Preparation and Properties of Polylactic Acid (PLA)/Nano-SiO2 Composite Master Batch with Good Thermal Properties
2015-01-12LIUShuqiang刘淑强WUGaihong吴改红GUOHongxia郭红霞ZUOZhong左中鹅DAIJinming戴晋明
LIU Shu-qiang (刘淑强), WU Gai-hong(吴改红), GUO Hong-xia(郭红霞), ZUO Zhong-e(左中鹅), DAI Jin-ming (戴晋明)
1 College of Textile Engineering, Taiyuan University of Technology, Taiyuan 030021, China2 Fashion Institute, Donghua University, Shanghai 200051, China
Preparation and Properties of Polylactic Acid (PLA)/Nano-SiO2Composite Master Batch with Good Thermal Properties
LIU Shu-qiang (刘淑强)1*, WU Gai-hong(吴改红)1, 2, GUO Hong-xia(郭红霞)1, ZUO Zhong-e(左中鹅)1, DAI Jin-ming (戴晋明)1
1CollegeofTextileEngineering,TaiyuanUniversityofTechnology,Taiyuan030021,China2FashionInstitute,DonghuaUniversity,Shanghai200051,China
In order to improve the thermal properties of polylactic acid (PLA) master batch, the nano-SiO2was applied to mixing with PLA. The structure and thermal properties of the composite master batches were studied. The results showed that the nano-SiO2modified by 3% coupling agent KH-570 could be dispersed evenly in PLA in small scale. The thermal decomposition temperature of composite master batches increased by 6.20-10.80℃, the glass transition temperature increased by 0.22-5.16℃, and the heat enthalpy at the glass transition temperature increased by 0.574-2.437 J/g, compared with pure PLA. The composite master batch possessed superior thermal stability and heat resistance.
compositemasterbatch;nano-SiO2;polylacticacid(PLA);thermalproperty
Introduction
Polylactic acid (PLA) possesses some excellent performances, including biocompatibility, biodegradability, biological absorbability and so on[1]. The raw material sources of PLA are renewable grains, such as corn, wheat and rice. Therefore, PLA is different from other traditional synthetic materials, such as polyester (PET) and nylon, which depend very heavily on petroleum, natural gas and natural gas liquids as sources of raw materials[2]. Moreover, the PLA material in water or soil can be completely decomposed into water (H2O) and carbon dioxide (CO2), which causes no pollution. Thence PLA is a kind of environment-friendly materials, and is different from other traditional synthetic materials which are degraded difficultly and cause many environmental pollutions[3].
PLA is often used as surgical sutures, scaffolds, drug slow release and tissue culture. But PLA exists some shortcomings, such as high brittleness and poor thermal property especially[4]. The pure PLA and its products are able to be used at room temperature. However, once the temperature rises over the PLA’s glass transition temperature (commonly 57℃), the PLA and its products would become easy to distort, wrinkle or tear, and the mechanical properties would fall sharply[5]. This is due to PLA’s poor thermal properties, so it is particularly important to improve the thermal properties of PLA.
In order to improve the thermal properties of PLA, some scholars mixed PLA with other heat-resistant polymers. For instance, Chenetal.[6]mixed PET, which was grafted with long-chain carboxylic acid and had good heat-resistance, with PLA to make composites with higher heat-resistance. Mamun and Bledzki[7]applied polypropylene (PP) to enhance the thermal properties of PLA. However, the mixed polymers with higher heat-resistance are usually non-degradable so that they would affect the degradation of PLA. Some heat-resistant inorganic nanoparticles were usually used as a new method to modify PLA in recent years[8]. For instance, the montmorillonite, rare earths nanoparticles, nanometer calcium carbonate and hydroxyl apatite were frequently reported to modify PLA[9]. The interface treatment between inorganic material and PLA matrix is very important, which is due to that the interface between them affects the dispersion of inorganic material in PLA matrix, the mechanical properties of composites, and other properties. The nano-SiO2was relatively less reported to enhance the thermal properties of PLA. The nano-SiO2is a kind of white, non-toxic and amorphous powder with micro porous morphology, lightweight, good chemical stability, high temperature resistance, non-flammability, and about 1750 ℃ melting point[10].
In this article, the PLA is mixed with nano-SiO2to form composite master batch. In order to improve the dispersibility of nano-SiO2throughout PLA and the interfacial bonding between nano-SiO2and PLA, the silane coupling agent KH-570 was applied to modifying nano-SiO2. The structure and thermal properties of PLA/nano-SiO2composite master batches were measured, and the effects of nano-SiO2on properties of composite master batch were studied. This provided a theoretical basis for preparing PLA/nano-SiO2composites with good thermal properties.
1 Experimental
1.1 Materials
PLA (6202D) was kindly supplied by Nature Works Industry (U.S.). The number average molecular weight (Mn) of PLA was 51000.
Nano-SiO2with particle size of 20-180 nm was provided by Fengcheng Chemical Industry, Tianjin, China.
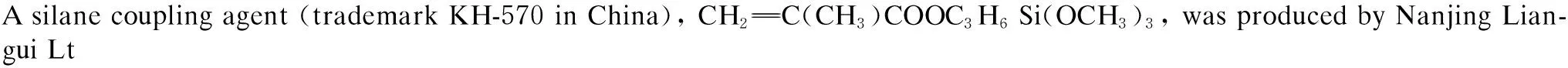
1.2 Surface modification of nano-SiO2
In order to improve the dispersion of nano-SiO2in PLA and increase their compatibility, the nano-SiO2should be modified by the coupling agent KH-570.
The process of modification was as follows: 0.04-0.20 g KH-570 and 15 mL anhydrous ethanol were put into a beaker together, and then 0.1 mol/L HCl was added into the beaker to adjust pH value to 5-6. After that, the beaker was heated in water bath at 70℃ for 30 min, and then 2 g nano-SiO2was added to the solution. The mixtures were shocked by using sonication at 30℃ and 30 kHz for 30 min. After that, the mixed solution was dried under 120 ℃ for 8 h by using the drum (101-2A,Beijing Zhongxing Weiye Instrument Co., Ltd., China). Then the dried mass was crushed and grinded into fine particles by using ball mill (QM-1SP, Nanjing Daxue Instrument Company, China).
1.3 Processing of PLA/nano-SiO2composite master batch
Before melt-blending, the pure PLA was dried at 100 ℃ for 20 h under vacuum. Then the dried pure PLA and 3% treated nano-SiO2were fed into the double screw extruder (CET35-40D, Coperion (Nanjing) Machine Co., Ltd., China) during main feed opening (Fig.1a) and auxiliary feed opening (Fig.1b). The temperatures of ten heating zones (Fig.1d) in extruder were set in Table 1. The melt was extruded from extruder into a cool water bath (Fig.1f) to be solidified. At last, the solid band (Fig.1g) was cut as small chips (Φ: 3.175 mm, long: 3.175 mm) by a chip cutter (Fig.1h, LQ, Coperion (Nanjing) Machine Co., Ltd., China). The cut chips were PLA/nano-SiO2master batches.

a—main feed opening; b—auxiliary feed opening; c—single-screw; d—heating zones; e—connecting with vacuum pump; f—a cool water bath; g—golid band; h—chip cutter; i—basket; j—composite master batchFig.1 PLA/nano-SiO2 composite master batch processing
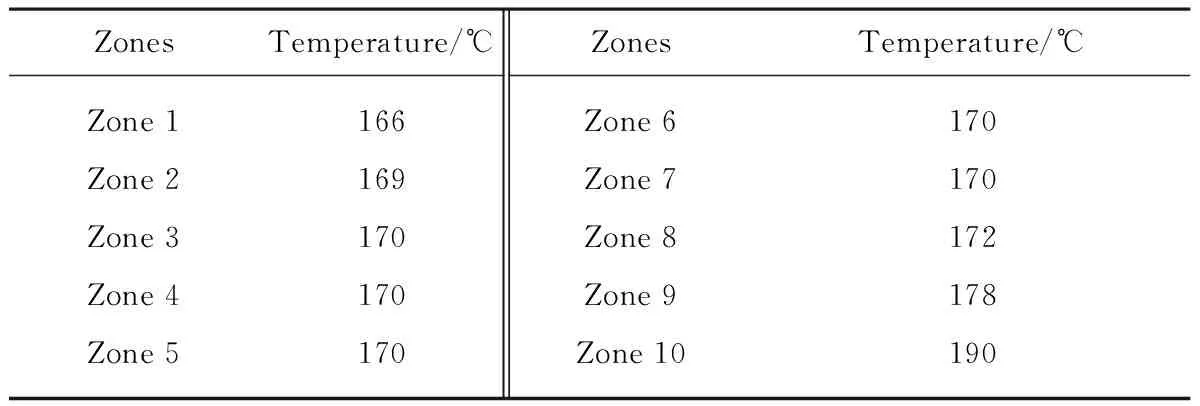
Table 1 Temperatures of ten heating zones in extruder
1.4 Scanning electron microscopy (SEM)
The dispersion of nano-SiO2and the fracture surfaces of pure PLA and PLA/nano-SiO2composite master batches, were studied by a JEOL JSM-6700F SEM under an acceleration voltage of 10 kV. Prior to the SEM examination, the pure PLA and PLA/nano-SiO2composite master batches were submerged in liquid nitrogen and broken to expose the internal structure for SEM studies. And all the surfaces were sputtered with gold.
1.5 Thermogravimetric analysis (TGA)
Thermogravimetric analysis (TGA, Germany Netzsch TG 209 F3) was performed on pure PLA and PLA/nano-SiO2composite samples as follows: 2.5 mg weight samples, nitrogen flow (600 mL/min), temperature range from 40 to 700 ℃, 10℃/min heating rate.
1.6 Differential scanning calorimetry (DSC)
The thermal properties of samples (6-9 mg) were measured by a Q100 V9.4 Build 287 DSC (TA Instruments Company, USA) using aluminium oxide as the standard. The melting point (Tm) and glass-transition temperature (Tg) of each sample were measured from 10 to 210℃ under nitrogen at a heating rate of 10℃/min.
2 Results and Discussion
2.1 Effect of KH-570 dosage on dispersion of nano-SiO2
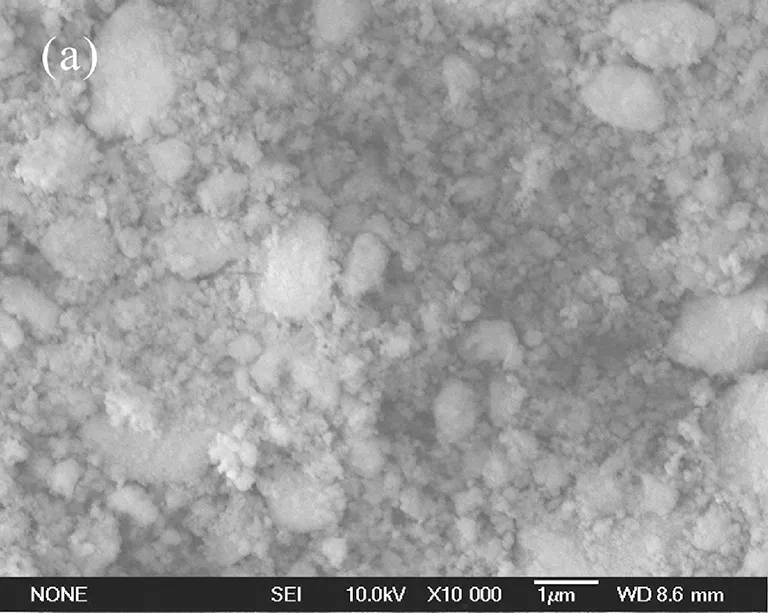
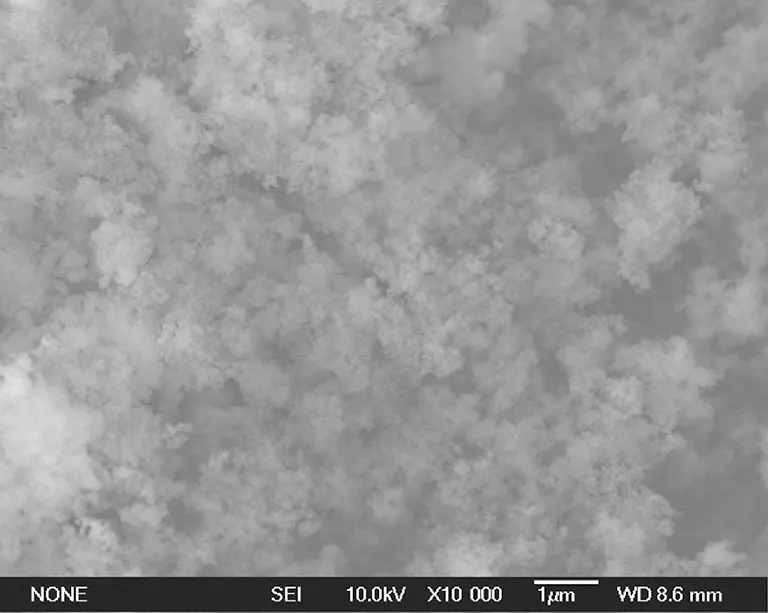
The morphological characterizations of unmodified and modified nano-SiO2were measured. The SEM images of nano-SiO2are shown in Fig.2.
(a)
(b)

(c)Fig.2 SEM images (×10000) of nano-SiO2: (a) unmodified; (b) modified by 3% KH-570; (c) modified by 5% KH-570
Figure 2(a) is the image of unmodified nano-SiO2, which showed that the nano-SiO2was agglomerated as big mass. Figure 2(b) is the image of nano-SiO2modified by 3% KH-570, which showed that the nano-SiO2was dispersed evenly and loosely in nano-scale. Figure 2(c) is the image of nano-SiO2modified by 5% KH-570, which shows that the nano-SiO2was agglomerated as big mass, even bigger than that of unmodified nano-SiO2(Fig.2(a)).
The unmodified nano-SiO2was covered with many hydroxyl groups (—OH) as shown in Fig.3. The hydroxyl groups (—OH) between SiO2nanoparticles could form hydrogen bonds. Then the SiO2nanoparticles were linked by hydrogen bonds. So the unmodified nano-SiO2was easy to be agglomerated as big mass. However, if the SiO2nanoparticles were modified by 3% coupling agent KH-570, some hydroxyl groups on its surface would be replaced by organic chain of coupling agent KH-570, and the number of hydroxyl groups (—OH) on surface of nano-SiO2would decrease[11]. So the agglomerating of nano-SiO2which was caused by hydrogen bonds (—OH) would weaken. Therefore, the nano-SiO2modified by 3% KH-570 could disperse evenly and loosely in nano-scale. Moreover, if the nano-SiO2was modified by excessive coupling agent KH-570 (i.e., 5%), there was too much KH-570 between SiO2nanoparticles, and the long-chained KH-570 could link nano-SiO2particles like some bridges between nano-SiO2particles. So the excessive KH-570 (i.e., 5%) would lead to agglomerate mass of nano-SiO2.
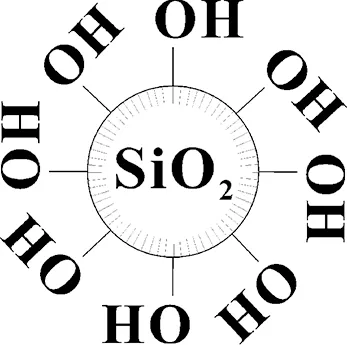
Fig.3 Diagram of nano-SiO2 covered with hydroxyl groups
2.2 Effect of KH-570 dosage on dispersion of nano-SiO2in composite master batch
The morphological characterizations of pure PLA and PLA/nano-SiO2composite master batches are shown in Fig.4. Figure 4(a) shows a plane fracture section of pure PLA. Figure 4(b) is the image of fracture section of PLA/unmodified nano-SiO2composite master batch, which showed that the unmodified nano-SiO2were unevenly distributed in the PLA matrix, and in some areas, some nano-SiO2particles (circles) were agglomerated in large scale. This indicated that the nano-SiO2without modifying were easy to be agglomerated in the PLA matrix. Figure 4(c) is the image of fracture section of PLA/3% nano-SiO2modified by 3% KH-570, which shows a uniform dispersion of nano-SiO2in the PLA matrix. Moreover, nano-SiO2still remained small scale (most of them are less than 1m in diameter) in the PLA polymer and was not separated from PLA matrix. This declared that the right amount of KH-570 (i.e., 3%) to modify nano-SiO2was beneficial to the dispersability of nano-SiO2in the PLA matrix and the link between nano-SiO2and PLA. Figure 4(d), the image of fracture section of PLA/3% nano-SiO2modified by 5% KH-570, showed that SiO2was agglomerated as bigger particles (circles) over 1.0 μm embedding in PLA. This image declared that too much KH-570 (i.e., 5%) to modify nano-SiO2, in fact, would lead to bigger agglomerations.
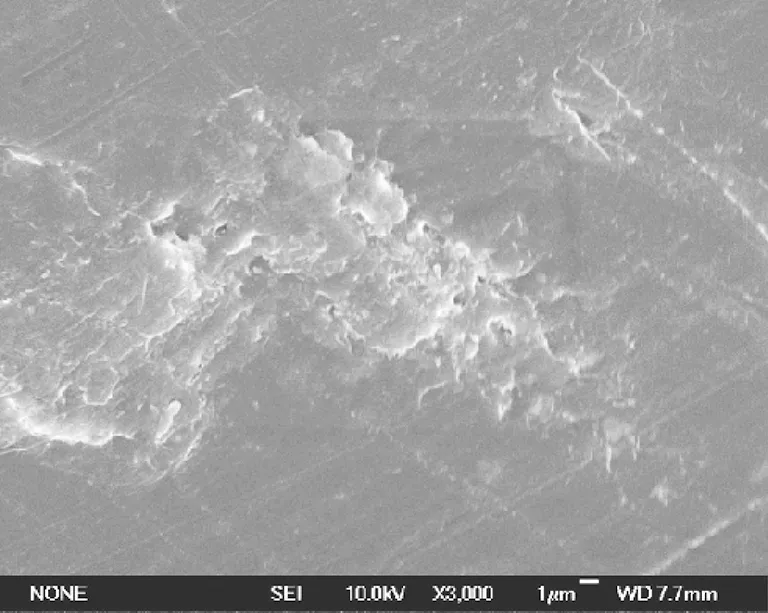
(a)

(b)

(c)

(d)
The KH-570 silane coupling agent has two functions in the master batch of PLA/nano-SiO2composite. One is that they could improve the dispersion of nano-SiO2, for details see section 2.1 above. The other is that they could connect nano-SiO2with the PLA matrix. The molecular structure of KH-570 silane coupling agent could be abbreviated as RnSiX3, in which R is an organic functional group which can form a chemical bond with the PLA polymer, and X is an easily hydrolyzable group which can react with the hydroxyl groups (—OH) on the surface of nano-SiO2and then connect KH-570 with nano-SiO2[12]. So the KH-570 silane coupling agent, as a bridge, connects the PLA polymer with nano-SiO2together. The process of connecting is shown in Fig.5.
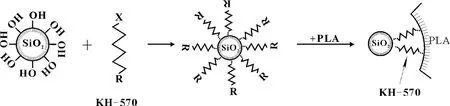
Fig.5 The process of connecting nano-SiO2 with PLA by KH-570
2.3 Dispersion of nano-SiO2in composite master batch
The morphological characterizations of PLA/nano-SiO2composite master batch with different nano-SiO2dosages are shown in Fig.6. From Figs.6(a)-(d), the nano-SiO2dosages are 1%, 3%, 5% and 10%.
Fig.6 SEM images (×3000) of PLA/nano-SiO2composite master batches with different nano-SiO2dosages: (a) PLA/1% nano-SiO2;
(b) PLA/3% nano-SiO2; (c) PLA/5% nano-SiO2; (d) PLA/10% nano-SiO2
Figure 6 shows that 1%-5% nano-SiO2, can distribute evenly in small scale throughout the PLA matrix, but 10% nano-SiO2formed some dense band (circles). These declare that when the PLA matrix includes less nano-SiO2(generally lower than 5%), the nano-SiO2, modified by appropriate amount of KH-570 silane coupling agent, can distribute evenly throughout the PLA matrix, so the KH-570 is effective. When the PLA matrix includes too much nano-SiO2(e.g., 10%), although the nano-SiO2is modified by KH-570 silane coupling agent, the nano-SiO2is still agglomerated as some dense band, and then the KH-570 is ineffective. Because the nano-SiO2nanoparticles have large surface energy, and the dosage of nano-SiO2increases, the density of nano-SiO2particles enhances, and the chances for nano-SiO2particles to contact with each other also increase, so they tend to get together. On the contrary, the KH-570 gives the nano-SiO2a tendency of separating from each other. When less nano-SiO2in PLA, the tendency of separating from each other occupied the main position, and when too much nano-SiO2in PLA, the tendency of getting together occupied the main position.
2.4 Thermal characterization of composite master batches
PLA can be thermal-decomposed easily at high temperature, due to its poor thermal stability. The thermo-decomposing temperature is a parameter of evaluating material’s thermal stability.
The TGA curves of pure PLA and composite master batches including different nano-SiO2are shown in Fig.7. The results show that the pure PLA and composite master batches lost weight mainly at 300-400℃. The four curves all have only one knee, which means that the PLA samples were all broken down in just one step. According to the four curves, the thermo-decomposing temperatures, which means the maximum slope of curve, are measured and shown in Table 2. The thermo-decomposing temperature of PLA composited with 1% to 5% nano-SiO2was higher than that of pure PLA by 6.20℃ to 10.80℃. This results declare that the added nano-SiO2can improve the thermo-decomposing temperature of composite master batches, and the more nano-SiO2, the higher thermo-decomposing temperature. For this there were many causes. The nano-SiO2itself was a kind of heat resistant material whose melting point was as high as 1750 ℃. Also its heterogeneous-nucleation-effect increased the crystallinity degree of PLA matrix. The bonds of “Si—O—Si” and “Si—O—C” also formed when nano-SiO2was reacted with PLA, and the energy of “Si—O” bond formed in composites was higher than that of “C—C” bond in pure PLA. All above were helpful to the thermal stability of composite master batches. Therefore, the thermo-decomposing temperature of composite master batches was higher than that of pure PLA.

Fig.7 TGA curves of pure PLA and composite master batches
The DSC heating curves of pure PLA and composite master batches are shown in Fig.8. According to these DSC curves, the glass transition temperature (Tg), melting temperature (Tm) and heat enthalpy are measured and shown in Table 2. The results indicated that the glass transition temperature (Tg) of composite master batches with 1%-5% nano-SiO2is higher than that of pure PLA by 0.22-5.16℃, and the melting temperature (Tm) of composite master batches with 1%-5% nano-SiO2are higher than that of pure PLA by 0.83-2.01℃, and the heat enthalpy atTgof composite master batches is higher than that of pure PLA by 0.574-2.437 J/g. Moreover, the more nano-SiO2added in PLA matrix, the higherTg,Tmand heat enthalpy atTg. It was due to the increase of crystallinity-degree of PLA caused by nano-SiO2, and the more nano-SiO2, the higher crystallinity-degree of composites, then the higherTg,Tmand heat enthalpy atTg. Moreover, the higher heat enthalpy atTgindicated that it needed more heat to soften the composites. All above results show that the nano-SiO2could improve the heat resistance of PLA.
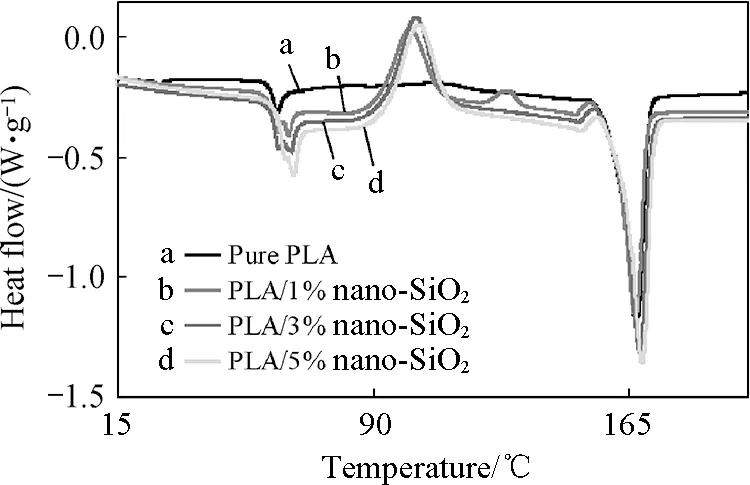
Fig.8 DSC curves of pure PLA and composite master batches
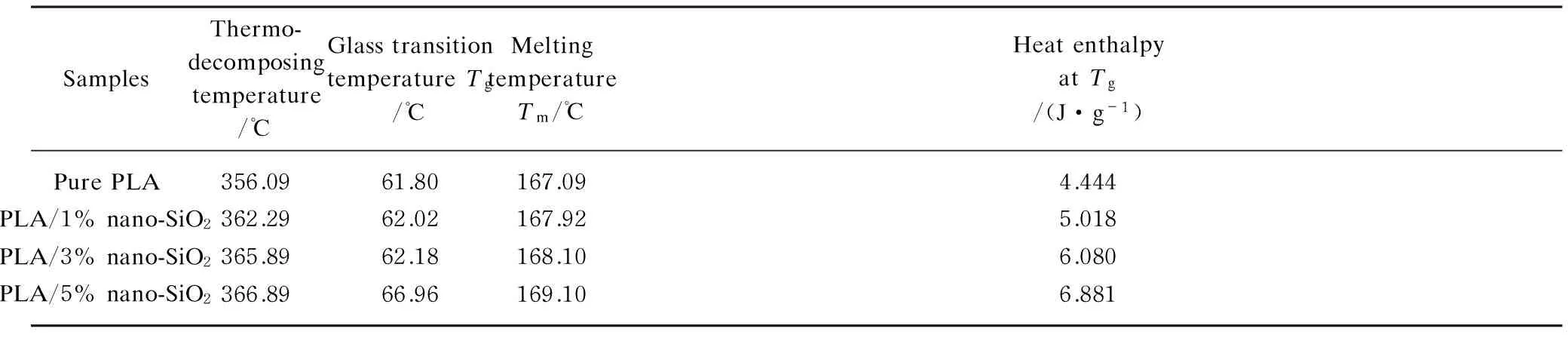
Table 2 Parameters of TGA and DSC curves of pure PLA and composite master batches
4 Conclusions
The nano-SiO2modified by 3% KH-570 were evenly distributed in the PLA matrix in small scale, and the nano-SiO2were not separated from the PLA matrix. The nano-SiO2unmodified or modified by too much KH-570 (i.e., 5%) were agglomerated as bigger particles over 1.0 μm embedding in PLA.
When the PLA matrix included less nano-SiO2(generally lower than 5%), the nano-SiO2could distribute evenly throughout the PLA matrix. However, when the PLA matrix included too much nano-SiO2(e.g., 10%), the nano-SiO2was agglomerated as some dense band.
The thermo-decomposing temperature of PLA composited with 1% to 5% nano-SiO2was higher than that of pure PLA by 6.20-10.80℃. The added nano-SiO2could improve the thermo-decomposing temperature of batches, and the more nano-SiO2, the higher thermo-decomposing temperature.
The glass transition temperature (Tg) of composites with 1%-5% nano-SiO2increased by 0.22-5.16℃, and the melting temperature (Tm) increased by 0.83-2.01℃, and the heat enthalpy atTgincreased by 0.574-2.437 J/g. Moreover, the more nano-SiO2added in the PLA matrix, the higherTg,Tmand heat enthalpy atTg. So the nano-SiO2could improve the heat resistance of PLA.
[1] Tsuji H, Kidokoro Y, Mochizuki M. Enzymatic Degradation of Poly (L-lactic acid) Fibers: Effects of Small Drawing [J].JournalofAppliedPolymerScience, 2007, 103(3): 2064-2071.
[2] Chen X Q, Lu R H, Meng D,etal. Preparation and Characterization of Magnetic Star-Shaped Amphiphilic Copolymer Nanoparticles of S-Fe3O4-PLA-b-MPEG [J].PolymerComposites, 2012, 33(12): 2134-2139.
[3] Zhao F W, Liu Y, Yuan H L,etal. Orthogonal Design Study on Factors Affecting the Degradation of Polylactic Acid Fibers of Melt Electrospinning [J].JournalofAppliedPolymerScience, 2012, 125(4): 2652-2658.
[4] Kontou E, Georgiopoulos P, Niaounakis M. The Role of Nanofillers on the Degradation Behavior of Polylactic Acid [J].PolymerComposites, 2012, 33(2): 282-294.
[5] Oi T, Shinyama K, Fujita S. Electrical Properties of Heat-Treated Polylactic Acid [J].ElectricalEngineeringinJapan, 2012, 180(1): 1-8.
[6] Chen H P, Pyda M, Cebe P. Non-isothermal Crystallization of PET/PLA Blends [J].ThermochimicaActa, 2009, 492(1/2): 61-66.
[7] Mamun A A, Bledzki A K. Micro Fibre Reinforced PLA and PP Composites: Enzyme Modification, Mechanical and Thermal Properties [J].CompositesScienceandTechnology, 2013, 78: 10-17.
[8] Farhoodi M, Dadashi S, Mousavi S M A,etal. Influence of TiO2Nanoparticle Filler on the Properties of PET and PLA Nanocomposites [J].Polymer-Korea, 2012, 36(6): 745-755.
[9] Murariu M, da Silva Ferreira A, Phuta M,etal. Polylactide (PLA)-CaSO4Composites Toughened with Low Molecular Weight and Polymeric Ester-like-Plasticizers and Related Performances [J].EuropeanPolymerJournal, 2008, 44(11): 3842-3852.
[10] Shang Q Q, Wang M Y, Liu H,etal. Facile Fabrication of Superhydrophobic Raspberry-like SiO2/Polystyrene Composite Particles [J].PolymerComposites, 2013, 34(1): 51-57.
[11] Wu G H, Liu S Q, Guo H X,etal. Surface Modification of Nano-SiO2and Application in the Poly lactic Acid (PLA) [J].BulletinoftheChineseCeramicSociety, 2014, 33(3): 506-510. (in Chinese)
[12] Zhang Y H, Zhai L L, Wang Y,etal. Surface Modification of Nano-SiO2by Silane Coupling Agent 3-(methacryloyloxy) Propyltrimethoxysilane [J].JournalofMaterialsScience&Engineering, 2012, 30(5): 752-756. (in Chinese)
Foundation items: Shanxi Province Science Foundation for Youths, China (No.2014021020-2); the Projects of Taiyuan University of Technology, China (Nos. 2012L074, 2013T020, 2013T021, and 2013T022); Shanxi Province College Students Training Program, China (No. 2013067)
TQ342+.8 Document code: A
1672-5220(2015)01-0097-06
Received date: 2014-03-21
* Correspondence should be addressed to LIU Shu-qiang, E-mail: liushuqiang8866@126.com
猜你喜欢
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Mechanical Property and Crystal Structure of Poly(p-phenylene terephthalamide) (PPTA) Fibers during Heat Treatment under Tension
- Diatomite Precoated Nonwoven Membrane Bioreactor for Domestic Wastewater Reclamation
- Supported Manganese Oxide on Graphite Oxide: Catalytic Oxidation of Nitrogen Oxide in Waste Gas
- Scheduling Rules Based on Gene Expression Programming for Resource-Constrained Project Scheduling Problem
- Dynamic Analysis of Some Impulsive Fractional-Order Neural Network with Mixed Delay
- Software Maintainability Evaluation Based on Quantitive Model
