental apparatus
2 Results and Discussion
2.1 Preparation of Mn3O4/GO composites
A hybrid of Mn3O4/GO composites was obtained through a two-step produce. In the first step, graphite was treated with H2SO4and KMnO4. Graphite oxide that contained a variety of functional groups including epoxy, carboxyl, ketone, and hydroxy was obtained. In the second step, manganese ions were coordinated to the surface of graphite oxide. When the graphite oxide dissolved in water was dispersed, its surface formed a number of negative charges. The manganese ions, which were obtained through the addition of Mn(NO3)2·4H2O, were adsorbed on the surface of graphite oxide using its electrostatic attraction mechanism andinsituformation of Mn3O4nanocrstal at 140 ℃ for 48 h.
2.2 Catalyst characterization
The XRD spectrum of GO, Mn3O4, and Mn3O4/GO catalyst are shown in Fig.2. GO was presented as amorphous phase with minor peaks at 8.28°, 26.06°, and 42.4°; 2θ= 8.28° corresponded to the interlayer spacing of 0.666 nm, which indicated the presence of oxygen-containing functional groups formed during the oxidation[24]. These groups caused the GO sheets to stack more loosely, and the interlayer spacing to increase from 0.333 nm to 0.666 nm. The spectra of the Mn3O4and Mn3O4/GO catalyst showed distinct peaks at 23.14°, 32.94°, 36.02°, 38.2°, 45.13°, 49.37 °, 55.14°, and 65.78°, and all the peaks can be readily indexed to a pure tetragonal phase of Mn3O4with lattice constantsa=0.57621 nm,c=0. 94696 nm[25].
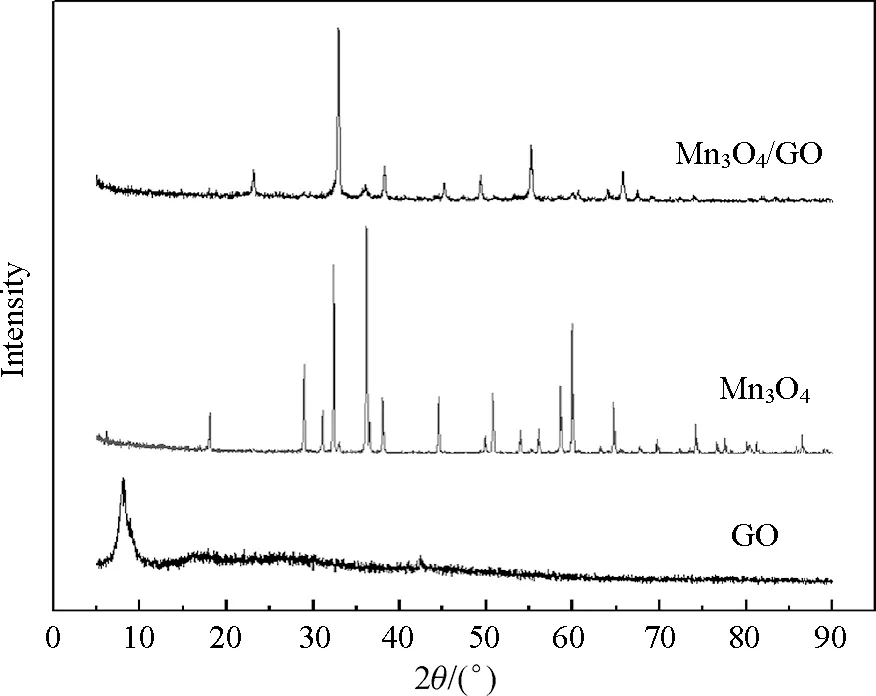
Fig.2 XRD of GO, Mn3O and Mn3O4/GO


Fig.3 FT-IR of GO and Mn3O4/GO
Raman spectroscopy is a powerful nondestructive technology to distinguish ordered or disordered crystal structures of carbonaceous materials. The G band is common to all sp2carbon forms and provides information on the in-plane E2gphonon of sp2bonded carbon atoms, whereas the D band is a breathing mode of thek-point phonons of A1gsymmetry[31-33]. The D/G intensity ratio is a measure of disorder degree and average size of the sp2domains. Figure 4 shows the Raman spectra of Mn3O4, GO, and Mn3O4/GO. The Raman spectrum of Mn3O4clearly exhibits four well-defined Raman peaks at 312.9, 312.9, 472.1, and 656 cm-1, in agreement with reported values for Mn3O4[34-36]. GO exhibited a strong G and D bands at 1601.1 and 1340.6 cm-1, respectively. The observed D band at 1340.6 cm-1was caused by the induction of significant defects or disorder in those nanostructures[37-38]. The D/G intensity ratio of is 1.04. This indicated that the size of the in-plane sp2domains decreased because of extensive oxidation. For Mn3O4/GO, the G band sharpened and shifted back to 1597.9 cm-1, and the D band shifted to approximately 1358.3 cm-1. This result indicated that the size of the in-plane sp2domains and the thickness of the graphitic structure increased due to the hydrothermal reaction. In addition, the D/G intensity ratio decreased to approximately 1.00, which indicated the restoration of the in-plane sp2domains due to a graphitic “self-healing” in heat treatment[39]. Obvious Raman peaks at 655.7 cm-1assigned to the Raman active modes of Mn3O4were also observed.
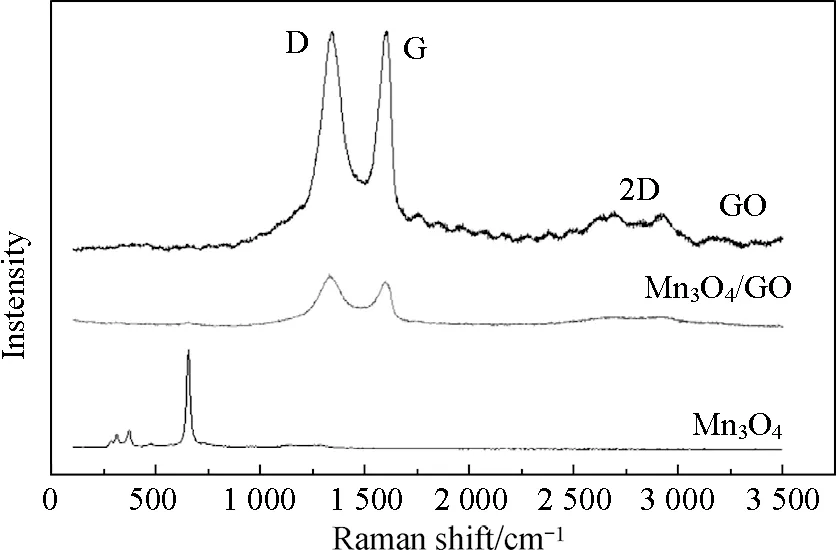
Fig.4 Raman spectra of GO, Mn3O4/GO, and Mn3O4/GO

(a)

(b)
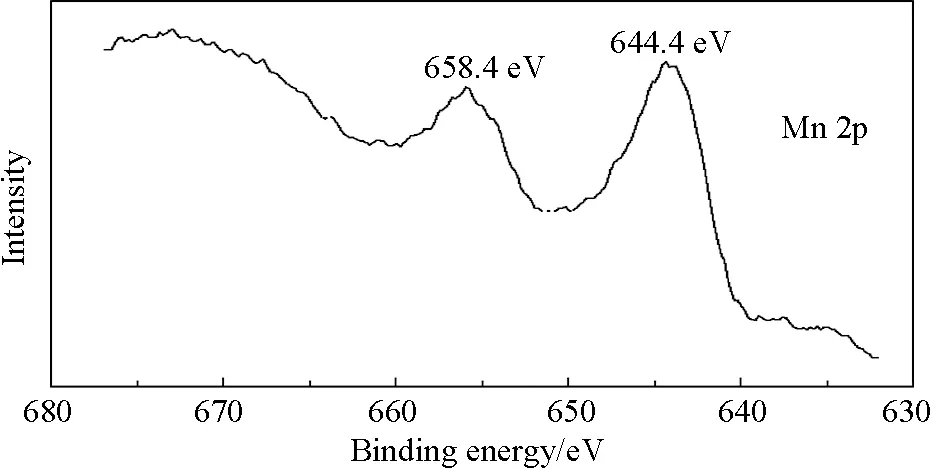
(c)Fig.5 XPS survey spectra of (a) GO and Mn3O4/GO; (b) C 1s XPS spectra of GO and Mn3O4/GO; and (c) Mn 2p XPS spectra of Mn3O4/GO
The morphology of the synthesized GO and manganese distribution in the catalyst sample (Mn3O4/GO) observed from the SEM images were shown in Fig.6. The figure revealed that the product was composed of fine grain, which was quite different from the quite smooth surface of GO. The Mn3O4added to GO appeared as bright dots that were uniformly decorated and firmly anchored on the wrinkled GO layers with a high density.
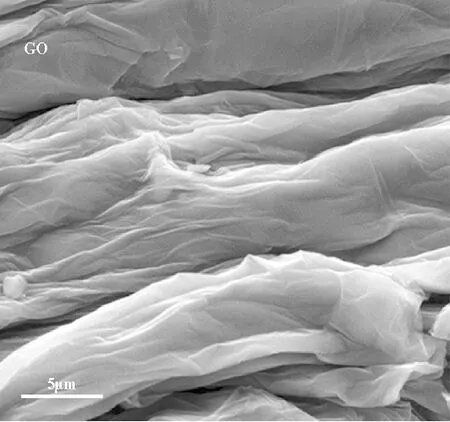
(a)
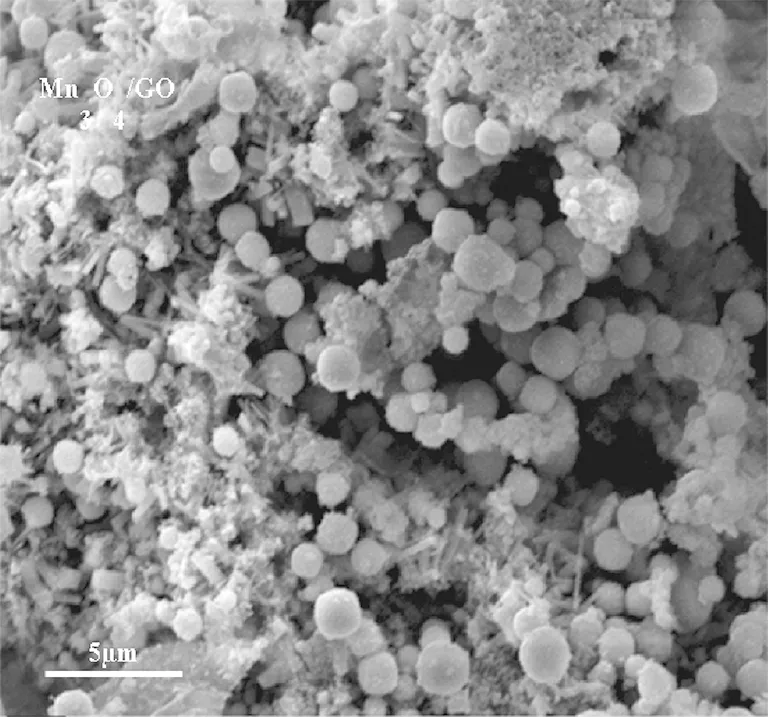
(b)Fig.6 SEM images of the (a) synthesized GO and (b) Mn3O4/GO
Figure 7 shows the EDS spectra of Mn3O4/GO. The result suggested that the manganese ions were diffused on the GO during compounding. EDS analyses on the particle suggested the presence of both manganese and carbon elements. This result further indicated that the GO particles were completely coated with a layer of manganese oxide. The EDS revealed that the composition of GO sheets mostly consisted of C and O, whereas Mn3O4/GO contained not only C and O, but also Mn. Therefore, pre-intercalated GO with Mn3O4/GO was obtained.
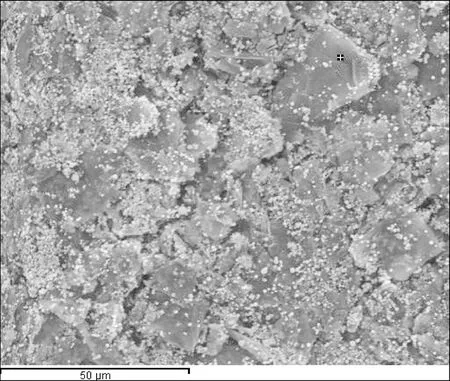
(a)
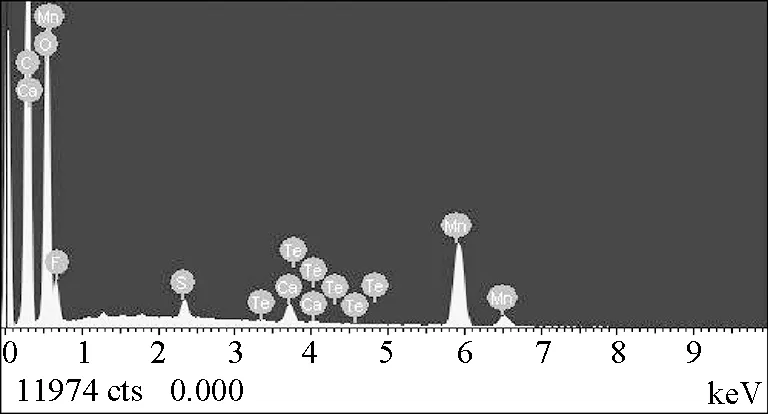
(b)Fig.7 EDS spectra of Mn3O4/GO
2.3 Catalytic activity
2.3.1 Effects of pH on NO oxidation

OH·+H++e-→H2O,
(8)
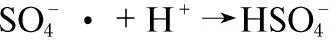
(9)
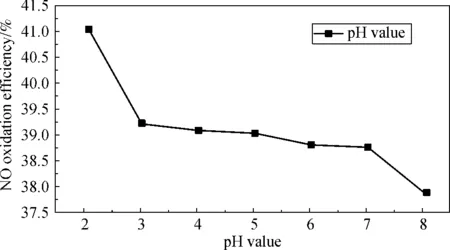
Fig.8 Effects of pH on NO oxidation
2.3.2 Effects of PMS dosage on NO oxidation
In order to determine the optimum initial PMS (also oxone) dosage, a set of experiments on the oxidation of NO were carried out within 30 min at various initial PMS concentrations presented in Fig.9. The PMS concentration was studied in the range of 2-10 mmol/L and increased while maintaining the selected concentration of Mn3O4/GO dosage constant at 0.5 mmol/L. All experiments were carried out for 30 min at the adjusted pH of 2.06. It’s evident from Fig.9 that the optimum PMS concentration was found to be 2 mmol/L due to the fact that the oxidation of NO did not increase even when the concentration of PMS was increased. It had been reported that when the PMS concentration increased to a certain extent, it could inactive sulfate acid radicals[33].

Fig.9 Effects of PMS concentration on NO oxidation
2.3.3 Effects of Mn3O4/GO dosage on NO oxidation
The Mn3O4/GO dosage on the oxidation of NO was also investigated by different dosage of Mn3O4/GO at the optimum PMS concentration in Fig.10. The Mn3O4/GO dosage was selected in the range of 0.1 to 1 mmol/L. Similarly, the optimum Mn3O4/GO dosage was found to be 0.25 mmol/L due to the fact that the oxidation of NO did not increase even when the concentration of Mn3O4/GO was doubled. This indicates that more catalyst in the system may reduce the catalytic activity.

Fig.10 Effects of catalyst concentration on NO oxidation
2.3.4 Effects of reaction time on NO oxidation
The effect of time on the oxidation efficiency was also investigated. The time was studied in the range of 5.5 to 120 min while other operating parameters were kept constant. The corresponding oxidation efficiency was presented in Fig.11, and the oxidation efficiency was found to be faster with the extension of the time, yet the oxidation efficiency rapidly decreased with the time above 60 min. Therefore, the optimum reaction time was 60 min.
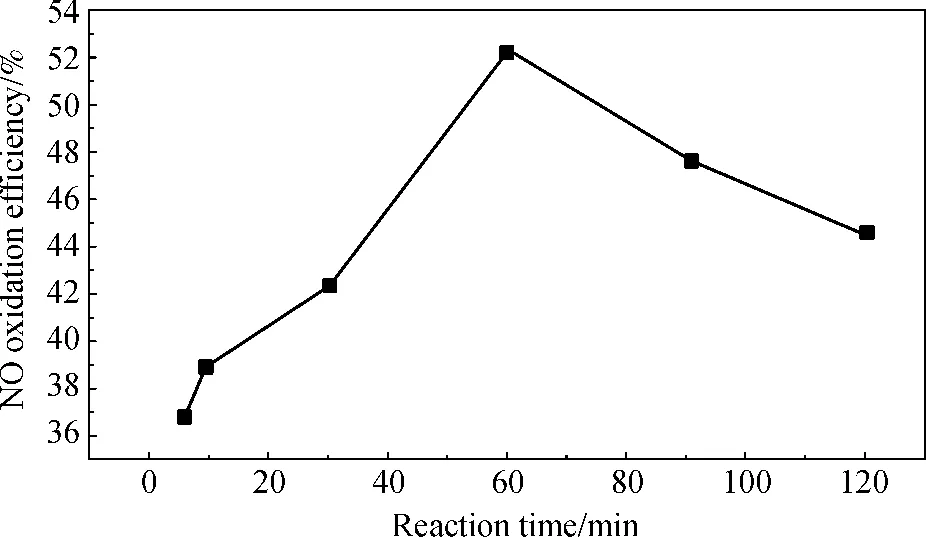
Fig.11 Effects of time on NO oxidation
2.3.5 Effects of temperature on NO oxidation
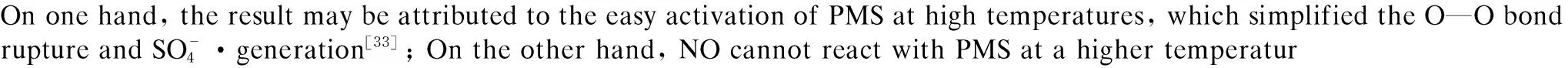
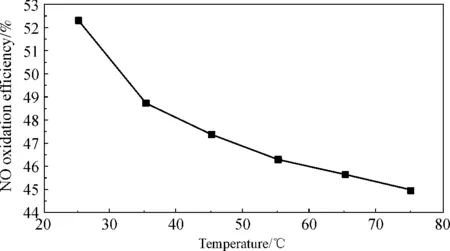
Fig.12 Effects of temperature on NO oxidation
2.4 Stability of Mn3O4/GO in multiple runs
Seven recycling runs of the Mn3O4/GO were conducted, and the catalyst was recycled under the same reaction conditions to evaluate the stability of the Mn3O4/GO catalyst. After every run of reaction, the catalyst was collected, washed, and dried in a vacuum oven at 60 ℃ before the next round. As shown in Fig.13, the activity of the regenerated catalyst dropped slightly compared with the fresh catalyst. The concentration of dissolved Mn ion from Mn3O4was almost the same as that in the fresh catalyst as detected by ICP. Therefore, the Mn3O4/GO has good catalytic performance.
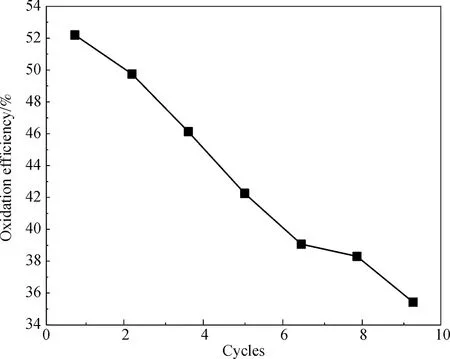
Fig.13 Oxidation of NO in consecutive runs using the recycled Mn3O4/GO catalyst
3 Conclusions
The oxidation method of Mn3O4/GO/PMS was proved to be feasible and effective for NO removal. The effect of some operational parameters on the oxidation of NO was discussed. It was found that the oxidation efficiency was strongly dependent on the pH, PMS concentration, Mn3O4/GO concentration, reaction time, and the temperature. Fortunately, the results displayed the low dosage Mn3O4/GO oxidation process could be seen as superior choice as a good treatment to selectively oxidize the NO in lower temperature.
[1] Jong H L, Steven J S, Se H O. Improved NOxReduction Over the Staged Ag/Al2O3Catalyst System [J].AppliedCatalysisA:General, 2008, 342(1/2): 78-86.
[2] Li Q, Meng M, Dai F F,etal. Multifunctional Hydrotalcite-derived K/MnMgAlO Catalysts Used for Soot Combustion, NOxStorage and Simultaneous Soot-NOxRemoval [J].ChemicalEngineeringJournal, 2012, 184(3): 106-112.
[3] Setiabudi A, Makkee M, Moulijn J A. An Optimal NOxAssisted Abatement of Diesel Soot in an Advanced Catalytic Filter Design [J].ApplliedCatalysisB:Environmental, 2003, 42(1): 35-45.
[4] Castoldi L, Artioli N, Matarrese R,etal. Study of DPNR Catalysts for Combined Soot Oxidation and NOxReduction [J].CatasisToday, 2010, 157(1/2/3/4): 384-389.
[5] Fritz A, Pitchon V. The Current State of Research on Automotive Lean NOxCatalysis [J].ApplliedCatalysisB:Environmental, 1997, 13(1): 1-25.
[6] Min K, Eun D P, Ji M K,etal. Manganese Oxide Catalysts for NOxReduction With NH3at Low Temperature [J].AppliedCatalysisA:General, 2007, 327(2): 261-269.
[7] Teraoka Y, Kanada K, Kagawa S. Synthesis of La-K-Mn-O Perovskite-type Oxides and Their Catalytic Property for Simultaneous Removal of NOxand Diesel Soot Particulates.ApplliedCatalysisB:Environmental, 2001, 34(1): 73-78.
[8] Fino D, Fino P, Saracco G,etal. Studies on Kinetics and Mechanism of La2-xKxCu1-yVyO4Layered Perovskites for the Combined Removal of Diesel Particulate and NOx[J].ApplliedCatalysisB:Environmental, 2003, 43(3): 243-259.
[9] Lin H, Li Y J, Shangguan W F. Soot Oxidation and NOxReduction Over BaAl2O4Catalyst [J].CombustionandFlame, 2009, 156(11): 2063-2070.
[10] Liu J, Zhao Z, Xu C M,etal. Simultaneous Removal of NOxand Diesel Soot over Nanometer Ln-Na-Cu-O Perovskite-like Complex Oxide Catalysts [J].ApplliedCatalysisB:Environmental, 2008, 78(1/2): 61-72.
[11] Li Z Q, Meng M, Li Q,etal. Fe-substituted Nanometric La0.9K0.1Co1-xFexO3-δPerovskite Catalysts Used for Soot Combustion, NOxStorage and Simultaneous Catalytic Removal of Soot and NOx[J].ChemicalEngineeringJournal, 2010, 164(1): 98-105.
[12] Wang K, Qian L, Zhang L,etal. Simultaneous Removal of NOxand Soot Particulates Over La0.7Ag0.3MnO3Perovskite Oxide Catalysts [J].CatalysisToday, 2010, 158(3/4): 423-426.
[13] Tikhomirov K, Krocher O, ElsenerM,etal. MnOx-CeO2Mixed Oxides for the Low-Temperature Oxidation of Diesel Soot [J].AppliedCatalysisB:Environmental, 2006, 64(1/2): 72-78.
[14] Saab E, Aouad S, Abi-Aad E,etal. Carbon Black Oxidation in the Presence of Al2O3, CeO2, and Mn Oxide Catalyst: an EPR Study [J].CatalysisToday, 2007, 119(1/2/3/4): 286-290.
[16] Li W B, Yang X F, Chen L F,etal. Adsorption/Desorption of NOxon MnO2/ZrO2Oxides Prepared in Reverse Microemulsions [J].CatalysisToday, 2009, 148(1/2): 75-80.
[17] Qi G, Yang R T. Performance and Kinetics Study for Low-Temperature SCR of NO with NH3over MnOx-CeO2Catalyst [J].JournalCatalysis, 2003, 217(2): 434-441.
[18] Anipsitakis G P, Dionysiou D D. Degradation of Organic of Organic Contaminants in Water with Sulfate Radicals Generated by the Conjunction of Peroxymonosulfate With Cobalt [J].EnvironmentalScience&Technology, 2003, 37(20): 4790-4797.
[19] Fernandez J, Maruthamuthu P, Renken A,etal. Bleaching and Photobleaching of Orange II Within Seconds by the Oxone/Co2+Reagent in Fenton-Like Processes [J].ApplliedCatalysisB:Environmental, 2004, 49(3): 207-215.
[20] Liang C, Huang C F, Chen Y J. Potential for Activated Persulfate Degradation of BETX Contamination [J].WaterResearch, 2008, 42(15): 4091-4100.
[21] Liang C, Lee I L, Hsu I Y,etal. Persulfate Oxidation of Trichloroethylene With and Without Iron Activation in Porous Media [J].Chemosphere, 2008, 70(3): 426-435.
[22] Hummers W S, Offeman R E. Preparation of Graphitic Oxide [J].JournaloftheAmericanChemicalSociety, 1958, 80(6): 1339-1339.
[23] He T, Chen D R, Jiao X L. Controlled Synthesis of Co3O4Nanoparticles through Oriented Aggregation [J].ChemistryofMaterials, 2004, 16(4): 737-743.
[24] Sasha S, Richard D P, SonBinh T N,etal. Synthesis and Exfoliation of Isocyanate-Treated Graphene Oxide Nanoplatelets [J].Carbon, 2006, 44(15): 3342-3347.
[25] Wang H W, Hu Z A, Chang Y Q,etal. Preparation of Reduced Graphene Oxide/Cobalt Oxide Composite and Their Enhanced Capacitive Behaviors by Homogeneous Incorporation of Reduced Graphene Oxide Sheets in Cobalt Oxide Matrix [J].MaterialsChemistryandPhysics, 2011, 30(1/2): 672-679.
[26] Ishii M, Nakahira M. Infrared Absorption Spectra and Cation Distributions in (Mn, Fe)3O4[J].SolidStateCommunications, 1972, 11(1): 209-212.
[27] Gillot B, Guendouzi M, Laarj M. Particle Size Effects on the Oxidation-reduction Behavior of Mn3O4Hausmannite [J].MaterialsChemistryandPhysics, 2001, 70(1): 54-60.
[28] Sagheer F A, Hasan M A, Pasupulety L,etal. Low-Temperature Synthesis of Hausmannite Mn3O4[J].JournalofMaterialsScienceLetters, 1999, 18(3): 209-211.
[29] Nam I, Kim N D, Kim G P,etal. One Step Preparation of Mn3O4/Graphene Composites for Use as an Anode in Li ion Batteries [J].JournalofPowerSources, 2013, 244: 56-62.
[30] Askarinejad A, Bagherzadeh M, Morsali A.Catalysis Performance of Mn3O4and Co3O4Nanocrystals Prepared by Sonochemical Method in Exopxidation of Styrene and Cyclooctene [J].AppliedSurfaceScience, 2010, 256(22): 6678-6682.
[31] Dresselhaus M S, Dresselhaus G, Jorio A. Raman Spectroscopy of Carbon Nanotubes in 1997 and 2007 [J].TheJournalofPhysicalChemistryC, 2007, 111(48): 17887-17893.
[32] Zhang W X, Cui J C, Tao C A,etal. A Strategy for Producing Pure Single-Layer Graphene Sheets Based on a Confined Self-assembly Approach [J].AngewandteChemieInternationalEdition, 2009, 48(32): 5864-5868.
[33] Shi P H, Su R J, Zhu S B,etal. Supported Manganese Oxide on Graphene Oxide: Highly Efficient Catalysts for the Removal of Orange II from Water [J].JournalofHazardousMaterials, 2012, 229/230: 331-339.
[34] Chen W, Fan Z L, Gu L,etal. Enhanced Capacitance of Manganese Oxide via Confinement Inside Carbon Nanotubes[J].Communication, 2010, 46(22): 3905-3907.
[35] Buciuman F, Patcas F, Cracium R,etal. Vibrational Spectroscopy of Bulk and Supported Manganese Oxides [J].PhysicalChemistryChemicalPhyssics, 1999, 1(1): 185-120.
[36] Kapteijn F, Van Langeveld A D, Moulijin J A,etal. Alumina-Supported Manganese Oxide Catalysts: I. Characterizatio: Effect of Precursor and Loading [J].JournalofCatalysis, 1994, 150(60): 94.
[37] Eklund P C, Holden J M, Jishi R A. Vibrational Modes of Carbon Nanotubes-Spectroscopy and Theory [J].Carbon, 1995, 33(7): 959-972.
[38] Zhang H B, Lin G D, Zhou Z H,etal. Raman Spectra of MWCNTs and MWCNT-Based H2-Adsorbing System [J].Carbon, 2002, 40(13): 2429-2436.
[39] Huang Y F, Huang Y H. Identification of Produced Powerful Radicals Involved in the Mineralization of Bisphenol A Using a Novel UV-Na2S2O8/H2O2-Fe (II, III) Two-Stage Oxidation Process [J].JournalofHazardousMaterials, 2009, 162(2/3): 1211-1266.
[40] Stankovich S, Dikin D A, Piner R D,etal. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide [J].Carbon, 2007, 24(7): 1558-1656.
[41] Ardizzone S, Bianchi C L, Tirelli D. Mn3O4andγ-MnOOH Powders, Preparation, Phase Composition and XPS Characterization [J].ColloidsandSurfaces, 1998, 134(3): 305-312.
[42] Huang Y F, Huang Y H. Behavioral Evidence of the Dominant Radicals and Intermediates Involved in Bisphenol A Degradation Using an Efficient Co2+/PMS Oxidation Process [J].JournalofHazardousMaterials, 2009, 167(1/2/3): 418-426.
[43] Schward H A. Schward.An Introduction to Radiation Chemistry [J].JournaloftheAmericanChemicalSociety, 1964, 86(19): 4227-4228.
Foundation items: Education Innovation Project of Shanghai, China (No. 12ZZ069); Natural Science Foundation of Shanghai, China (No.11ZR1400400); Doctoral Fund of Ministry of Education of China (No. 20130075110006); Modification Fiber Materials Project of the National Key Laboratory of China (No. LK1203)
X701.7 Document code: A
1672-5220(2015)01-0103-06
Received date: 2013-11-16
* Correspondence should be addressed to LI Deng-xin, E-mail: lidengxin@dhu.edu.cn
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Mechanical Property and Crystal Structure of Poly(p-phenylene terephthalamide) (PPTA) Fibers during Heat Treatment under Tension
- Diatomite Precoated Nonwoven Membrane Bioreactor for Domestic Wastewater Reclamation
- Preparation and Properties of Polylactic Acid (PLA)/Nano-SiO2 Composite Master Batch with Good Thermal Properties
- Scheduling Rules Based on Gene Expression Programming for Resource-Constrained Project Scheduling Problem
- Dynamic Analysis of Some Impulsive Fractional-Order Neural Network with Mixed Delay
- Software Maintainability Evaluation Based on Quantitive Model
