反气相色谱法测定聚环己基丙烯酸甲酯的热力学性质
2014-12-24IsmetKAYACigdemYigitPALA
Ismet KAYA,Cigdem Yigit PALA
(1.Canakkale Onsekiz Mart University,Faculty of Sciences and Arts,Department of Chemistry,Polymer Synthesis and Analysis Laboratory,Canakkale 17020,Turkey;2.Kaleseramik AR-GE Research,Kaleseramik R &D Center,Kaleseramik Canakkale Kalebodur Ceramic Industries Inc.,Canakkale 17020,Turkey)
The methacrylate polymers are widely used in the manufacture of prostheses,contact lenses,adhesives,coatings,etc.[1].The thermodynamic properties and solubility of polymers are parameters that must be known for application of polymer synthesis,economic production of polymeric materials and the process that used for this purpose.These parameters can be investigated with the inverse gas chromatography (IGC)technique.In this technique,the polymer is coated onto supported material and then filled into chromatographic columns.The solvents with known properties pass through the column by carrier gas and leave the column at different times according to the interest of the polymer.IGC technique has been used for the determination of some properties of the polymers such as the solubility parameters,melting point and glass transition temperature[2].This method is of convenience and economics of operation.The basic tools for IGC are inexpensive,rugged,widely available,and suitable for routine laboratory applications.IGC data might be collected quite rapidly over extended temperature ranges[3].As the molecular weights of the polymeric substances are very high and the polymeric substances are non-volatile,IGC method has been used to investigate the properties of these substances instead of normal gas chromatography.IGC was developed by Smidsrod and Guillet[4]and applied to many polymeric systems.The information in the processing steps of polymers are important parameters for higher polymer quality.The thermodynamic of polymer systems affects how these processing steps can be carried out.Therefore,the knowledge of thermodynamic data of polymer solutions is a necessity for the improvement of industrial processes.

1 Theoretical
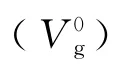
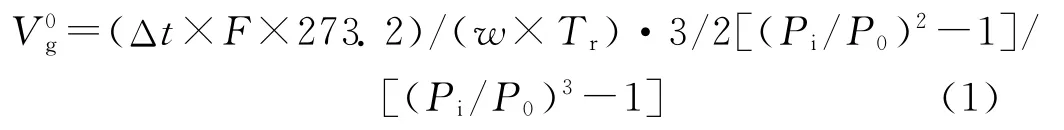
whereΔt(=tp-tg)is the difference between the retention times of the probe(tp)and the methane(tg);Fis the flow rate of the carrier gas measured at room temperature(Tr);wis the mass of the polymeric stationary phase;Piand P0are the inlet and outlet pressures,respectively.
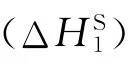

By incorporating Equation(2)and(3)we calculated the entropy of sorption of solutes as follows:

The adsorption heat of probes adsorbed by the poly(cyclohexyl methacrylate)is given by the follow-ing equation whereΔHais the adsorption enthalpy and Ris the ideal gas constant[9]:

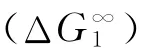
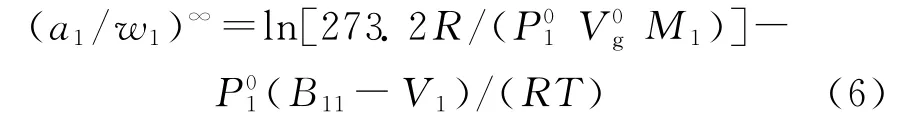

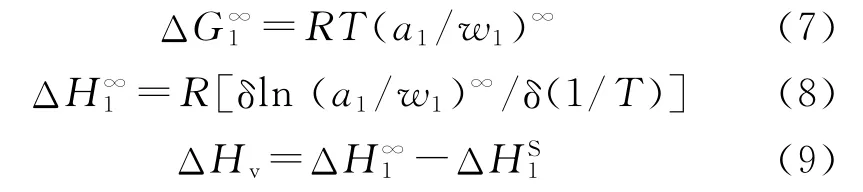
The molar volume of the solute(V1)was calculated using the following equation[11]:

where Vcis the critical molar volume and qris the reduced density of the solute given by the following equation.
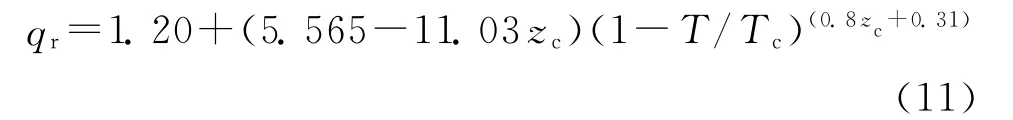
where zcis the critical compressibility factor and Tcis critical temperature[13].

where Ris the gas constant;v2is the specific volume of the polymer.
Solubility parameter of the probe is calculated as follows[14]:

whereδ1is solubility parameter of probes;ΔHvis the molar enthalpy of vaporization for the probe at temperature T (K).
The solubility parameter of the polymer(δ2)can be calculated by using the following equation:
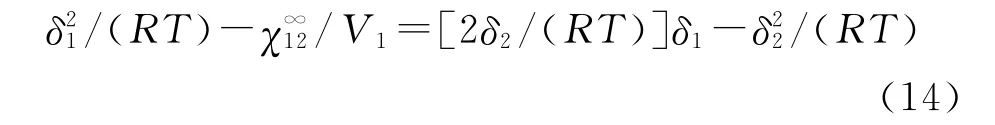
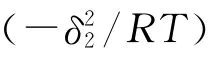
2 Experimental
2.1 Materials
Fourteen polar and non-polar probes were used in this study.They were selected to provide different chemical natures and polarities.n-Pentane,n-hexane,n-heptane,n-octane,n-decane,methanol,ethanol,2-propanol,butanol,acetone,ethyl methyl ketone,benzene,toluene and o-xylene,were from Aldrich Chemical Co.Poly(cyclohexyl methacrylate)was supplied by Across Organics in powder form of Registry No.1849402.Poly(cyclohexyl methacrylate)was in white powder form.Refractive index(n20/D)and density of poly(cyclohexyl methacrylate)were 1.506 5 and 1.1 g/mL at 25 ℃,respectively.Chromosorb W (45-60 mesh)was supplied from Sigma Chemical Co.
2.2 Instrumentation and procedure of thermodynamic studies
A Shimadzu GC-2010 model gas chromatograph equipped with a dual flame ionization detector was used.Dried nitrogen gas(research grade)was used as carrier gas.Methane was used as a non-interacting marker to correct the dead volume in the column.Pressures at inlet of the column read from GC were used to compute corrected retention volumes by the usual procedure.Flow rates were measured with a soap bubble flow meter at the end of the column.A flow rate of about 15 cm3/min was used throughout our experiment.The glass tube(2.1 m×3.2 mm i.d.)was washed with acetone and was annealed prior to use.A column packing material was prepared by coating 45-60 mesh size Chromosorb W treated with polymer.An amount of 0.5 g poly(cyclohexyl methacrylate)was dissolved in 100 mL of tetrahydrofuran(THF).An amount of 5 g of the solid supporting material was added to this solution and kept stirring afterwards.The solvent was removed by continuous stirring and slow evaporation under partial vacuum in a rotary evaporator.The prepared material was packed into the glass tube[14,16].The column was conditioned with fast carrier gas(N2)flow rate for 48 h prior to use.The probes were injected into the column with an auto sampler.Three consecutive injections were made for each probe at each set of measurement and three values of retention time with inverse gas chromatography method were averaged.An injection volume was selected as 0.1μL.Methane was synthesized in the laboratory by the reaction of sodium acetate with sodium hydroxide[10].DSC analyses were carried out between 20 - 250 ℃ (in N2,10℃/min)using Perkin Elmer Pyris Sapphire DSC.
3 Results and Discussion

Table 1 Variation of of selected organic solvent systems at different column temperatures using poly(cyclohexyl methacrylate)as stationary phase

Table 1 Variation of of selected organic solvent systems at different column temperatures using poly(cyclohexyl methacrylate)as stationary phase
EMK:ethyl methyl ketone.
Probe V0g/(cm3/g)353 K 363 K 373 K 383 K 393 K 403 K 413 K 423 K 433 K 443 K 453 K 11 n-Hexane 10.32 8.64 7.99 8.78 8.79 8.33 7.88 7.46 7.25 6.74 6.37 n-Heptane 12.15 9.54 8.66 9.92 10.40 9.37 8.40 7.63 7.72 7.08 6.80 n-Octane 19.69 15.73 13.83 11.20 10.72 9.77 9.11 8.58 8.14 7.51 7.03 n-Decane 32.79 23.83 19.17 24.11 23.07 20.42 18.71 16.62 15.49 13.89 12.62 Methanol 17.93 13.24 11.56 10.24 8.98 8.13 7.45 6.97 6.59 6.27 5.81 Ethanol 23.59 18.86 14.29 12.91 11.13 9.55 8.51 7.69 7.29 6.61 6.19 2-Propanol 28.58 19.25 17.19 14.47 12.31 10.43 9.13 8.18 7.67 6.93 6.47 Butanol 90.38 72.19 47.73 38.28 28.02 21.00 16.54 13.49 11.38 9.88 8.67 Acetone 18.14 15.88 13.41 12.06 10.72 9.45 8.43 7.67 7.20 6.68 6.24 EMK 31.49 26.25 20.40 18.16 15.00 12.46 10.55 9.26 8.45 7.61 6.98 Benzene 44.49 33.46 29.04 25.38 20.34 16.42 13.53 11.62 10.21 9.18 8.15 Toluene 85.16 61.78 52.61 44.47 32.49 25.61 20.10 14.64 12.99 11.90 10.33 o-Xylene 160.60 106.79 94.12 66.48 53.42 39.38 30.69 22.29 19.72 16.32 13.n-Pentane 9.21 8.59 7.42 8.08 8.39 7.77 7.33 6.96 6.82 6.46 6.64

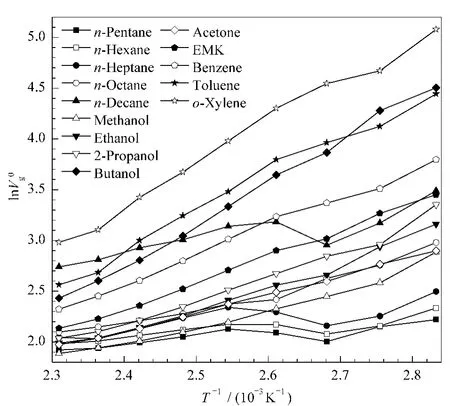
Fig.1 of selected probes at different temperatures
Table 2 Poly(cyclohexyl methacrylate)-solute interaction coefficientand weight fraction activity coefficients(a1/w1)∞of selected organic solvents at various temperatures
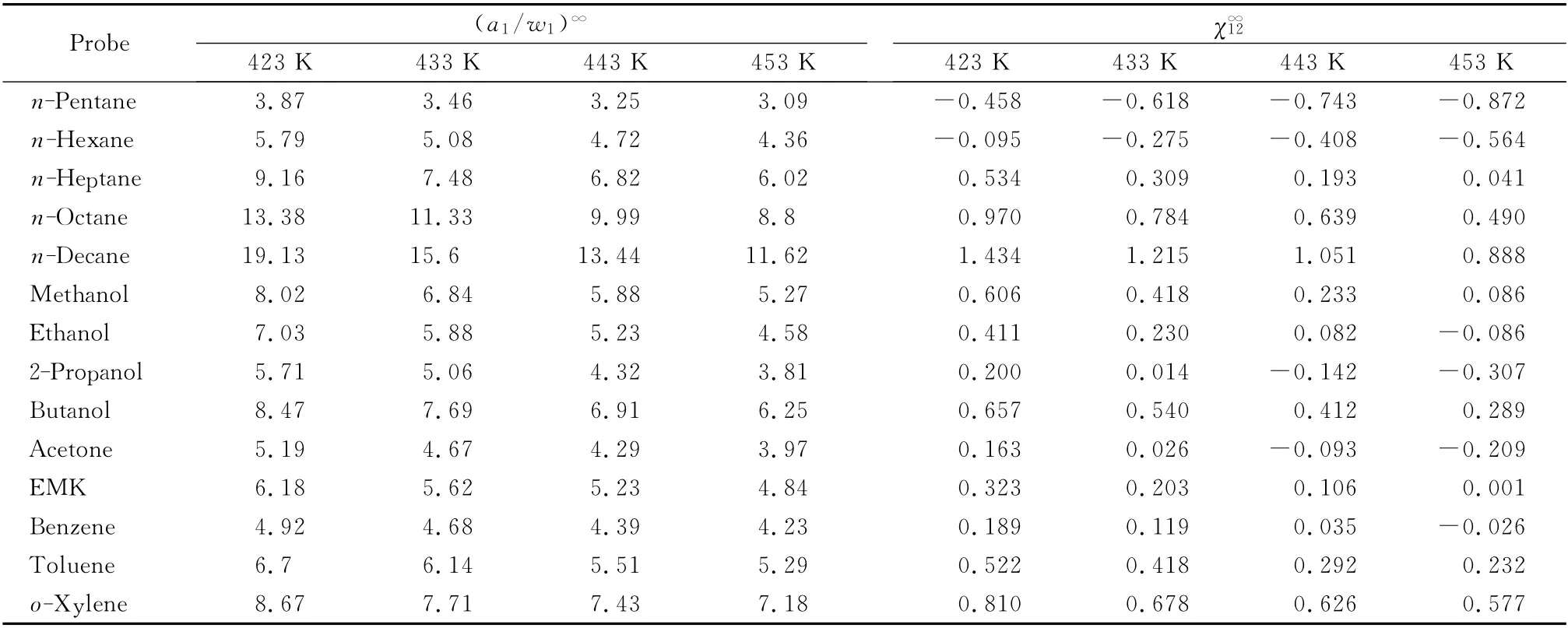
Table 2 Poly(cyclohexyl methacrylate)-solute interaction coefficientand weight fraction activity coefficients(a1/w1)∞of selected organic solvents at various temperatures
Probe(a1/w1)∞χ∞12 423 K 433 K 443 K 453 K 423 K 433 K 443 K 453 K 872 n-Hexane 5.79 5.08 4.72 4.36 -0.095 -0.275 -0.408 -0.564 n-Heptane 9.16 7.48 6.82 6.02 0.534 0.309 0.193 0.041 n-Octane 13.38 11.33 9.99 8.8 0.970 0.784 0.639 0.490 n-Decane 19.13 15.6 13.44 11.62 1.434 1.215 1.051 0.888 Methanol 8.02 6.84 5.88 5.27 0.606 0.418 0.233 0.086 Ethanol 7.03 5.88 5.23 4.58 0.411 0.230 0.082 -0.086 2-Propanol 5.71 5.06 4.32 3.81 0.200 0.014 -0.142 -0.307 Butanol 8.47 7.69 6.91 6.25 0.657 0.540 0.412 0.289 Acetone 5.19 4.67 4.29 3.97 0.163 0.026 -0.093 -0.209 EMK 6.18 5.62 5.23 4.84 0.323 0.203 0.106 0.001 Benzene 4.92 4.68 4.39 4.23 0.189 0.119 0.035 -0.026 Toluene 6.7 6.14 5.51 5.29 0.522 0.418 0.292 0.232 o-Xylene 8.67 7.71 7.43 7.18 0.810 0.678 0.626 0.n-Pentane 3.87 3.46 3.25 3.09 -0.458 -0.618 -0.743 -0.577


Table 3 (ΔG)∞1 andof sorption by using poly(cyclohexyl methacrylate)as the stationary phase and selected organic solvents as mobile phase

Table 3 (ΔG)∞1 andof sorption by using poly(cyclohexyl methacrylate)as the stationary phase and selected organic solvents as mobile phase
Probe(ΔG)∞1/(cal/mol)ΔGS1/(cal/mol)423 K 433 K 443 K 453 K 383 K 393 K 403 K 20 n-Hexane 1476.43 1398.71 1365.51 1324.59 2579.29 2645.53 2755.23 n-Heptane 1861.30 1731.35 1690.51 1615.34 2371.57 2396.44 2540.90 n-Octane 2180.32 2088.28 2026.24 1957.45 2179.00 2270.28 2402.61 n-Decane 2480.51 2363.44 2287.20 2207.47 1428.77 1500.63 1643.31 Methanol 1749.69 1654.61 1558.98 1495.33 3214.53 3401.00 3567.44 Ethanol 1639.31 1524.45 1457.05 1369.42 2761.86 2949.78 3147.60 2-Propanol 1464.38 1395.49 1288.25 1203.32 2473.09 2663.95 2863.92 Butanol 1795.60 1754.55 1701.28 1649.08 1573.02 1857.78 2136.03 Acetone 1384.58 1325.49 1282.21 1240.59 2637.35 2798.47 2970.26 EMK 1531.13 1484.61 1456.66 1419.81 2161.57 2367.24 2576.04 Benzene 1339.41 1327.67 1302.81 1297.47 1845.92 2067.12 2290.90 Toluene 1599.16 1561.34 1502.87 1499.05 1293.36 1572.16 1802.89 o-Xylene 1815.67 1757.25 1751.68 1746.22 879.59 1073.39 1344.n-Pentane 1137.51 1069.13 1036.91 1014.34 2777.02 2820.91 2953.82

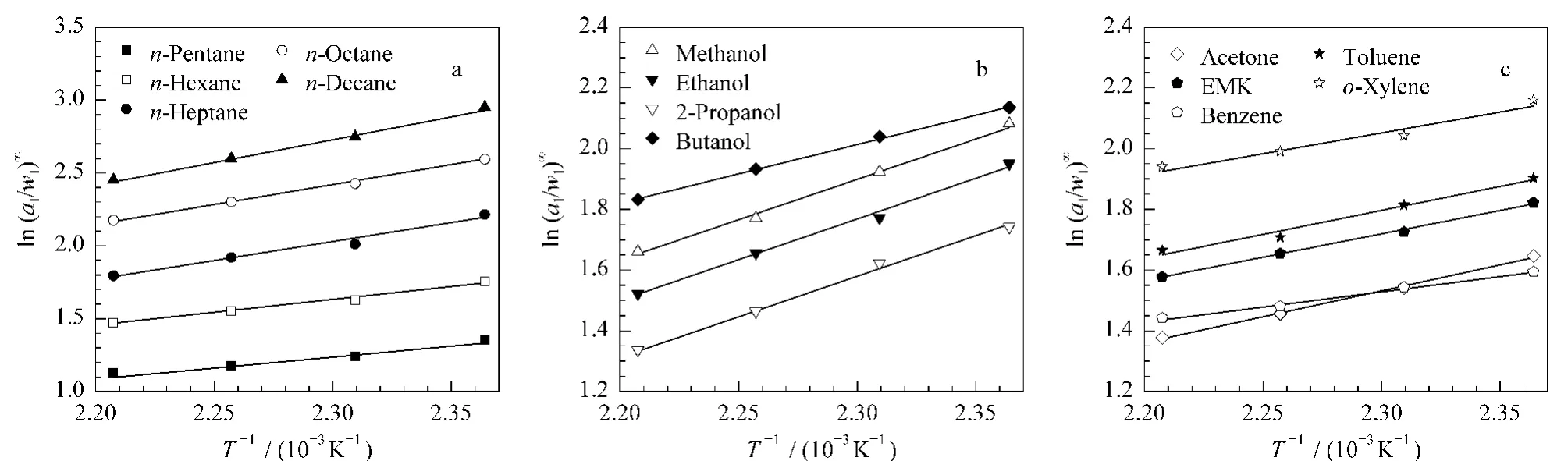
Fig.2 Variation of(a1/w1)∞with T-1/K-1 for(a)n-pentane,n-hexane,n-heptane,n-octane,n-decane;(b)methanol,ethanol,2-propanol,butanol;(c)acetone,ethyl methyl ketone,benzene,toluene and o-xylene

ΔH∞1values of n-hydrocarbons changed from 3.84 to 6.27 kcal/mol as seen from Table 4.ΔH∞1values of alcohols changed from3.88 to 5.39 kcal/mol,while the values of ketones changed from3.40 to 3.88 kcal/mol and the values of aromatics changed from 1.36 to 1.98 kcal/mol.Based upon these results the probes with low ΔH∞1values were accepted as solvent-polymer systems and the others were taken as non-solvent-polymer systems.
Table 4 (383-403 K),ΔH∞1 (423-453 K),ΔHa(353-373 K),andΔHvof selected organic solvents on poly(cyclohexyl methacrylate)

Table 4 (383-403 K),ΔH∞1 (423-453 K),ΔHa(353-373 K),andΔHvof selected organic solvents on poly(cyclohexyl methacrylate)
Solvent ΔHS1/(kcal/mol)ΔHa/ΔHv/(kcal/mol)(kcal/mol)ΔH∞1/(kcal/mol)Calculated according to Equation(9) From Ref.[13]16 n-Hexane -0.78 -3.36 3.55 4.33 6.9 n-Heptane -0.85 -4.44 5.16 6.01 7.58 n-Octane -2.10 -4.64 5.27 8.48 8.23 n-Decane -2.67 -7.03 6.27 6.43 9.39 Methanol -3.55 -5.76 5.39 8.94 8.43 Ethanol -4.62 -6.55 5.35 9.97 9.26 2-Propanol -5.01 -6.68 5.23 10.24 9.52 Butanol -9.21 -8.33 3.88 13.09 10.3 Acetone -1.99 -3.95 3.4 5.38 6.96 EMK -5.78 -5.67 3.06 8.84 7.46 Benzene -6.67 -5.60 1.98 8.65 7.35 Toluene -8.47 -6.32 1.46 9.93 7.93 o-Xylene -8.70 -7.02 1.36 10.98 8.n-Pentane -0.58 -2.82 2.84 3.42 6.8
DiPaola-Baranyi et al[7]determined thatΔH∞1values for aromatic solvents changed from -0.01 kcal/mol to 0.3 kcal/mol in polystyrene(PS),and from0.3 kcal/mol to 1.1 kcal/mol in polymethyl acrylate(PMA).These values for the same polymers changed from 0.6 kcal/mol to 2.5 kcal/mol and from2.5 kcal/mol to 4.1 kcal/mol in n-hydrocarbons.According to these results the probes with smallΔH∞1values were suitable for solvent-polymer systems and those with largeΔH∞1values were suitable for nonsolvent-polymer systems[7].
The solubility parameter(δ2)of a polymer can be determined by Equation(14)[18].δ2was determined from either slope or intercept of a straight line obtained by plotting the left-hand-side of Equation(14)versusδ1(Fig.3).δ2of poly(cyclohexyl methacrylate)was found as 5.17,4.60,4.18,3.74 (cal/cm3)0.5and 5.17,4.38,3.87,3.22(cal/cm3)0.5at 423,433,443 and 453 K,respectively(Table 5).

Table 5 Variation ofδ2 with poly(cyclohexly methacrylate)temperature
These values were in compliance with the values found previously [20].The solubility parameters obtained from the slopes and intercepts of the plots were in good agreement with each other.Comparing theδ2values of poly(cyclohexyl methacrylate)at different temperatures,it showed that the solubility parameters decreased with increasing temperature.

Fig.3 Variation of the term /(RT)-/V1]withδ1at the column temperatures of(a)423 K,(b)433 K,(c)443 K and(d)453 K for poly(cyclohexyl methacrylate)
4 Conclusions
Inverse gas chromatography technique was successfully applied to determine the glass transition temperature of poly(cyclohexyl methacrylate).Some thermodynamic properties were obtained for poly(cyclohexyl methacrylate)-solute systems such as adsorption heat,partial molar heat of sorption,partial molar free energy of sorption,partial molar heat of mixing at infinite dilution,partial molar free energy of mixing at infinite dilution,Flory-Huggins interaction parameter and weight fraction activity coefficient values.The solubility parameter of the polymer was determined with inverse gas chromatography technique.The results obtained are in good agreement with those of polymer-solvents and polymer-non-solvents systems.The technique is relatively uncomplicated and the data reduction is carried out by a computer.
[1] Jang Y S,Kang J W,Byun H S.J Ind Eng Chem,2010,16(4):598
[2] Aydin S.[MS Dissertation].Istanbul,Turkey:Yildiz Technique University,2005
[3] Shyamala M,Pragati Ranjan S,Sharma J V C.International Journal of Pharma Sciences,2013,3(2):201
[4] Guillet J E,Purnel J H.New Developments in Gas Chromatography:322 Progress in Gas Chromatography.New York,USA:Wiley-Interscience,1973:323,187
[5] Smidsrod O,Guillet J E.Macromolecules,1969,2(3):272
[6] Braun J M,Guillet J E.Macromolecules,1977,10(1):101
[7] DiPaola-Baranyi G,Guillet J E.Macromolecules,1978,11(1):228
[8] Galin M,Maslinco L.Macromolecules,1985,18(11):2192
[9] Kaya I,Ozdemir E.Polymer,1999,40(9):2405
[10] Kaya I.[PhD Dissertation].Elazig,Turkey:Firat University,1995
[11] Kaya I,Demirelli K.Polymer,2000,41(8):2855
[12] Papadopoulou S K,Panayiotou C.J Chromatogr A,2012,1229:230
[13] Reid C R,Prausnitz J M,Sherwood T K.The Properties of Gases and Liquids.2nd ed.New York,USA:McGraw-Hill,1977
[14] Kaya I,Ozdemir E,Coskun M.J Macromol Sci A,1996,A33(1):37
[15] Guillet J E,Purnel J H.Advances in Analytical Chemistry and Instrumentation Gas Chromatography.New York,USA:John Wiley &Sons,1973
[16] Pala C Y.[MS Dissertation].Canakkale,Turkey:Canakkale Onsekiz Mart University,2013
[17] Klein J,Jeberien H E.Macromol Chem Phys,1980,181(6):1237
[18] Kaya I,Ilter Z,Senol D.Polymer,2002,43(24):6455
[19] Emam M N,Ahmed E E.Chinese Journal of Chromatography,2007,25(6):871
[20] Bicerano J.Prediction of Polymer Properties.3rd ed.New-York,USA:Marcel Dekker,Inc.,2002
