氧化石墨烯:一种用于癌症诊断与治疗的新型纳米试剂
2014-12-13刘智明郭周义
刘智明,郭周义
(华南师范大学生物光子学研究院,激光生命科学教育部重点实验室,国家中医药管理局中医药与光子技术三级实验室,广州510631)
纳米技术已经成为癌症治疗的一种新型而有巨大前景的手段[1-2].传统的化学疗法、放射疗法以及外科切除手术等在治疗癌症时往往存在许多局限,比如:由于到达肿瘤部位的药物剂量不足导致的疗效不佳;因非特异性的抗癌药物递送导致的毒副作用;长期单一疗法导致的药物耐受;以及不完全的外科手术切除导致的肿瘤复发等[2]. 因此,对于一次有效的癌症治疗的关键在于到达肿瘤位点的药物浓度足以杀死癌变细胞,而对周边正常细胞损害很小,要求采用适当的策略以达到靶向的癌症治疗. 纳米粒子能够以被动及主动的方式在肿瘤部位富集,这为肿瘤的最优化治疗提供了极大的便利. 尺寸在几十至几百纳米大小的粒子能够通过增强的渗透和潴留(EPR)作用穿透缺陷的肿瘤血管而在肿瘤组织内富集,从而实现被动的肿瘤靶向[3-5];同时,纳米粒子亦含有巨大的活性表面提供给各种各样的靶向连接分子结合,以实现纳米粒子的主动肿瘤靶向功能[6].超大的比表面积同样赋予了纳米材料作为优秀的抗癌药物载体的能力[7]. 另一方面,部分纳米材料本身也能作为一种治疗试剂用于癌症的治疗,如光热治疗[8-10].
石墨烯自2004年被发现以来,已经迅速成为纳米科学领域中的一颗明星[11]. 石墨烯是一种二维(2D)的以sp2 杂化碳原子排列成蜂窝状的六角平面碳网络,是目前已知的最薄的材料.鉴于其独特的表面化学以及光学性质,石墨烯及其氧化衍生物——氧化石墨烯(GO)在生物医学领域中表现出了巨大的潜能[12-14]. 相对于其他纳米材料,GO 在生物医学方面的应用有着独特的优势:(1)制备成本低,将廉价的碳原料通过简单的化学方法即可制得大量的GO 材料,有利于GO 在临床上的应用[15];(2)2D 平面状的碳结构使得GO 拥有巨大的比表面积,可以用于各种治疗药物的高通量运载[16];(3)GO 具有很好的水溶性,源于其表面富含负电荷的含氧基团(环氧基、羟基和羧基)[17-18];(4)独特的2D 结构同样适合于以GO 为基础的纳米复合材料的构建[19];(5)GO 的生物毒性还存在着一些争议,但从现有资料来看,相对于其他应用于生物医学领域的纳米材料,如金纳米材料、碳纳米管和量子点等,GO 展现出了更为良好的生物相容性[20-21]. 鉴于此,GO 及其衍生材料已经被发展为一种很有前景的纳米生物材料用于癌症研究中,包括肿瘤诊断、成像及治疗等.
1 GO 的生物毒性及低毒改良
作为一种新型的碳纳米材料,GO 的生物相容性还存有争议. 近期的研究报道显示GO 存在一定的生物毒性,并依赖时间及剂量[22]. 石墨烯的主要功能源于石墨烯会在细胞内引发一定量的活性氧(ROS),从而激活细胞凋亡通路,引起细胞凋亡[21,23-25].石墨烯的毒性跟粒子大小、形状、氧含量、表面修饰情况以及周围环境有关[26].Duch 等[27]认为GO 上的共价氧含量是导致GO 毒性的主要原因.Singh 等[28]也认为因氧基团的存在GO 容易引发血管内血栓生成,而当GO 还原后(rGO),这种现象明显减少.但是,同其他纳米粒子相比,如与结构相似的SWNTs 相比,石墨烯引发的细胞反应要小[21,25].
使用生物相容性好的高聚物或者生物大分子对石墨烯进行表面修饰能够显著降低它的毒性. 研究得最多的是对石墨烯进行聚乙二醇(PEG)化,斯坦福大学的戴宏杰团队及苏州大学刘庄课题组对此做了很大贡献.体外实验表明,PEG-G/GO 对多种细胞(Raji,HCT-116,OVCAR-3,U87MG,MDA-MB-435 和MCF-7 等)没有发现明显毒性[29-30]. 体内实验以Balb/c 小鼠为模型,刘庄团队发现20 mg/kg 剂量的PEG-GO 处理小鼠40 天并不会对其各个脏器造成明显的损伤[31].而没有修饰的GO 在10 mg/kg 的剂量下就有明显的毒性[32].进一步采用放射性示踪法对GO-PEG 在体内的药代动力学、长期的分布以及毒性进行了系统研究,发现GO-PEG(20 mg/kg)主要分布在网状内皮系统中,长达3个月的观察没有发现明显的毒性[33].其他生物相容性好的大分子修饰(如葡聚糖、壳聚糖、PVP 及血清蛋白等)同样能够降低石墨烯的毒性;另一方面,大分子的修饰又能显著地提高石墨烯在生理环境中的分散性[34-37].
2 基于GO 的肿瘤光学诊断
2.1 GO 依赖的荧光淬灭技术
GO 已经被证明能够对吸附在其表面的荧光染料有荧光淬灭效应[38-39]. 同样的现象也发生于GO材料与其他荧光物质(如量子点、共轭聚合物和上转换荧光粒子等)之间[40-42]. 这种淬灭效应可能的机制可以被归结于石墨烯材料与紧附着于其上荧光基序间的荧光共振能量转移作用(FRET)[38,40]. 根据这种荧光淬灭现象,如果用一肿瘤识别分子将GO 与荧光染料连接起来,则可制备出一种可用于诊断肿瘤的生物传感器(图1).Yue 等[43]设计出一种新颖的探针将近红外(NIR)荧光染料Cy5 通过一段基质金属蛋白酶(MMPs)敏感的肽链共价地连接在了GO 表面. 由于MMPs 在肿瘤组织中是高表达的,大量的MMPs 能够将肽链剪断使得Cy5 从GO的束缚中挣脱出来,恢复荧光;而在正常组织中,由于Cy5 与GO 间的FRET 效应,导致Cy5 的荧光沉默.同样地,根据肿瘤细胞内谷胱甘肽水平高这一特点,Feng 等制备出了一种以二硫键连接的GO-罗丹明B 荧光“开-关”纳米探针用于探测及成像叶酸受体阳性的肿瘤细胞[44].
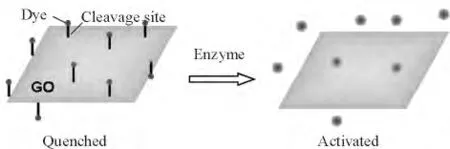
图1 基于GO 的FRET 生物传感器示意图Figure 1 A schematic model of GO-based FRET biosensor
2.2 以GO 为活性基底的表面增强拉曼技术
表面增强拉曼散射(SERS)技术已经发展为一种强有力的方法用于肿瘤组织及其细胞组分的鉴定[45-46].金和银纳米粒子(NPs)是常见的SERS 活性底物,因为在激光照射下在金属结构周围能够产生局部表面等离子共振[47].然而,制备出高重复性、高灵敏的SERS 底物仍旧是一个长久性课题. 近年来,构建石墨烯- 金属纳米复合物作为高灵敏的SERS 底物已经被证明是一种很好的策略,这源于石墨烯对于被分析物的富集作用,导致了附着在石墨烯片上的纳米粒子周围的被测分子的浓度提高[48-49].在最新发表的一篇文章中,Manikandan 等报道了一种GO 与金纳米六边形复合结构(Au@G)用于正常乳腺细胞、乳腺癌细胞和乳腺癌干细胞的探测与鉴别[50].与离散的Au NPs 相比,复合物能够激发出更为丰富和强烈的细胞SERS 信号,对癌细胞和干细胞的增强因子分别可达5.4 和4.8.
采用化学合成的策略制备出的纳米粒子一般以被动摄入的方式进入细胞内,然而,这种被细胞內吞的粒子往往被截留在一些膜状细胞器中,如溶酶体、内含体等,而在细胞核内基本上看不到纳米粒子存在[51].细胞内合成的金纳米粒子(IGAuNs)制备简单环保,且能够在细胞内随机分布,是一个较为理想的SERS 活性底物用于检测细胞内不同区域(细胞质及细胞核)的拉曼信号[52].按照这一思路,本课题组通过在细胞孵育液中加入聚乙烯吡咯烷酮(PVP)功能化的GO 以及氯金酸,利用细胞自身的氧化还原体系合成出了GO/PVP/IGAuNs 复合物[53]. 在透射电镜(TEM)下可以观察到人肺腺癌(A549)细胞内杂合子的生成速率远远高于没有GO-PVP 辅助的IGAuNs 生成,经过24 h 的孵育时间,GO/PVP/IGAuNs 的生成量明显高于IGAuNs,且随机地分布在细胞质、核质及核仁等区域(图2). 同时GO/PVP/IGAuNs 还展现出了超高的对癌细胞内不同区域化学组分的SERS 活性.
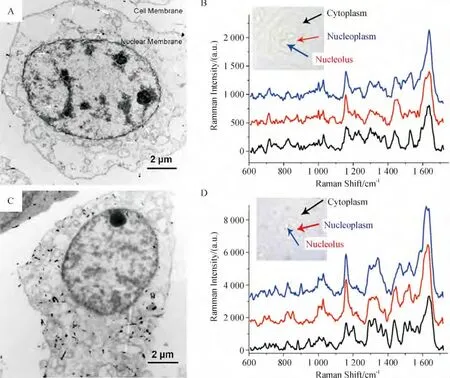
图2 细胞内合成的金纳米结构及其对A549 细胞的SERS 活性Figure 2 Intracellular growth of gold nanostructures and their SERS activities to human lung adenocarcinoma epithelial (A549)cells
3 基于GO 的肿瘤成像
3.1 荧光成像
由于存在异质的原子和电子结构,导致GO 在紫外、可见及近红外区域有着强烈的内在可调的荧光[54].GO 作为荧光标记有低毒性、无光漂白现象等优点,利用其近红外光致发光的特点可以有效降低荧光背景及减少激发光照射时对细胞的损伤. Sun等[29]对进入Raji B 细胞内的NGO 粒子进行了NIR荧光成像(激发光:658 nm,发射光:1 100~2 200 nm),观察了NGO 在细胞内的分布. 同时作为一种强有力的载药平台,将这一荧光探针运用于DOX 的运载,实现了肿瘤成像与治疗的一体化. 最近,Li等[55]又成功地运用NGO 于双光子荧光成像(激发光:800 nm,发射光:400~650 nm)与肿瘤光热治疗中.当然,同样可以在GO 上连接外源荧光分子进行常规的荧光成像[30-31,56].
当石墨烯的尺寸减小至100 nm 以下时,将会产生强烈的量子限制效应和边缘效应[57].如果石墨烯的大小减少到量子大小,即石墨烯量子点(GQDs,~10 nm),这2 种效应将会更加强烈. 鉴于此,GQDs能够展现出强烈而不淬灭的荧光. Pan 等[58]利用水热反应手段(200 ℃,1 h)制备出了一种发出亮蓝色荧光的GQDs.在日光下,这种GQDs 溶液呈浅黄色,在320 nm 处有一定的吸收. 采用320 nm 的紫外光照射溶液,则会激发出强烈的亮蓝色荧光(430 nm).GQDs 的荧光强度与激发光波长密切相关,320 nm 激发能够得到最高的荧光强度,随着波长红移,GQDs 的荧光强度逐渐减弱.GQDs 的荧光颜色是可调控的,采用不同的制备方法,获得的GQDs 的荧光发射谱不尽相同. GQDs 的制备有2 种方式:“从上而下”剥离和“自下而上”组装. 水热反应法属于前者,除此之外,其他如电化学剥离法[59-60]、再次氧化法[61-62]、微波切割法[63]以及对富勒烯开笼处理[64]等均可以得到GQDs.“自下而上”的策略就是以小分子有机物为原料,采用化学合成的方法从零开始一步一步地组装出GQDs[65]. 目前已发展出了一些简便的、绿色的、有机物质为原料的GQDs 合成方法,具有极大的实际应用价值. 如:以葡萄糖为唯一原料,微波辅助的水热法一步合成GQDs[66];以及直接加热柠檬酸至200 ℃,在30 min 内即可生成GQDs[67].与微米级GO 相比,GQDs 展现出更为良好的生物相容性,揭示了其在生物医学领域中的巨大潜能[68].Zhu 等[69]采用DMF 为溶剂,将GO 的DMF 溶液置于水热反应釜内进行水热反应(200 ℃,5 h),得到了能够发射强烈绿色荧光的GQDs,并成功地应用于人肉骨瘤(MG-63)细胞的荧光成像中.GQDs也可以作为一种非常有效的双光子荧光探针用于细胞及深层组织成像.Liu 等[70]报道了一种N 掺杂的GQDs(N-GQDs),展示出超大的双光子吸收截面(48 000).对孵育有N-GQDs 的宫颈癌(HeLa)细胞进行双光子荧光成像,可以看到非常明亮的绿色荧光图像,且自发荧光背景低. 另外,采用这种荧光探针亦可实现深达1 300 μm 的组织荧光成像;相对应地,单光子激发仅能实现400 μm 处的组织有效成像.
3.2 SERS 成像
GO 展现了明显的内在拉曼信号,分别是位于1 350 cm-1代表石墨晶格的D 峰和代表石墨边缘缺陷的G 峰(1 600 cm-1)[71]. 另外,当金属纳米粒子附着于GO 表面后,这一拉曼信号显著增强[72-74],这一特征为GO 作为新颖的拉曼标记运用于癌细胞的快速成像提供了巨大的可能性. 在本课题组近期的工作中,以PVP 为还原剂和稳定剂采用原位还原法制备出GO-Ag NPs,该复合物展现出了强烈的对GO 本身的SERS 活性,最大增强因子可达48.4[75].采用这种新型SERS 标记,仅需1 μg/mL 即可实现对HeLa 细胞的快速SERS 成像,扫描一个像素仅花费0.06 s(图3A).从细胞内不同区域提取出的拉曼谱线来看,GO-Ag NPs 在细胞内的分布是不均匀的.接着,将靶向分子——叶酸(FA)共价地连接在GO上,制备出FA-GO-Ag NPs 用于肿瘤细胞的靶向标记与SERS 成像. 图3B 显示了孵育有FA-GO-Ag NPs(2 h)的癌细胞成像图,可以发现在FR 阳性细胞(HeLa)表面存在着非常明显的SERS 信号,而在FR 阴性细胞(A549)上基本上没有发现GO 的信号,表明FA-GO-Ag NPs 能够实现对FR 阳性细胞的快速靶向识别与SERS 成像. Liu 等[76]选用GO-Au NPs 实现了对HeLa 229 细胞的SERS 成像.
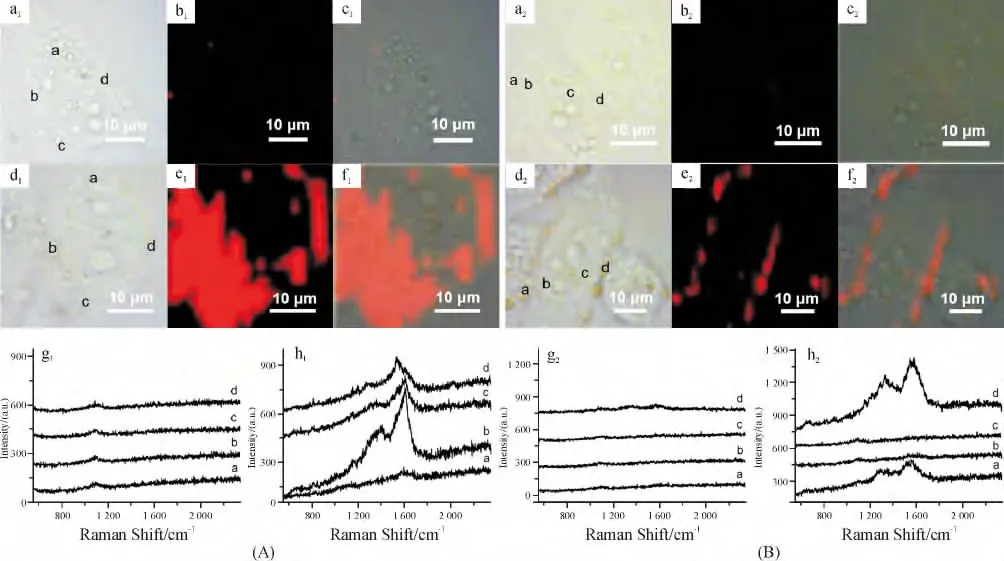
图3 基于GO 的癌细胞SERS 成像Figure 3 GO-based SERS imaging of cancer cells
4 基于GO 的肿瘤治疗
4.1 肿瘤近红外光热治疗
GO 有着一个极宽的从紫外至近红外(UVNIR)的光吸收,并且这一吸收特性会随着GO 的还原程度增大而增强[77].在NIR 区内在且强烈的光吸收使得GO 在肿瘤的NIR 光热治疗中具有潜在的价值.Yang 等[31]第一次发表了关于GO 在肿瘤光热治疗的应用研究. 他们将PEG 修饰后的NGO 通过小鼠尾静脉注射进入小鼠体内. 荧光成像实验结果表明,NGO-PEG 在24 h 后即能够在肿瘤区域富集,表现出高效的被动靶向效应,并且网状内皮系统中停留得较少.用808 nm 低强度激光照射后,NGO-PEG处理的小鼠肿瘤能够被有效消除,而肿瘤周围正常组织却没有损伤. 毒性实验显示出NGO-PEG 具有良好的生物相容性. 随后,Robinson 等[78]指出rGO的NIR 吸收高出GO 6 倍,具有更强的光热治疗效果.同时,他们还在rGO 上分别连接了靶向分子RGD 和荧光标记Cy5,实现了肿瘤细胞的靶向光热杀伤与实时荧光成像.NGO 的表面修饰以及大小同样也对NGO 的光热治疗效果有重要影响[79].近期,Akhavan 等[80]报道了一种新颖的还原性纳米氧化石墨烯网状结构(rGONM)用于神经胶质瘤在体超高效光热治疗. rGONM 对808 nm 激光的吸收分别高出rGO(~60 nm)及GO(~2 μm)的4.2 倍和22.4 倍.
在体外实验中,Markovic 等[81]比较了石墨烯及CNTs 在光热杀伤癌细胞的能力. 结果表明,石墨烯在对808 nm 光的吸收上会比CNTs 低,但其产生的热量却比CNTs 多.在光热杀伤人胶质瘤细胞U251的能力上,石墨烯表现出更强的杀伤能力,这可能与石墨烯有着比CNTs 更好的分散性有关. 流式细胞分析实验指出,石墨烯介导的光热杀伤肿瘤细胞的分子机制可能与细胞中产生的氧化压力及线粒体膜去极化有关.当然,更为详细的机制续在后的研究中进一步阐明.
4.2 肿瘤化学治疗
共轭π 键连接的2D 碳网状结构赋予了GO 在抗癌药物递送领域中的巨大潜能. 未经过修饰的GO 往往会在生理溶液中发生聚集,因此,采用一些生物相容性好的大分子物质(如PEG、PVP、PEI 和PVA 等等)对GO 进行功能化,可以防止这种聚集的发生,同时提高了GO 的生物相容性.当然,GO 的表面功能化同样会对其药物递送效率造成一定的影响.表1 总结了近期发表的一些GO 纳米载体及其药物递送性能.石墨烯是一个用途广泛的纳米载体,包括水溶性及疏水性有机小分子抗癌药物、DNA 和siRNA 等[35,82-84].对于靶向的肿瘤化学治疗,GO 纳米载体能够通过被动及主动2 种方式寻找到靶标.相比于碳纳米管,GO 表现出更为高效的被动靶向能力,可能跟GO 独特的形状和大小有助于EPR 效应的发生有关[85]. 另外,丰富的含氧功能团同样有利于GO 的表面共价的靶向分子修饰,以实现主动肿瘤靶向的功能.

表1 一些典型的GO 纳米载体及其药物递送性能Table 1 A brief summary of typical GO-based nanocarriers and their delivery performances
阿霉素(DOX)是石墨烯肿瘤化疗研究中最常用的抗癌药物模型. 斯坦福大学戴宏杰团队在该领域中有着开创性的研究[29-30]. 他们最早采用多臂PEG 对纳米级别的氧化石墨烯(NGO)进行共价修饰,以增强NGO 在生理环境中的溶解性并降低NGO 的毒性.水溶性芳香族抗癌药物DOX 通过ππ 键的堆叠作用吸附在NGO 的表面而被运送至肿瘤细胞Raji B 中.在细胞内,药物只有从载体上释放下来才能起到抑癌作用.在这里,药物从NGO 上释放是pH 依赖的.肿瘤细胞内的pH 是酸性的,这使得DOX 得以大量释放(释放率~40%). 而在正常生理环境下(pH 约7.4)DOX 的释放量很少(~15%),这一策略在很大程度上避免了药物导致的非特异性损伤[29]. 不仅仅单一药物,中科院苏州纳米所张智军团队研究发现,GO 亦能够同时装载多种药物分子,同时装载的DOX 和CPT 的装载率分别可达400%和4.5%,这种多种药物装载方式能够有效提高药物对肿瘤细胞的杀伤效率,同时降低了肿瘤对单一药物的耐药性[88].
GO 表面大分子修饰物的存在常常会对吸附其上的药物释放产生阻碍. 近期,Wen 等[94]发展出了依赖于氧化还原反应和pH 两种因素的药物释放体系.他们采用双硫键将PEG 共价连接在NGO 上(NGO-SS-mPEG),并用于运载药物(DOX). 由于肿瘤细胞内氧化还原活力高于正常组织,当载体进入肿瘤细胞时,双硫键在高氧化还原体系下发生断裂,使得PEG 从GO 片上脱离,这样就为随后DOX 的释放扫除了阻碍.加之,肿瘤细胞中pH 值呈酸性,π-π结合于NGO 上的DOX 因此得到大量释放. 这种双保险型的药物释放体系能够更好地确保药物在运送过程中不会意外释放,也是今后石墨烯载药体系研究的方向.
4.3 肿瘤光化疗
多种手段联合治疗肿瘤是目前临床上广泛采用的一种应对肿瘤细胞耐药性的策略. 考虑到石墨烯在药物运载中的优势及其在NIR 光热治疗中的应用,本课题组首次尝试了将NGO 应用于肿瘤的化疗联合光热治疗[86]. 采用酰胺化反应将PEG 共价连接在NGO 上,制备出的NGO-PEG 表现出了很好的NIR 光吸收性以及对DOX 的负载能力(载药率142.5%).细胞实验(图4A)显示,在NGO 浓度在10 μg/mL 时,NGO-PEG-DOX +NIR 处理组的细胞抑制能力明显强于仅用单一处理方式组,表明只有在合适的NGO 及药物剂量下,化疗和光热治疗间才存在着协同效应.进一步在动物实验中证明了化疗和光热治疗联合处理能够有效地消融种植在Balb/c小鼠背部的乳腺瘤(图4B 和C). 同时在这一剂量下,NGO-PEG-DOX 并不会对小鼠的主要器官造成损伤.为了增强NGO 在肿瘤组织中的富集能力,减弱载体对机体存在的可能损伤,在随后的研究中采用FA 靶向分子制备出了肿瘤靶向纳米载体(FANGO-PVP),这一载体能够特异地将药物以及热量运送至目标肿瘤细胞中,起到了很好的肿瘤细胞杀伤效果[87].Wang 等[95]也报道了一种靶向分子修饰的石墨烯-介孔硅纳米复合物(GSPI)用于体外神经胶质瘤细胞的光化疗.由于介孔结构的存在,GSPI展现出了更为高效的药物负载效率,同时其释药能力被发现是热敏感及pH 依赖的,这为肿瘤的光化疗提供了一个更为有效的平台.
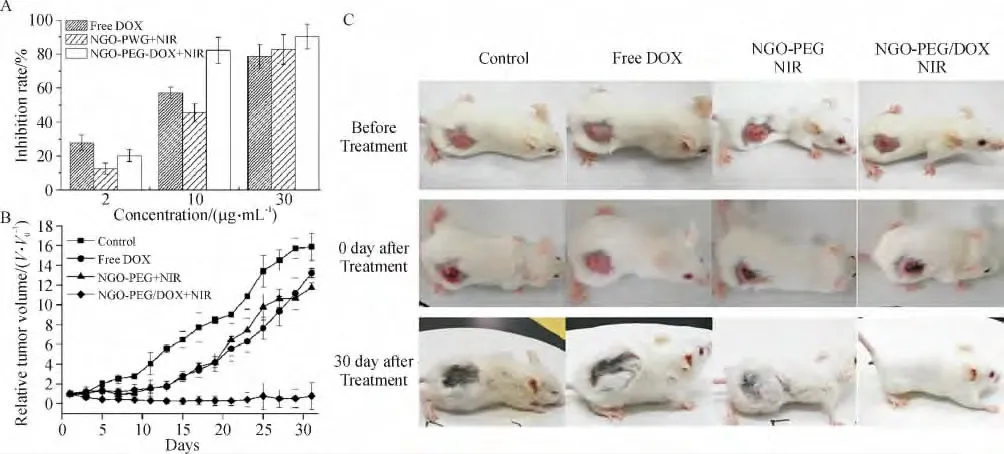
图4 基于NGO-PEG 的肿瘤NIR 光化疗Figure 4 Synergistic effect of chemo-photothermal therapy using NGO-PEG
5 总结与展望
GO 及其衍生物展示出了极好的作为一种新型肿瘤诊断、成像及治疗纳米试剂的潜能.在肿瘤诊断方面,不仅能够作为一种极好的荧光淬灭平台用于荧光相关的生物传感器,还能与贵金属纳米材料复合作为新颖的SERS 活性底物用于肿瘤细胞的诊断;成像方面,GO 展现出良好的光致发光特性和内在的拉曼特征,在肿瘤的荧光成像及拉曼成像方面均有很好的应用价值;同时,GO 还是一个优异的肿瘤治疗媒介,用于肿瘤的化学治疗和光热治疗. 当然,今后的研究仍然有许多内容值得进一步探讨.比如:GO 材料的进一步低毒化,特别是对于一些基于GO 的复合材料来说,生物相容性更是其在生物医学领域应用中亟待解决的问题;GO 在体内的分布以及最终去向也是关系到GO 能否最终应用到人体的重要问题;真正利用GO 于肿瘤的诊断治疗一体化的实验研究还很少,也是今后GO 研究的重点.
[1]Nie S. Understanding and overcoming major barriers in cancer nanomedicine[J]. Nanomedicine (Lond),2010,5(4):523-528.
[2]Misra R,Acharya S,Sahoo S K. Cancer nanotechnology:Application of nanotechnology in cancer therapy[J].Drug Discovery Today,2010,15(19/20):842-850.
[3]O'neal D P,Hirsch L R,Halas N J,et al. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles[J]. Cancer Letters,2004,209(2):171-176.
[4]Minelli C,Lowe S B,Stevens M M. Engineering nanocomposite materials for cancer therapy[J]. Small,2010,6(21):2336-2357.
[5]Maeda H,Wu J,Sawa T,et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics:A review[J]. Journal of Controlled Release,2000,65(1/2):271-284.
[6]Jain K K. Advances in the field of nanooncology[J].BMC Medicine,2010,8:83.
[7]Parveen S,Sahoo S K. Polymeric nanoparticles for cancer therapy[J]. Journal of Drug Targeting,2008,16(2):108-123.
[8]Shen S,Tang H,Zhang X,et al. Targeting mesoporous silica-encapsulated gold nanorods for chemo-photothermal therapy with near-infrared radiation[J]. Biomaterials,2013,34(12):3150-3158.
[9]Gutwein L G,Singh A K,Hahn M A,et al. Fractionated photothermal antitumor therapy with multidye nanoparticles[J]. International Journal of Nanomedicine,2012,7:351-357.
[10]Thakare V S,Das M,Jain A K,et al. Carbon nanotubes in cancer theragnosis[J]. Nanomedicine (Lond),2010,5(8):1277-1301.
[11]Novoselov K S,Geim A K,Morozov S V,et al. Electric field effect in atomically thin carbon films[J]. Science,2004,306(5696):666-669.
[12]Goenka S,Sant V,Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering[J].Journal of Controlled Release,2014,173(0):75-88.
[13]Yang K,Feng L,Shi X,et al. Nano-graphene in biomedicine:Theranostic applications[J]. Chemical Society Reviews,2013,42(2):530-547.
[14]Li J L,Tang B,Yuan B,et al. A review of optical imaging and therapy using nanosized graphene and graphene oxide[J]. Biomaterials,2013,34(37):9519-9534.
[15]Park S,Ruoff R S. Chemical methods for the production of graphenes[J]. Nature Nanotechnology,2009,4(4):217-224.
[16]Pan Y,Sahoo N G,Li L. The application of graphene oxide in drug delivery[J]. Expert Opinion on Drug Delivery,2012,9(11):1365-1376.
[17]Dreyer D R,Park S,Bielawski C W,et al. The chemistry of graphene oxide[J]. Chemical Society Reviews,2010,39(1):228-240.
[18]Compton O C,Nguyen S T. Graphene oxide,highly reduced graphene oxide,and graphene:Versatile building blocks for carbon-based materials[J]. Small,2010,6(6):711-723.
[19]Georgakilas V,Otyepka M,Bourlinos A B,et al. Functionalization of graphene:Covalent and non-covalent approaches,derivatives and applications[J]. Chemical Reviews,2012,112(11):6156-6214.
[20]Qu G,Wang X,Wang Z,et al. Cytotoxicity of quantum dots and graphene oxide to erythroid cells and macrophages[J]. Nanoscale Research Letters,2013,8(1):198.
[21]Yuan J,Gao H,Sui J,et al. Cytotoxicity evaluation of oxidized single-walled carbon nanotubes and graphene oxide on human hepatoma HepG2 cells:An iTRAQ-coupled 2D LC-MS/MS proteome analysis[J]. Toxicological Sciences,2012,126(1):149-161.
[22]Wang K,Ruan J,Song H,et al. Biocompatibility of graphene oxide[J]. Nanoscale Research Letters,2011,6:8.
[23]Chang Y,Yang S T,Liu J H,et al. In vitro toxicity evaluation of graphene oxide on A549 cells[J]. Toxicology Letters,2011,200(3):201-210.
[24]Zhang Y,Ali S F,Dervishi E,et al. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells[J]. ACS Nano,2010,4(6):3181-3186.
[25]Yuan J,Gao H,Ching C B. Comparative protein profile of human hepatoma HepG2 cells treated with graphene and single-walled carbon nanotubes:An iTRAQ-coupled 2D LC-MS/MS proteome analysis[J]. Toxicology Letters,2011,207(3):213-221.
[26]Liao K H,Lin Y S,Macosko C W,et al. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts[J]. ACS Applied Materials & Interfaces,2011,3(7):2607-2615.
[27]Duch M C,Budinger G R,Liang Y T,et al. Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung[J]. Nano Letters,2011,11(12):5201-5207.
[28]Singh S K,Singh M K,Nayak M K,et al. Thrombus inducing property of atomically thin graphene oxide sheets[J]. ACS Nano,2011,5(6):4987-4996.
[29]Sun X,Liu Z,Welsher K,et al. Nano-graphene oxide for cellular imaging and drug delivery[J]. Nano Research,2008,1(3):203-212.
[30]Liu Z,Robinson J T,Sun X,et al. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs[J]. Journal of the American Chemical Society,2008,130(33):10876-10883.
[31]Yang K,Zhang S A,Zhang G X,et al. Graphene in mice:Ultrahigh in vivo tumor uptake and efficient photothermal therapy[J]. Nano Letters,2010,10(9):3318-3323.
[32]Zhang X,Yin J,Peng C,et al. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration[J]. Carbon,2011,49(3):986-995.
[33]Yang K,Wan J,Zhang S,et al. In vivo pharmacokinetics,long-term biodistribution,and toxicology of PEGylated graphene in mice[J]. ACS Nano,2011,5(1):516-522.
[34]Zhang S,Yang K,Feng L,et al. In vitro and in vivo behaviors of dextran functionalized graphene[J]. Carbon,2011,49(12):4040-4049.
[35]Bao H,Pan Y,Ping Y,et al. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery[J]. Small,2011,7(11):1569-1578.
[36]Zhang Y,Hu W,Li B,et al. Synthesis of polymer-protected graphene by solvent-assisted thermal reduction process[J]. Nanotechnology,2011,22(34):345601.
[37]Hu W,Peng C,Lv M,et al. Protein corona-mediated mitigation of cytotoxicity of graphene oxide[J]. ACS Nano,2011,5(5):3693-3700.
[38]Kim J,Cote L J,Kim F,et al. Visualizing graphene based sheets by fluorescence quenching microscopy[J].Journal of the American Chemical Society,2010,132(1):260-267.
[39]Treossi E,Melucci M,Liscio A,et al. High-contrast visualization of graphene oxide on dye-sensitized glass,quartz,and silicon by fluorescence quenching[J]. Journal of the American Chemical Society,2009,131(43):15576-15577.
[40]Dong H,Gao W,Yan F,et al. Fluorescence resonance energy transfer between quantum dots and graphene oxide for sensing biomolecules[J]. Analytical Chemistry,2010,82(13):5511-5517.
[41]Wang Y,Kurunthu D,Scott G W,et al. Fluorescence quenching in conjugated polymers blended with reduced graphitic oxide[J]. Journal of Physical Chemistry C,2010,114(9):4153-4159.
[42]Wu S,Duan N,Ma X,et al. Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins[J]. Analytical Chemistry,2012,84(14):6263-6270.
[43]Yue Z,Lv P,Yue H,et al. Inducible graphene oxide probe for high-specific tumor diagnosis[J]. Chemical Communications,2013,49(37):3902-3904.
[44]Feng D,Song Y,Shi W,et al. Distinguishing folate-receptor-positive cells from folate-receptor-negative cells using a fluorescence off-on nanoprobe[J]. Analytical Chemistry,2013,85(13):6530-6535.
[45]Kneipp J,Kneipp H,Mclaughlin M,et al. In vivo molecular probing of cellular compartments with gold nanoparticles and nanoaggregates[J]. Nano Letters,2006,6(10):2225-2231.
[46]Qian X,Peng X H,Ansari D O,et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags[J]. Nature Biotechnology,2008,26(1):83-90.
[47]Campion A,Kambhampati P. Surface-enhanced Raman scattering[J]. Chemical Society Reviews,1998,27(4):241-250.
[48]Ren W,Fang Y,Wang E. A binary functional substrate for enrichment and ultrasensitive SERS spectroscopic detection of folic acid using graphene oxide/Ag nanoparticle hybrids[J]. ACS Nano,2011,5(8):6425-6433.
[49]Xu W,Ling X,Xiao J,et al. Surface enhanced Raman spectroscopy on a flat graphene surface[J]. Proceedings of the National Academy of Sciences,2012,109(24):9281-9286.
[50]Manikandan M,Nasser A H,Talib A,et al. Facile synthesis of gold nanohexagons on graphene templates in Raman spectroscopy for biosensing cancer and cancer stem cells[J]. Biosensors and Bioelectronics,2014,55:180-186.
[51]Chithrani B D,Ghazani A A,Chan W C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells[J]. Nano Letters,2006,6(4):662-668.
[52]Shamsaie A,Jonczyk M,Sturgis J,et al. Intracellularly grown gold nanoparticles as potential surface-enhanced Raman scattering probes[J]. Journal of Biomedical Optics,2007,12(2):020502.
[53]Liu Z,Hu C,Li S,et al. Rapid intracellular growth of gold nanostructures assisted by functionalized graphene oxide and its application for surface-enhanced Raman spectroscopy[J]. Analytical Chemistry,2012,84(23):10338-10344.
[54]Loh K P,Bao Q,Eda G,et al. Graphene oxide as a chemically tunable platform for optical applications[J].Nature Chemistry,2010,2(12):1015-1024.
[55]Li J L,Bao H C,Hou X L,et al. Graphene oxide nanoparticles as a nonbleaching optical probe for two-photon luminescence imaging and cell therapy[J]. Angewandte Chemie International Edition,2012,51(8):1830-1834.
[56]Peng C,Hu W,Zhou Y,et al. Intracellular imaging with a graphene-based fluorescent probe[J]. Small,2010,6(15):1686-1692.
[57]Ponomarenko L A,Schedin F,Katsnelson M I,et al.Chaotic dirac billiard in graphene quantum dots[J]. Science,2008,320(5874):356-358.
[58]Pan D,Zhang J,Li Z,et al. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots[J]. Advanced Materials,2010,22(6):734-738.
[59]Markovic Z M,Ristic B Z,Arsikin K M,et al. Graphene quantum dots as autophagy-inducing photodynamic agents[J]. Biomaterials,2012,33(29):7084-7092.
[60]Zhang M,Bai L,Shang W,et al. Facile synthesis of water-soluble,highly fluorescent graphene quantum dots as a robust biological label for stem cells[J]. Journal of Materials Chemistry,2012,22(15):7461-7467.
[61]Shen J,Zhu Y,Chen C,et al. Facile preparation and upconversion luminescence of graphene quantum dots[J]. Chemical Communications,2011,47(9):2580-2582.
[62]Jiang F,Chen D,Li R,et al. Eco-friendly synthesis of size-controllable amine-functionalized graphene quantum dots with antimycoplasma properties[J]. Nanoscale,2013,5(3):1137-1142.
[63]Li L L,Ji J,Fei R,et al. A facile microwave avenue to electrochemiluminescent two-color graphene quantum dots[J]. Advanced Functional Materials,2012,22(14):2971-2979.
[64]Lu J,Yeo P S E,Gan C K,et al. Transforming C60 molecules into graphene quantum dots[J]. Nat Nano,2011,6(4):247-252.
[65]Yan X,Cui X,Li L S. Synthesis of large,stable colloidal graphene quantum dots with tunable size[J]. Journal of the American Chemical Society,2010,132 (17):5944-5945.
[66]Tang L,Ji R,Cao X,et al. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots[J]. ACS Nano,2012,6(6):5102-5110.
[67]Dong Y,Shao J,Chen C,et al. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid[J]. Carbon,2012,50(12):4738-4743.
[68]Wu C,Wang C,Han T,et al. Insight into the cellular internalization and cytotoxicity of graphene quantum dots[J]. Advanced Healthcare Materials,2013,2(12):1613-1619.
[69]Zhu S,Zhang J,Qiao C,et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications[J]. Chemical Communications,2011,47(24):6858-6860.
[70]Liu Q,Guo B,Rao Z,et al. Strong two-photon-induced fluorescence from photostable,biocompatible nitrogendoped graphene quantum dots for cellular and deep-tissue imaging[J]. Nano Letters,2013,13(6):2436-2441.
[71]Kudin K N,Ozbas B,Schniepp H C,et al. Raman spectra of graphite oxide and functionalized graphene sheets[J]. Nano Letters,2008,8(1):36-41.
[72]Hu C,Rong J,Cui J,et al. Fabrication of a graphene oxide-gold nanorod hybrid material by electrostatic selfassembly for surface-enhanced Raman scattering[J].Carbon,2013,51:255-264.
[73]Kim Y K,Na H K,Lee Y W,et al. The direct growth of gold rods on graphene thin films[J]. Chemical Communications,2010,46(18):3185-3187.
[74]Xu C,Wang X. Fabrication of flexible metal-nanoparticle films using graphene oxide sheets as substrates[J].Small,2009,5(19):2212-2217.
[75]Liu Z,Guo Z,Zhong H,et al. Graphene oxide based surface-enhanced Raman scattering probes for cancer cell imaging[J]. Physical Chemistry Chemical Physics,2013,15(8):2961-2966.
[76]Liu Q,Wei L,Wang J,et al. Cell imaging by graphene oxide based on surface enhanced Raman scattering[J].Nanoscale,2012,4(22):7084-7089.
[77]Li D,Muller M B,Gilje S,et al. Processable aqueous dispersions of graphene nanosheets[J]. Nature Nanotechnology,2008,3(2):101-105.
[78]Robinson J T,Tabakman S M,Liang Y,et al. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy[J]. Journal of the American Chemical Society,2011,133 (17):6825-6831.
[79]Yang K,Wan J,Zhang S,et al. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power[J].Biomaterials,2012,33(7):2206-2214.
[80]Akhavan O,Ghaderi E. Graphene nanomesh promises extremely efficient in vivo photothermal therapy[J].Small,2013,9(21):3593-3601.
[81]Markovic Z M,Harhaji-Trajkovic L M,Todorovic-Markovic B M,et al. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes[J]. Biomaterials,2011,32(4):1121-1129.
[82]Lu C H,Zhu C L,Li J,et al. Using graphene to protect DNA from cleavage during cellular delivery[J]. Chemical Communications,2010,46(18):3116-3118.
[83]Zhang L,Lu Z,Zhao Q,et al. Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide[J]. Small,2011,7(4):460-464.
[84]Sahu A,Choi W I,Lee J H,et al. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy[J]. Biomaterials,2013,34(26):6239-6248.
[85]Feng L,Liu Z. Graphene in biomedicine:opportunities and challenges[J]. Nanomedicine (Lond),2011,6(2):317-324.
[86]Zhang W,Guo Z,Huang D,et al. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide[J]. Biomaterials,2011,32(33):8555-8561.
[87]Qin X C,Guo Z Y,Liu Z M,et al. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy[J]. Journal of Photochemistry and Photobiology B,2013,120:156-162.
[88]Zhang L,Xia J,Zhao Q,et al. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs[J]. Small,2010,6(4):537-544.
[89]Huang P,Xu C,Lin J,et al. Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy[J]. Theranostics,2011,1:240-250.
[90]Wang C,Li J,Amatore C,et al. Gold nanoclusters and graphene nanocomposites for drug delivery and imaging of cancer cells[J]. Angewandte Chemie International Edition,2011,50(49):11644-11648.
[91]Yang X,Wang Y,Huang X,et al. Multi-functionalized graphene oxide based anticancer drug-carrier with dualtargeting function and pH-sensitivity[J]. Journal of Materials Chemistry,2011,21(10):3448-3454.
[92]Ma X,Tao H,Yang K,et al. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery,photothermal therapy,and magnetic resonance imaging[J]. Nano Research,2012,5(3):199-212.
[93]Misra S K,Kondaiah P,Bhattacharya S,et al. Graphene as a nanocarrier for tamoxifen induces apoptosis in transformed cancer cell lines of different origins[J]. Small,2012,8(1):131-143.
[94]Wen H,Dong C,Dong H,et al. Engineered redox-responsive PEG detachment mechanism in PEGylated nanographene oxide for intracellular drug delivery[J]. Small,2012,8(5):760-769.
[95]Wang Y,Wang K,Zhao J,et al. Multifunctional mesoporous silica-coated graphene nanosheet used for chemophotothermal synergistic targeted therapy of glioma[J].Journal of the American Chemical Society,2013,135(12):4799-4804.
[96]Liu G,Shen H,Mao J,et al. Transferrin modified graphene oxide for glioma-targeted drug delivery:In vitro and in vivo evaluations[J]. ACS Applied Materials & Interfaces,2013,5(15):6909-6914.
[97]Zhang G,Chang H,Amatore C,et al. Apoptosis induction and inhibition of drug resistant tumor growth in vivo involving daunorubicin-loaded graphene-gold composites[J]. Journal of Materials Chemistry B,2013,1(4):493-499.
