Water Repellent Finishing on Cotton Fabric via Atom Transfer Radical Polymerization
2015-01-12LIShiwei李时伟XINGTieling邢铁玲LIZhanxiong李战雄CHENGuoqiang陈国强
LI Shi-wei (李时伟), XING Tie-ling (邢铁玲), 2, LI Zhan-xiong (李战雄), CHEN Guo-qiang (陈国强)*
1 National Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, China 2 Xinyuan Cocoon and Silk Group Co., Ltd., Hai’an 226600, China
Water Repellent Finishing on Cotton Fabric via Atom Transfer Radical Polymerization
LI Shi-wei (李时伟)1, XING Tie-ling (邢铁玲)1, 2, LI Zhan-xiong (李战雄)1, CHEN Guo-qiang (陈国强)1*
1NationalEngineeringLaboratoryforModernSilk,CollegeofTextileandClothingEngineering,SoochowUniversity,Suzhou215123,China2XinyuanCocoonandSilkGroupCo.,Ltd.,Hai’an226600,China
In order to enhance the water repellence property of cotton fabric, cotton fabric was grafted using hexafluorobutyl methacrylate (HFMT) monomer via atom transfer radical polymerization (ATRP) method. Water repellent cotton fabric was successfully prepared, and characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR), and X-ray photoelectron spectroscopy (XPS). The SEM images of the HFMT-treated cotton displayed significant difference from the untreated one. FT-IR characterization of the HFMT-treated cotton indicated that HFMT was successfully grafted onto the surface of the cotton fabric. XPS analysis indicated that the fluorine element of the HFMT-treated cotton existing on the surface of the cotton fabric. The surface contact angle test as well as the water repellence rating test showed that the water repellence of the HFMT-treated cotton fabric was much better than that of the untreated cotton fabric. The surface contact angle of the HFMT-treated cotton fabric could reach (132.4 ± 2.2)°, and the water repellence rating could achieve grade 3. The washing durability of the HFMT-treated fabric was also investigated. The surface contact angle of the HFMT-treated cotton fabric could reach (121.1 ± 2.1)° after 20 washing times. Furthermore, the whiteness, air permeability, breaking strength, and breaking elongation of the HFMT-treated cotton fabric decreased slightly compared with the untreated cotton fabric. Finally, cotton fabric with good water repellence property and excellent washing durability could be obtained with little effect on the intrinsic properties of cotton fabric.
cottonfabric;structureproperties;atomtransferradicalpolymerization(ATRP);hexafluorobutylmethacrylate(HFMT);waterrepellence
Introduction
Cotton, one of the most abundant organic natural materials in the world, has been widely studied because of its biodegradable and renewable nature. It also exhibits very good physical and chemical properties. Although cotton has large-scale application in many fields, it has bad water repellence property and resistance to abrasion, which limits the extensive utilization of cotton in other areas. Therefore, different functional groups grafted onto the surface of cotton have been largely investigated for extending application areas of the traditional material[1-4].
The fluorine atom owns some advantages, such as low electronegativity, small diameter, and high C—F bond energy. Many properties of materials are dependent on the surface structure and the chemical composition of the outermost surface layer. When the surface is uniformly covered with fluorine containing finishing agent, the surface tension of the materials can significantly be reduced for formulating non-wettable surfaces[5]. Surface tension reduction has been reported to be attained by copolymers of fluorinated monomers or by chemically grafting perfluoroalkyl groups to polymers[6-7]. In contrast to organic silicone and hydrocarbon finishing agent, fluorine containing finishing agent has the characteristics of low concentration and high effectiveness, which can endow the treated fabric with good handle, air permeability, and moisture permeability. Therefore, fluorine containing finishing agent is widely applied in textiles, leather, paper, and other substrates[8-9].
There are many methods for incorporation of fluorine containing finishing agent into a substrate, including plasma treatment, grafting reaction, and living polymerization. The structure of the substrate greatly influences its surface property; accordingly, it is very important to control the distribution of the polymer introducing to the substrate surface[10]. Living/controlled radical polymerization (CRP) has attracted much interest due to its great significance in academics and potential applications in industry. CRP techniques can provide a high degree of control over the functionality, grafting density, and thickness of the grafted polymer brush. The CRP approaches mainly include nitroxide-mediated radical polymerization (NMP), reversible addition-fragmentation chain transfer (RAFT) polymerization, atom transfer radical polymerization (ATRP), and single-electron transfer living radical polymerization (SET-LRP)[11-13]. The CRP technique was recently employed to synthesize well-defined degradable polymers by the incorporation of hydrolytically, photochemically, or electrochemically labile chemical bonds into the polymer backbone[14-15]. As one of the most important CRP methods, ATRP was often catalyzed by a Cu II/Cu I system in which the success of this process relied on a rapid and reversible activation/deactivation step[16-19]. ATRP has been widely studied owing to its advantages such as controllable polymer molecular weight, narrow molecular weight distribution, adaptability to most monomers, designable molecular structures, and commercially available ATRP initiators[20]. Moreover, ATRP approach can tolerate many functional groups; thus, using this method, some functionalized molecules have been designed and prepared[21]. ATRP has been successfully used to prepare various co-polymers with any essentially desired complex macromolecular structure, including polymers with controlled topology, ranging from linear chains with precisely controlled dimensions and controlled dispersity to various branched structures being grafted onto functional backbones or grafted from multifunctional backbones[22-23]. Recent studies show that ATRP can be used advantageously to graft polymers onto various substrates, such as gold, silica, silicon, and porous substrates[24]. For example, introducing fluorine-containing substituents into a silica/polymer could result in dramatic changes in the properties of the material with low surface energy and good chemical/thermal stability[25]. ATRP is a feasible technique for cellulose grafting since the available hydroxyl groups on the surface can easily be converted to α-bromoesters, which are known to be excellent initiators for ATRP[26]. By grafting various fluorine containing polymers onto the cellulose surface via ATRP, the hydrophobicity can be enhanced.
In the present work, hexafluorobutyl methacrylate (HFMT) monomer was grafted onto the surface of cotton fabric through ATRP in aqueous solution. The structure and properties of the HFMT-treated cotton fabric were analyzed by different techniques.
1 Experimental
1.1 Materials and reagents
Desized cotton fabric (twill weave, 138 g/m2) was purchased from Suzhou Kaisheng Silk Dress Company, China. HFMT was purchased from Harbin Xeogia Fluorine-Silicon Chemical Company, China. Triethylamine (TEA) and tetrahydrofuran (THF) were dehydrated by CaH2overnight, and then distilled under reduced pressure before use. CuBr, N, N, N′, N″, N″-pentamethyldiethylene triamine (PMDETA), and 4-(dimethylamino) pyridine (DMAP) were used as received. The 2-bromoisobutyryl bromide (BriB-Br) (98%, Alfa Aesar) and all other reagents were used without further purification.
1.2 Grafting procedure
The cotton fabric (1 g) was cleaned with certain amount of THF, and dried in a vacuum drying oven for 2 h at 80 ℃. Then cotton fabric was placed in the dryer to cool down for 30 min. Cotton macroinitiator (C-Br) was prepared by reacting BriB-Br with the hydroxyl groups presented on cotton in the presence of TEA, and DMAP catalysis at 30 ℃. Typically, 50 mL dehydrated THF, 2.46 mL TEA, and 0.5 g DMAP were mixed in a 100 mL round bottom flask under oscillating. The flask was cooled down to 10 ℃ and 1.11 mL of BriB-Br was added into the flask. And then 1 g of dried cotton fabric was added into the mixed solution. The reaction mixture was stirred at 10 ℃ for 1 h, then left to be warmed up to 30 ℃ and reacted for another 24 h. The cotton sample was thereafter thoroughly washed with THF, then with water, and finally dried at 80 ℃ in a vacuum oven. Thus the C-Br was prepared. The emulsifier fluorocarbon surfactant FSO and Tween 60 (the dosages of FSO and Tween 60 were 20% and 30%, respectively, both of which were ratios of mass to HFMT) were dissolved in a certain amount of water and stirred with the emulsifying machine for 1 min, and then the required fluorinated monomers were added by dropping. The grafting was accomplished by immersing the C-Br into the reaction mixture containing certain amount of emulsified fluorinated monomer, CuBr/PMDETA and deionized water in a 100 mL round-bottom flask. After sealing it with a three-way stopcock, the flask was evacuated and back-filled with nitrogen, which was repeated three times. The mixture was placed in a water bath and polymerized under oscillating at certain temperature for some time. After the polymerization was completed, the sample was subjected to intense washing in hydrochloric acid, and was extracted with acetone to reach constant weight by Soxhlet extraction method for 4 h. Then the sample was washed with water and dried under a vacuum oven. Thus the HFMT-treated cotton sample was obtained. The grafting condition was as follows: HFMT pH = 8; mole ration(PMDETA)∶n(CuBr)=2∶1; reaction time 120 min; reaction temperature 60, 70, and 80 ℃; monomer concentration 150% (o.w.f), and the concentration of catalyst CuBr 0.32 mmol/L.
1.3 Characterization and measurements
The graft yield(G) was calculated as
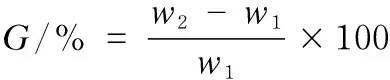
wherew1andw2denote the weights of C-Br and HFMT-treated cotton, respectively.
The surfaces of the treated and untreated samples were examined, after gold coating, with a Hitachi S-4800 scanning electron microscopy (SEM) at an acceleration voltage of 3 kV.
The infrared spectra of cotton fabric were recorded on a Nicolet 5700 Fourier transform infrared spectroscopy (FT-IR) equipped with a single reflection attenuated total reflection system.
The chemical compositions were studied by the Axis-Ultra X-ray photoelectron spectroscopy (XPS) using a 100 W AlKα X-ray source. The pressure in the analysis chamber was maintained at 4.0×10-9Pa.
Whiteness index was measured by WSD III whiteness instrument. The calculation formula of hunter whiteness was Wh/%=100-[(100-L)2+a2+b2]1/2. HereLdenotes the hunter lightness, andaandbdenote the hunter chromaticity indexes, respectively. The result was the average of three measurements.
Tensile properties were measured in standard conditions with a Model YG026A electricity fabric tester machine (ISO 13934-1: textiles tensile properties of fabric part 1: determination of maximum force and elongation at maximum force using the strip method, 1999).
The air permeability of cotton fabric was measured by YG461E-Ⅲ digital fabric ventilation instrument in line with the state standard of ISO 9073-15-2008. The result was the average of three measurements.
Fabric contact angle was measured by the 322W surface tension contact angle instrument.
The test of water repellence of cotton fabric was carried out according to AATCC Test Method 22-2005.
The washing durability of cotton fabrics was tested by SW-12A washing fastness testing machine. Washing conditions are soapflakes (special made for testing of textiles) 2 g/L, liquor ratio 1∶50, 40 ℃, and 10 min.
2 Results and Discussion
2.1 Surface morphology
Figure 1 showed the surface morphologies of the untreated and HFMT-treated cotton fabrics. The surface of cotton fiber was composed of layered microfibrils with a helix orientation[27]. It could be seen from Fig.1 (a) that the surface of the untreated cotton fiber was very smooth. When HFMT was employed, a surface with partly covered polymer-like granules was observed [Fig.1(b)], which showed slight changes on the surface of the treated cotton fabric. Initiator is of prime importance for successful ATRP because it can initiate the radical polymerization reaction[28]. In ATRP grafting system, there was no initiator in the grafting solution, and theoretically the monomer could only react with the macroinitiator.


(a) Untreated (b) HFMT-treated
2.2 FT-IR spectra
FT-IR analysis was used to demonstrate the presence of the polymer grafted onto the cotton fabric surface. FT-IR results of different cotton fabrics were given in Fig.2. The differences between the untreated and HFMT-treated cotton fabrics existed in the absence or presence of ester carbonyl groups and C—F bond. The HFMT-treated cotton sample showed adsorption peaks at 1751.67 and 1108.29 cm-1, which corresponded to the stretching vibrations of the ester carbonyl group and C—F bond. It could be concluded that HFMT was grafted onto cotton fabric successfully. Moreover, the new absorption peak at 836.61 cm-1, which was not seen in the spectrum of the untreated cotton fabric, was assigned to the stretching vibration absorption of —CH2—, —CH2—CH2—, and C—H groups. The FT-IR results clearly indicated that HFMT was grafted onto the surface of the cotton fabric.

Fig.2 FT-IR of untreated and HFMT-treated cotton fabrics
2.3 Surface analysis by XPS

Table 1 Elemental composition of the cotton fabric surface

SamplesC/%O/%F/%Untreatedcotton76.5923.410.00HFMT-treatedcotton63.8618.6717.47


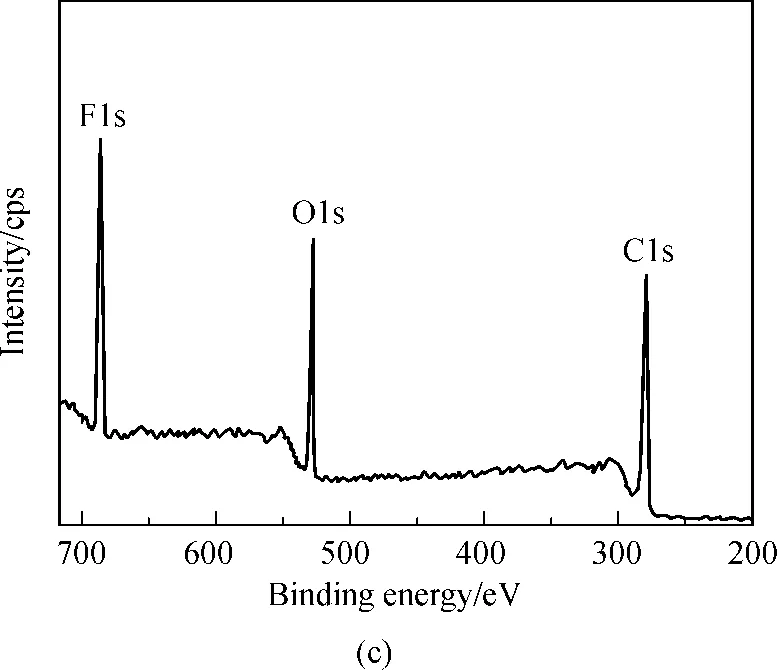

Fig.3 XPS wide-scan and C1s core-level spectra of untreated ((a) and (b)) and HFMT-treated cotton fabrics ((c) and (d))
2.4 Surface contact angle
Figure 4 showed the photos of water drops on HFMT grafted cotton fabric with different grafting yields, and the contact angles corresponding to the grafting yields were listed in Table 2. The contact angle of the untreated cotton fabric was 0°, which meant there existed no water repellence function of the untreated fabric. As grafting yield increased gradually, the contact angle of cotton fabric also increased. When grafting yield was 20.4%, the water contract angle was (132.40 ± 2.15)°, which indicated that the HFMT-treated cotton fabric obtained good water repellence property. Because fluorine atom has extremely low surface free energy and self-aggregated property, this property causes the fluorinated segments to be absorbed and oriented to the surface of the cotton fabric leading to the decrease of the surface tension. The low-energy fluorinated moieties formed a well-ordered structure at the outermost surface. The fluorine containing long carbon chain was surrounded by the fluorine atoms, and the formation of space shielding effect was more remarkable[31-33]. Thus, the HFMT-treated cotton surface possessed excellent water repellence property.

Fig.4 Surface contact angles of cotton fabrics: (a) untreated cotton sample; HFMT-treated cotton samples with (b) 13.9%, (c) 16.3%, and (d) 20.4% grafting yield obtained by changing the incubating temperature at 60, 80, and 70 ℃, respectively
Table 2 Surface contact angle of cotton fabric

SamplesGraftingyield/%Surfacecontactangle/(°)Untreatedcotton00HFMT-treatedcotton13.9109.6±1.216.3121.9±1.720.4132.4±2.2
2.5 Water repellence rating
The water repellence rating of the HFMT-treated cotton fabric was displayed in Table 3. The water repellence property of the cotton fabric was improved by treating with fluorine-containing monomers. The water repellence rating of the HFMT-treated fabric achieved grade 3, which indicated that the HFMT-treated cotton fabric was provided with water repellence property. This could be explained by fluorinated carbon chain surrounded by fluorine atoms. Consequently, the water repellence property of the grafted cotton fabric was improved via HFMT monomer.
Table 3 Water repellence rating of cotton fabric
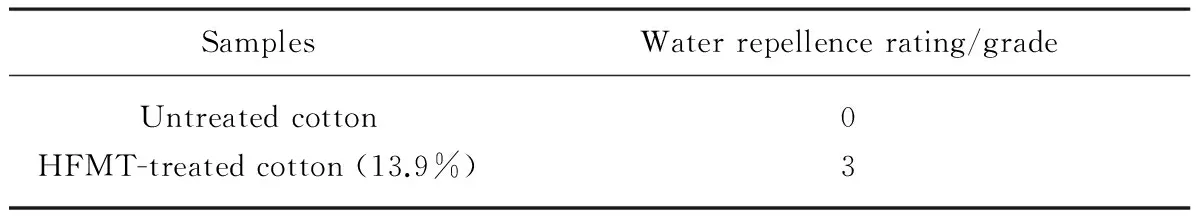
SamplesWaterrepellencerating/gradeUntreatedcotton0HFMT-treatedcotton(13.9%)3
2.6 Washing durability
The results of washing durability test after 0, 5, 10, 15, and 20 times of washing were given in Table 4. The results showed that the HFMT-treated cotton fabrics had better water repellence with the increase of the washing times. The surface contact angle of the HFMT-treated fabric slightly decreased after 20 washing times, indicating that the water repellence property of the HFMT-treated cotton fabrics was almost not affected when the washing times augmented. This could be explained by the hydroxyl groups of cotton reacting with 2-bromoisobutyryl bromide and generating initiator.Through a series of chain initiation and chain transfer, HFMT reacted with initiator in the grafting system[34]. Washing did not affect the covalent bond
Table 4 Surface contact angle of cotton fabric after repeated washing

SamplesGraftingyield/%Surfacecontactangleafterdifferentwashingtimes/(°)05101520Untreatedcotton000000HFMT-treatedcotton13.9109.6±1.2106.9±1.1104.5±0.8101.6±0.998.7±0.616.3121.9±1.7119.7±1.9117.1±1.7113.5±1.3111.5±1.520.4132.4±2.2129.9±2.6126.7±1.7123.4±0.7121.1±2.1
between the fabric and the fluorinated polymer. Consequently, the HFMT-treated cotton fabric possessed good washing durability. However, the water repellence property of the fabrics treated with the fluorine-containing acrylate via other methods, such as plasma treatment and coating method, suffered a significant decline after a few washing times[35-37]. This decline was ascribed to the destroy of the finishing agent on the surface of the fabrics which caused the loss of the fluorine-containing polymer after multi-times of washing. Therefore, the ATRP method was suggested to impart cotton fabrics with good and durable water repellence.
2.7 Physical properties
Table 5 revealed the whiteness of the HFMT-treated cotton fabric decreased to some extent due to the influences of TEA and the catalyst CuBr on cotton fabric, and the whiteness index fell by 16.5% when the grafting yield was 20.4%. The air permeability of the HFMT-treated cotton fabric decreased by 19.3% in contrast with the untreated cotton sample when the grafting yield was 20.4%. The grafting occurred mainly in the amorphous region of the cotton fabric, HFMT might distribute onto the surface of cotton fabric, fibers of the HFMT-treated cotton fabric became thicker, and the pore size between the interstices of cotton fibers became smaller, which decreased the air permeability property of the HFMT-treated cotton fabric[38-39]. Therefore, with the increase of the grafting yield, the air permeability of the cotton decreased. When the grafting yield of the HFMT-treated cotton fabric was 20.4%, the breaking strength dropped by about 25.7%. The decrease of strength should be contributed to the fiber damages in the grafting process. And the breaking elongation also reduced by 6.0% when the grafting yield of the HFMT-treated cotton was 20.4%. It could be explained that HFMT was grafted on the cotton fibers molecular chains, which would hinder the connection between the molecular chains and weaken the intermolecular forces among the molecular chains[40-41]. In general, the grafting had slight effect on the intrinsic properties of cotton fabric; cotton with good water repellence property could be obtained by properly controlling the grafting yield.
Table 5 Whiteness, breaking strength, breaking elongation, and air permeability of HFMT-treated cotton fabric

SamplesGraftingyield/%Whiteness/%Airpermeability/(mm·s-1)Breakingstrength/NBreakingelongation/%Untreatedcotton078.7129.2786.68.3HFMT-treatedcotton13.967.6114.3718.98.116.366.5112.6648.17.920.465.7104.3584.57.8
3 Conclusions
HFMT was successfully grafted onto the cotton fabric by ATRP method. All the analyses results including SEM, FT-IR, and XPS confirmed that HFMT was grafted onto the surface of the cotton fabric. The contact angle of the HFMT-treated cotton fabric significantly increased and good water repellence property was obtained. The contact angle of the HFMT-treated cotton fabric increased with the grafting yield increasing. Also, the contact angle of the HFMT-treated cotton fabric just slightly decreased after 20 washing times. The whiteness and the breaking strength as well as the air permeability of the HFMT-treated cotton decreased slightly, which nearly had no effect on the intrinsic properties of cotton fabric. ATRP method offers a feasible way for the preparation of the HFMT-treated cotton fabric and imparts cotton with functionality.
[1] Heinze T, Liebert T. Unconventional Methods in Cellulose Functionalization [J].ProgressinPolymerScience, 2001, 26(9): 1689-1762.
[2] Shen D W, Yu H, Huang Y. Synthesis of Graft Copolymer of Ethyl Cellulose through Living Polymerization and Its Self-assembly [J].Cellulose, 2006, 13(3): 235-244.
[3] Eichhorn S J. Cellulose Nanowhiskers: Promising Materials for Advanced Applications [J].SoftMatter, 2011, 7: 303-315.
[4] Roy D, Guthrie J T, Perrier S. Graft Polymerization: Grafting Poly(styrene) from Cellulose via Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerization [J].Macromolecules, 2005, 38(25): 10363-10372.
[5] Meissner E, Myszkowski J, Szymanowski J. Synthesis and Surface-Activity of Surfactants Containing Fluorocarbon Hydrophobes [J].TensideSurfactantsDetergents, 1995, 32(3): 261-271.
[6] Desimone J M, Guan Z, Elsbernd C S. Synthesis of Fluoropolymers in Supercritical Carbon Dioxide [J].Science, 1992, 257(5027): 945-947.
[7] Thomas R R, Anton D R, Graham W F,etal. Preparation and Surface Properties of Acrylic Polymers Containing Fluorinated Monomers [J].Macromolecules, 1997, 30(10): 2883-2890.
[8] Lu G H, Chen Z G, Cheng S Y,etal. Synthesis and Application of Perfluoroalkyl Acrylate Polymer [J].ChineseJournalofColloid&Polymer, 2000, 18(2): 31-34. (in Chinese)
[9] Zhou Y M, Huang J Y, Xu Q H. Advances in Fluorine-Containing Finishing Agents [J].ModernChemicalIndustry, 2001, 21(5): 9-12. (in Chinese)
[10] Li K, Wu P P, Han Z W. Preparation and Surface Properties of Fluorine-Containing Diblock Copolymers [J].Polymer, 2002, 43(14): 4079-4086.
[11] Badri A, Whittaker M R, Zetterlund P B. Modification of Graphene/Graphene Oxide with Polymer Brushes Using Controlled/Living Radical Polymerization [J].PolymerChemistry, 2012, 50(15): 2981-2992.
[12] Matyjaszewski K, Xia J H. Atom Transfer Radical Polymerization [J].ChemicalReviews, 2001, 101(9): 2921-2923.
[13] Wang J S, Matyjaszewski K. Controlled/“Living” Radical Polymerization. Atom Transfer Radical Polymerization in the Presence of Transition-Metal Complexes [J].JournaloftheAmericanChemicalSociety, 1995, 117(20): 5614-5615.
[14] Tsarevsky N V, Matyjaszewski K. Combining Atom Transfer Radical Polymerization and Disulfide/Thiol Redox Chemistry: a Route to Well-Defined (Bio)degradable Polymeric Materials [J].Macromolecules, 2005, 38(8): 3087-3092.
[15] Tsarevsky N V, Matyjaszewski K. Reversible Redox Cleavage/Coupling of Polystyrene with Disulfide or Thiol Groups Prepared by Atom Transfer Radical Polymerization [J].Macromolecules, 2002, 35(24): 9009-9014.
[16] Ding J X, Xiao C S, Tang Z H,etal. Highly Efficient “Grafting from” an α-Helical Polypeptide Backbone by Atom Transfer Radical Polymerization [J].MacromolecularBioscience, 2011, 11(2): 192-198.
[17] Bortolamei N, Isse1 A A, Magenau A J D,etal. Controlled Aqueous Atom Transfer Radical Polymerization with Electrochemical Generation of the Active Catalyst [J].AngewandteChemie, 2011, 123(48): 11593-11596.
[18] Wang J S, Matyjaszewski K. Controlled/“Living” Radical Polymerization. Halogen Atom Transfer Radical Polymerization Promoted by a Cu (Ⅰ)/Cu (Ⅱ) Redox Process [J].Macromolecules, 1995, 28(23): 7901-7910.
[19] Braunecker W A, Matyjaszewski K. Controlled/Living Radical Polymerization: Features, Developments and Perspectives [J].ProgressinPolymerScience, 2007, 32(1): 93-95.
[20] Tsarevsky N V, Matyjaszewski K. “Green” Atom Transfer Radical Polymerization: from Process Design to Preparation of Well-Defined Environmentally Friendly Polymeric Materials [J].ChemicalReviews, 2007, 107(6): 2270-2299.
[21] Shu J B, Cheng C J, Zheng Y,etal. “One Pot” Synthesis of Fluorinated Block Copolymers Using a Surface-Active ATRP Initiator under Emulsion Polymerization Conditions [J].PolymerBulletin, 2011, 67(7): 1185-1200.
[22] Matyjaszewski K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives [J].Macromolecules, 2012, 45(10): 4015-4039.
[23] Jiang C, Wang Q H, Wang T M. Tunable Wettability via Counterion Exchange of Polyelectrolyte Brushes Grafted on Cotton Fabric [J].NewJournalofChemistry, 2012, 36(8): 1641-1645.
[24] Carlmark A, Malmstrom E E. ATRP Grafting from Cellulose Fibers to Create Block-Copolymer Grafts [J].Biomacromolecules, 2003, 4(6): 1740-1745.
[25] Huang H P, He L. Silica-Diblock Fluoropolymer Hybrids Synthesized by Surface-Initiated Atom Transfer Radical Polymerization [J].RoyalSocietyofChemistryAdvances, 2014, 4(25): 13108-13118.
[26] Hansson S, Östmark E, Carlmark A,etal. ARGET ATRP for Versatile Grafting of Cellulose Using Various Monomers [J].AppliedMaterials&Interfaces, 2009, 1(11): 2651-2659.
[27] Yu W D. Textile Materials [M]. Beijing: China Textile Press, 2006: 50-51. (in Chinese)
[28] Coessens V, Pintauer T, Matyjaszewski K. Functional Polymers by Atom Transfer Radical Polymerization [J].ProgressinPolymerScience, 2001, 26(3): 349-354.
[29] Selli E, Mazzone G, Oliva C,etal. Characterisation of Poly(ethylene terephthalate) and Cotton Fibres after Cold SF6Plasma Treatment [J].JournalofMaterialsChemistry, 2001, 11(8): 1985-1991.
[30] Zhang F, Wu X L, Chen Y Y,etal. Application of Silver Nanoparticles to Cotton Fabric as an Antibacterial Textile Finish [J].FibersandPolymers, 2009, 10(4): 496-501.
[31] Ye H H, Li Z X, Chen G Q. Synthesis and Application Properties of Fluorinated Aromatic Copolymers [J].JournalofAppliedPolymerScience, 2013, 130(6): 4410-4418.
[32] Ni H G, Xue D W, Wang X F,etal. Composition and Solution Properties of Fluorinated Block Copolymers and Their Surface Structures in the Solid State [J].ScienceinChinaSeriesB:Chemistry, 2009, 52(2): 203-211.
[33] Zhao X S, Ding X B, Zhang J H,etal. Preparation and Surface Properties of Acrylate Copolymer Latex Containing Fluorine [J].ActaPolymericaSinica, 2004(2): 197-199. (in Chinese)
[34] Xia J H, Matyjaszewski K. Controlled/“Living” Radical Polymerization. Atom Transfer Radical Polymerization Using Multidentate Amine Ligands [J].Macromolecules, 1997, 30(25): 7697-7700.
[35] Shen L, Dai J J, Chen Q L. Surface Properties of Fluorocarbon Plasma-Treated Cotton Fabrics [J].JournalofTextileResearch, 2009, 30(12): 72-74. (in Chinese)
[36] Zhou L Z, Jiang H, Yang L,etal. Preparation of Hydrophobicity Modified Silica Nanoparticles and Their Performance in Deepening Dyed Silk Fabrics [J].ScienceofSericulture, 2013, 39(6): 1131-1138. (in Chinese)
[37] Zhang P Q, Zhu Y W, Peng T Z. Water- and Oil-Repellent Finishing of Nylon-Cotton Union Fabrics [J].TextileAuxiliaries, 2006, 23(11): 36-38. (in Chinese)
[38] Xing T L, Liu J, Li S W. Surface-Initiated Atom Transfer Radical Polymerization on Cotton Fabric in Water Aqueous [J].TextileResearchJournal, 2013, 83(4): 363-370.
[39] Xing T L. Studies on Silk Grafting Using Acrylate Monomers via ATRP Method in Aqueous Medium [D]. Suzhou: Soochow University, 2009: 79-80. (in Chinese)
[40] Malik Z A, Malik M H, Hussain T,etal. Development of Models to Predict Tensile Strength of Cotton Woven Fabrics [J].JournalofEngineeredFibersandFabrics, 2011, 6(4): 46-52.
[41] Reti C, Casetta M, Duquesne S,etal. Intumescent Biobased-Polylactide Films to Flame Retard Nonwovens [J].JournalofEngineeredFibersandFabrics, 2009, 4(2): 33-38.
Foundation items: National Natural Science Foundations of China (Nos. 51203107, 51273134, and 51273140); Jiangsu Province Project of Postgraduate Innovation Engineering, China (No. CXZZ13_0818); Qing Lan Project, Jiangsu, China; Priority Academic Program Development of Jiangsu Higher Education Institutions, China
TS195.5 Document code: A
1672-5220(2015)01-0007-06
Received date: 2013-09-16
* Correspondence should be addressed to CHEN Guo-qiang, E-mail: chenguojiang@suda.edu.cn
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Joint Optimization Strategy for Video Transmission over Distributed Cognitive Radio Networks
- Asymptotic Behavior of the Drift Coefficient Estimator of Stochastic Differential Equations Driven by Small Noises
- Adaptive Modulation and Coding Based on Fuzzy Logic Cognitive Engine
- Modeling and Simulation of P-Aloha, CSMA/CA and MACAW Protocols for Underwater Acoustic Channel
- Design and Analysis of Axial Thrust Roller-Exciting Vibrating Table and Its Motor-Control System Based on Co-simulation
- Effects of Compression Garments on Lower Limb Muscle Activation via Electromyography Analysis during Running
