BnWRI1 coordinates fatty acid biosynthesis and photosynthesis pathways during oil accumulation in rapeseed
2014-11-22XueLongWuZhiHongLiuZhangHuaHuandRuiZhiHuang
Xue-Long Wu,Zhi-Hong Liu,Zhang-Hua Hu and Rui-Zhi Huang
Institute of Virology and Biotechnology,Zhejiang Academy of Agricultural Sciences,Key Laboratory of Plant Metabolic Engineering of Zhejiang Province,Hangzhou 310021,China.*Correspondence:huangrz2001@aliyun.com
INTRODUCTION
Carbohydrates,oils,and specialized proteins are major storage compounds found in most seeds.These reserves sustain young seedlings during germination before photosynthesis begins and are involved in the maintenance of the species by connecting two distinct sporophytic generations(Sullivan and Deng 2003).Seed oils composed mainly of triacylglycerols(TAGs)have significant economic value in agricultural and industrial markets(Wang et al.2012;Jiang et al.2013).Understanding the genetic control of TAG biosynthesis in seeds is a major challenge as a step along the way to increasing yields.
The “green” oil seeds,such as Arabidopsis,soybean,and rapeseed,are green during embryogenesis,which is accompanied by TAG accumulation,demonstrating their light-utilizing capacity.Photosynthesis in “green” seeds plays a substantial role in the improved efficiency of oil synthesis by providing adenosine triphosphate(ATP),reductants,and increased metabolic flux(Schwender et al.2004;Goffman et al.2005;Hay and Schwender 2011).Transcriptomic and proteomic analyses of Arabidopsis and rapeseed during seed filling provided additional evidence that several photosynthesisrelated genes share the bell-shaped expression profile of the core fatty acid(FA)biosynthetic genes(Ruuska et al.2002;Hajduch et al.2006;Niu et al.2009),suggesting that some regulatory factors play roles in controlling their expression.Overexpression of the BnGRF2 gene from rapeseed caused upregulation of several genes related to FA modification,oil deposition,and photosynthesis in Arabidopsis(Liu et al.2012).However,considering that BnGRF2 preferentially expressed in silique wall and genes assessed in de novo FA synthesis in plastids did not include in BnGRF2 putative target genes(Liu et al.2012),it is apparent that other transcriptional regulators may contribute to coordinate FA biosynthesis and photosynthesis pathways in Brassica napus developing seeds.
The AP2/EREB subfamily transcription factor WRI1 is a key regulator of FA biosynthesis(Ruuska et al.2002;Baud et al.2007,2009;Maeo et al.2009).WRI1 regulates expression of the FA biosynthesis genes by directly binding to the AW-box sequence,which is conserved among proximal promoter regions of the genes(Ruuska et al.2002;Baud et al.2009;Maeo et al.2009;Pouvreau et al.2011;To et al.2012).The wri1-deficient mutants produce wrinkled seeds with an 80%reduction in seed oil content(Focks and Benning 1998).Overexpression of either WRI1 from Arabidopsis or a homolog from rapeseed under the control of the CaMV35S promoter in Arabidopsis can increase oil content in seed and TAGs in vegetative tissues as well due to the ectopic accumulation of WRI1 transcripts(Cernac and Benning 2004;Liu et al.2010;Sanjaya et al.2011).EgWRI1 from oil palm(Elaeis guineensis)also can restore the reduced oil phenotype of wri1(Ma et al.2013).Interestingly,ZmWRI1 from maize not only has a role in seed oil biosynthesis but also in amino acid(a.a.)biosynthesis(Pouvreau et al.2011),suggesting a conserved function of WRI1 in mono-and di-cotyledon plants and other roles in plants remaining to be elucidated.The master seed development regulators LEC1 and LEC2 directly regulate expression of WRI1(Baud et al.2007;Mu et al.2008).Additional roles of WRI1 and its homologs in regulating sugar metabolism during seed germinatiion and fiber development have also been investigated(Cernac et al.2006;Qu et al.2012),which suggest that WRI1 may have pleiotropic roles in plants.In the context that genes related to FA biosynthesis and photosynthesis are tightly correlated during seed development(Ruuska et al.2004;Goffman et al.2005;Li et al.2006;Han et al.2012),we hypothesized that WRI1 or its homolog from“green seed”crop is involved in the photosynthesis pathway to promote oil accumulation in developing seeds.
In this study,the role of BnWRI1,a homolog of WRI1 from B.napus,in FA biosynthesis was emphasized in the oilseed crop rapeseed.As expected,overexpression of BnWRI1 in a seedspecific manner with napin promoter could obviously increase seed oil content,thus showing possibilities for use of the transgenic rapeseed lines in breeding programs.However,increased chlorophyll(Chl)content in developing seeds and also seed mass in matured seeds was found in the seed-specific BnWRI1 transgenic lines.Actually,BnWRI1 was co-expressed with the marker genes of FA biosynthesis and photosynthesis,and overexpression of BnWRI1 upregulated these marker genes’expression.Further,the BnWRI1 protein could directly activate the transcription of genes involved in both FA biosynthesis and photosynthesis pathways by interacting with the GT1-element and/or GCC-box in their promoters.These results highlight the role of BnWRI1 gene in coordinating the FA biosynthesis and photosynthesis pathways in green seeds,which in turn promoted the flow of carbon to oil accumulation in seeds.
RESULTS
Seed-specific overexpression of BnWRI1 increases seed oil content and seed mass in rapeseed
Ectopic expression of WRI1 driven by the CaMV35S promoter has been shown to cause increased TAGs in vegetative tissues(Cernac and Benning 2004;Liu et al.2010;Sanjaya et al.2011),yet it may bring some aberrant development of the transgenic lines(Cernac and Benning 2004).In order to test whether overexpression of the WRI1-like gene could increase oil accumulation in rapeseed,the oilseed crop usually characterized with much higher oil content than Arabidopsis and maize,BnWRI1,the homolog of WRI1 in rapeseed,was isolated from B.napus.BnWRI1 seed-specific overexpression construct NAPINPr::BnWRI1 was generated and transformed into ZGY2,a rapeseed cultivar with an average oil content of approximately 49%when grown in the Yangtze River Valley(a major rapeseedproducing area of the world).Oil content(percentage on a dry weight basis)analysis of independent transgenic rapeseed lines demonstrated that the NAPINPr::BnWRI1 transgenic lines OE7,OE26,and OE27 have significantly higher oil content than that of untransformed controls(Figure 1A).
As the major storage compounds in rapeseed are TAGs and proteins(Li et al.2006),the increased oil content could be a result of either more TAG deposition or reduced protein levels in mature seeds.To elucidate it,the mature seeds’mass of the transgenic lines was individually collected and weighed.Compared to that of untransformed plants,the NAPINPr::BnWRI1 transgenic rapeseed plants had significantly increased average 1 000-seed weights(Figure 1B)and exhibited obviously bigger seeds(Figure 1C).However,there was no difference in the average yield per plant between the NAPINPr::BnWRI1 transgenic lines and untransformed plant(Table S1),indicating that the increased oil content is not a result of reduced yield,but partially a consequence of more carbon flux toward seed oil biosynthesis mediated by BnWRI1.It also proved that seedspecific overexpression of BnWRI1 is a potential approach to increase seed oil content in rapeseed.
Lipids in Brassica species are generally stored in oil bodies.The quantity and total volume of oil bodies in cotyledon storage cells of mature seeds were correlated with the seed oil content(Dong et al.2009;He and Wu 2009).To assess possible ultrastructural affects of altered BnWRI1 expression,the matured embryos of ZGY2 and NAPINPr::BnWRI1 transgenic rapeseed were further examined by transmission electron microscopy(TEM),and the representative micrographs are presented.As shown in Figure 1D,no obvious morphology alterations in oil bodies were observed,whereas the proportion of sum area of oil bodies per cell was increased in cotyledon cells of the NAPINPr::BnWRI1 transgenic plants compared with the untranformed controls.The TEM observation result was consistent with the elevated oil accumulation in BnWRI1 overexpressed rapeseed.Additionally,the FA content and composition in the transgenic seeds were further compared to evaluate which types of FAs were abundant.As shown in Table 1,most of the examined unsaturated FA content increased in the transgenic NAPINPr::BnWRI1 lines.However,the FA compositions had no statistical change(Table S2),indicating that overexpression of BnWRI1 only increased the total FA content but did not alter their relative ratios.
BnWRI1 is co-expressed with genes involved in FA biosynthesis and photosynthesis during seed development
In order to examine further the possible involvement of BnWRI1 in the regulation of FA biosynthesis in rapeseed,detailed investigation of the transcription profile of BnWRI1 during seed development was performed.The quantitative reverse transcription polymerase chain reaction(qRT-PCR)results exhibited a developmental regulation of BnWRI1 expression(Figure 2A).The transcripts were not detectable before 17 d after anthesis(DAA),then increased and remained constant until 23 DAA,when seeds were at mid-maturation stage.The transcripts peaked at 26–29 DAA when oil rapidly accumulated,but then declined substantially(Figure 2A,B).The temporal expression pattern of BnWRI1 during seed development mirrored those of type II FA biosynthesis genes previously described(Niu et al.2009).Actually,BnBCCP,a homolog of the FA biosynthesis pathway marker BCCP gene(Ruuska et al.2002),showed a similar expression profile to that of BnWRI1(Figure 2A),suggesting that BnWRI1 may simultaneously regulate expression of the marker gene in FA biosynthesis.Consistent with that is the FA content in developing seeds,which increased very slowly at the early stage and then rapidly from 26 DAA,and reached its maximum at 38 DAA(Figure 2B).
Based on that oil accumulation is not only tightly developmentally dependent,but also significantly influenced by light levels(Li et al.2006;Han et al.2012),and also on that light harvested by seeds within siliques strongly drives oil biosynthetic processes(Ruuska et al.2004;Goffman et al.2005),an interesting issue was whether BnWRI1 participates in the regulation of photosynthesis to promote the carbon flux to oil accumulation in developing seeds.For this purpose,the expression profile of the BnCAB gene,a homolog of the photosynthesis marker CAB gene(Ruuska et al.2002),was investigated comprehensively in the developing seeds from the very early stage to the final mature stage.Because the Chl content was positively related to its photosynthetic activity throughout B.napus seed development(Eastmond et al.1996),the Chl content was also determined to assay the effects of BnWRI1 overexpression on seed photosynthetic capacities.What is noteworthy is that the BnCAB gene also had a similar temporal expression pattern to that of BnWRI1 throughout seed development(Figure 2A),indicating that BnWRI1 may regulate its expression.Correspondingly,Chl content reflected the expression of genes in photosynthesis with a peak at 26–38 DAA(Figure 2C),which also corresponded with the period of rapid FA accumulation(Figure 2B,C).Chl content was almost consistent with the color phenotype of the developing seeds(Figure 2D).
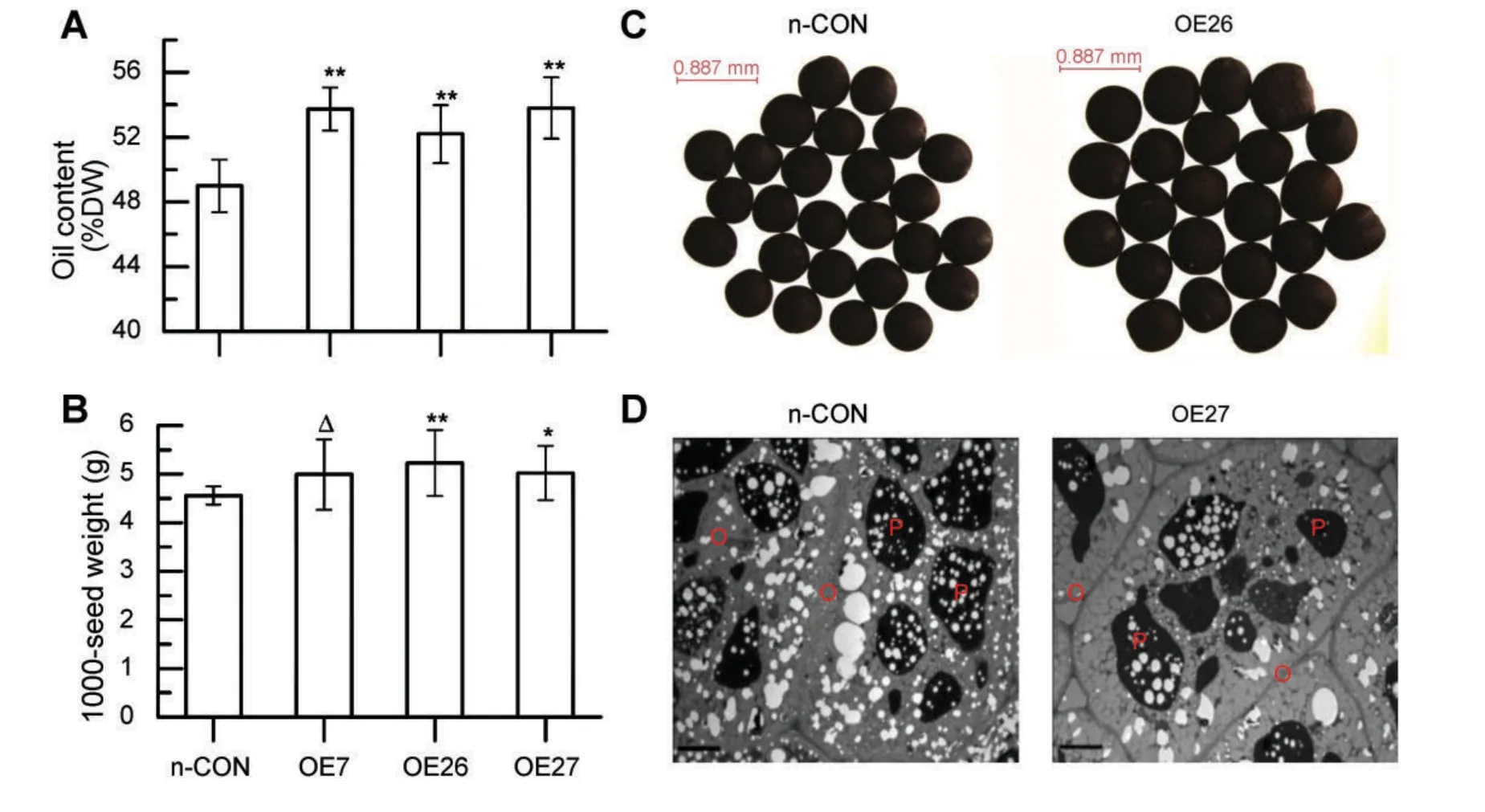
Figure 1.Seed-specific overexpression of BnWRI1 enhanced seed oil content and seed mass in rapeseed(A)Oil contents of untransformed rapeseed(n-CON)and three independent T3 NAPINPr::BnWRI1 transgenic lines(OE7,OE26,and OE27).The oil contents were determined by the Soxhlet method.DW,dry weight.(B)Matured seed weight of untransformed rapeseed and three transgenic lines.(C)Representative images of matured seeds of NAPINPr::BnWRI1 transgenic rapeseed and the untransformed control.(D)Representative transmission electron microscopy images of matured embryos of NAPINPr::BnWRI1 transgenic rapeseed and the untransformed control.O,oil body;P,protein body.Bars=5 μm.For all experiments,three biological replicates were performed.Each biological replicate consisted seeds from at least 10 plants.Values represented means±SD.Significant differences were determined by one-way ANOVA(Δ,P<0.1;*P<0.05;**P<0.01).For(C,D),three biological replicates were conducted,yielding similar results.Images from one representative repeat are shown.
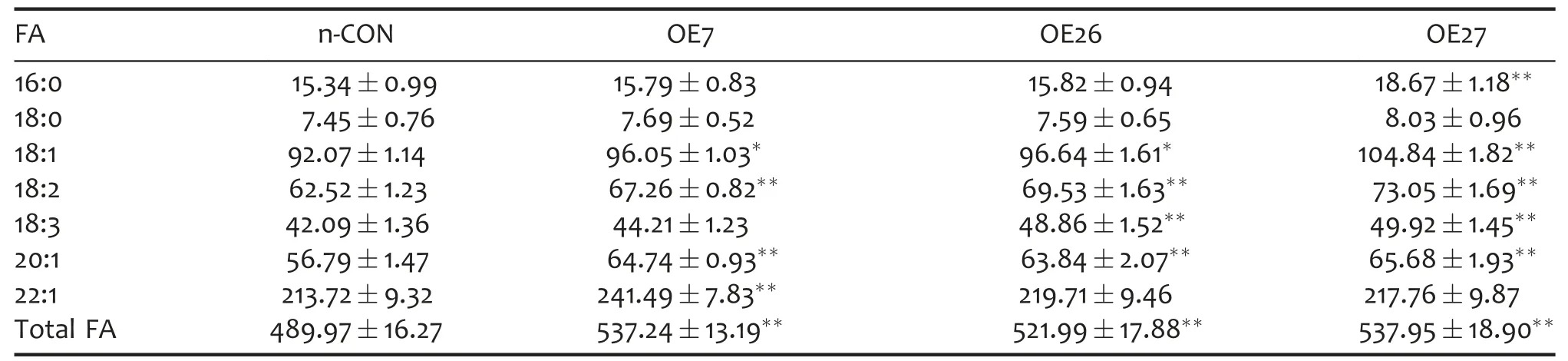
Table 1.FA contents(mg/g DW)in seeds of n-CON and NAPINPr::BnWRI1 transgenic rapeseed

Figure 2.BnWRI1 coordinated expression of genes involved in photosynthesis and FA biosynthesis(A)Quantitative reverse transcription polymerase chain reaction(qRT-PCR)analysis of expression of BnWRI1,BnBCCP,and BnCAB in developing seeds of Brassica napus L.cv.ZGY2.Transcript levels were normalized against that for the BnACT gene and relative expressions were compared with the expression of each gene in 20 d after anthesis(DAA)seed(set as 1).(B)The time courses of total fatty acid(FA)accumulation in developing seeds of rapeseed.(C)The time courses of chlorophyll contents in developing seeds of rapeseed.Values are the means of three replicate measurements.(D)Phenotypes of rapeseed seeds at different development stages.(E)Expressions of BnWRI1,BnBCCP,BnCAB,and BnrbcS in rapeseed developing seedswere dramaticallyrepressed by darkness.Brassica napus L.cv.ZGY2 plants were grown in a photoperiod of 14:10 of light:dark in greenhouse.Siliques(28 DAA)at the main axis were wrapped with aluminum foil alternately after 4 h light and harvested 0,4,8,or 24h after dark treatment.Samples without dark treatment at the indicated timepoints were used as controlsfor light treatment.Transcript levels were normalized to transcripts of the BnACT gene and relative expressions were compared with that at 0 h(set as 1).L,light;D,darkness.All data are presented as means±SD.Significant differences of the qRT-PCR data were determined by one-way ANOVA test(**P<0.01;***P<0.001).
The expression patterns of the genes and the accumulations of FA and Chl contents indicated that BnWRI1 is likely to function as a coordinator of both the FA biosynthesis and photosynthesis pathways.The potential effect of BnWRI1 on regulating the expression of FA biosynthesis and photosynthesis genes during light/dark treatment of the developing seeds was examined at 28 DAA,a development stage that the embryo occupies a vital portion of seed volume,oil accumulates rapidly,and most FA synthesis genes are highly expressed(Niu et al.2009).As the results shown in Figure 2E,dark treatment caused a dramatic reduction of BnWRI1 transcripts in developing seeds inside siliques,and the inhibitory effect increased with prolonged darkness.All three BnBCCP,BnrbcS,and BnCAB genes exhibited similar variation trends to that of BnWRI1 during the treatment(Figure 2E).The results implied a functional linkage among these genes and further supported the role of BnWRI1 in regulating both FA biosynthesis and photosynthesis pathways.

Figure 3.Overexpression of BnWRI1 enhanced transcriptions of fatty acid(FA)biosynthesis-and photosynthesis-related genes in developing seeds(A)Quantitative reverse transcription polymerase chain reaction(RT-PCR)analysis of BnWRI1,BnBCCP,and BnCAB in 28 d after anthesis(DAA)developing seeds of untransformed rapeseed(n-CON)and three independent NAPINPr::BnWRI1 transgenic lines OE7,OE26,and OE27.Transcript levels were normalized against the BnACT gene.The relative expression was calculated by setting the expression of each gene in developing seeds of n-CON as 1.0.(B)Chlorophyll(Chl)contents in 28 DAA developing seeds of three independent transgenic lines OE7,OE26,and OE27.(C)Chl contents per seed of three transgenic lines OE7,OE26,and OE27 at 28 DAA.All data are presented as means±SD of three biological replicates.Significant differences of the data were determined by one-way ANOVA test(**P<0.01;***P<0.001).
Overexpression of BnWRI1 promotes expression of the FA biosynthesis-and photosynthesis-related genes in seeds
Because BnWRI1 was co-expressed with both FA biosynthesisand photosynthesis-related genes,experiments were performed to test whether the genes could be co-regulated by BnWRI1.The marker genes BnBCCP and BnCAB for FA biosynthesis and light-harvesting pathways,respectively,in rapeseed were selected and their expression levels in the BnWRI1 overexpressed plants were compared at the crucial seed developmental stage of 28 DAA by qRT-PCR analysis.As shown in Figure 3A,the transcripts of BnWRI1 were substantially increased almost fourfold to fivefold in seeds of all three NAPINPr::BnWRI1 transgenic rapeseed lines OE7,OE26,and OE27 as compared to untransformed ZGY2,which were expected due to the seed-specific napin promoter and also highly consistent with the increased oil content for each line(Figure 1A).However,expression of the FA biosynthesis marker gene BnBCCP was upregulated threefold in the transgenic lines(Figure 3A).Synchronously,the photosynthesis marker gene BnCAB was also obviously upregulated by the NAPINPr::BnWRI1 transgene showing a twofold increase(Figure 3A).These results further confirmed that BnWRI1 coordinates the expression of at least some genes related to FA biosynthesis and light-harvesting pathways.
Seed-specific overexpression of BnWRI1 could promote both FA biosynthesis and photosynthesis,which had led to increased oil accumulation in the transgenic seeds(Figure 1A).Therefore,it was interesting to test whether the NAPINPr::BnWRI1 transgenic lines were accompanied by elevated photosynthesis capacity in the developing seeds.As shown in Figure 3B,C,the Chl contents in seeds of all three transgenic lines were obviously elevated by 19.5%–28.8%(on a fresh weight basis)and 32.86%–35.43%(on a seed basis),which indicated the photosynthetic capacity could be enhanced in seeds of the BnWRI1 overexpression transgenic plants.
BnWRI1 is nucleus localized and functions as a transcriptional activator
BnWRI1 is a homolog of WRI1 from B.napus.It belongs to the AP2/EREBP transcription factor family.However,except for a short stretch of basic a.a.(RPKRAKRAKK,a.a.30–39)in the N-terminal region(Figure S1),no conventional nuclear location sequence was found in the deduced BnWRI1 protein sequence by prediction (http://cubic.bioc.columbia.edu/services/loctree).To verify whether BnWRI1 is located in the nucleus,the BnWRI1-eGFP fusion protein was transiently expressed in Nicotiana benthamiana leaves via agroinfiltration.Observation of the green fluorescence by confocal laser scanning microscopy revealed that the BnWRI1-eGFP fusion protein was exclusively localized in the nucleus,contrasting with the cytoplasmic and nuclear distributions of the control GFP protein(Figure 4A),which is consistent with BnWRI1 as a transcription factor.
Sequence analysis indicated that BnWRI1 contains two putative transcriptional activation domains at the C terminus,one acidic residue-rich region and another serine/threonine residue-rich region at positions 247–291 and 377–408,respectively(Figure S1).To test whether BnWRI1 possessed transcriptional activation activity,the entire coding region of BnWRI1 and a series of deletion mutants were fused in-frame to the GAL4 DNA-binding domain according to the method of Meng et al.(2005).The fusion plasmids and the negative control plasmid pBD were transformed into yeast AH109.If BnWRI1 or its derivatives include transcriptional activation domain(s),the fusion protein would activate transcription and expression of the reporter genes(HIS3,Ade,and b-gal).The yeast transformants containing pBD-BnWRI1 or derivatives that included the C-terminal region of BnWRI1 grew well on the SD/–Trp/–Ade/–His medium,whereas transformants harboring pBD or fusion constructs without the C-terminal region did not grow on the same medium(Figure S2).The liquid βgalactosidase(β-gal)assay further confirmed that the C-terminal region contained transformants show much higher bgal activities than those without the C-terminal or pBD alone(Figure 4B).These results demonstrated that the transcriptional activation domain in the C-terminal region is essential for its transcriptional activity of BnWRI1.
BnWRI1 binds to both GCC-box and GT1-element
Next,it was further tested whether BnWRI1 directly binds to the promoter regions of the FA biosynthesis-and photosynthesis-related genes using a yeast one hybrid system.Because the whole genome sequence of B.napus is currently not available,and the wri1-like gene from B.napus were able to complement the wri1 Arabidopsis mutant defective in FA accumulation(Liu et al.2010),in the 180 bp upstream region of BCCP2 promoter(pBCCP2),which was previously verified to be able to interact with WRI1(Baud et al.2009;To et al.2012),and a fragment of the LHB1B2(a photosystem II(PSII)type I Chl a/bbinding protein)promoter with-1 614 to-1 226 bp relative to the ATG codon(pLHB1B2)from Arabidopsis were used as baits of the yeast one hybrid assay.The LHB1B2 promoter fragment included at least two GCC-boxes and one GT1-element,which could be specifically bound by AP2/EREBP family transcription factors(Shigyo et al.2006)and mediate light-related transcription(Green et al.1988),respectively.The selection medium of SD/-Leu/-Trp/-His supplemented with 10 mmol/L 3-amino-1,2,4-triazole(3-AT),a concentration enough to eliminate the leaky HIS3 expression,was used to test the interaction of BnWRI1 with the promoter baits.The yeast strains cotransformed with pGAD-BnWRI1 plus either pBCCP2 or pLHB1B2,grown well on the selection medium,whereas the yeast strain containing pGAD-BnWRI1 plus pHIS2,the negative controls,did not(Figure 5A).These results confirmed the direct interaction of BnWRI1 with FA biosynthesis-and photosynthesis-related gene promoters.
国防科技工业主要是指从事武器科研装备生产、研发工作的相关工业产业,具体包括航空、航天、核能、电子、船舶等多个部门,其对于我国国防事业的建设与发展有着直接的影响。在计划经济时期,军工企业本身所存在的竞争压力非常之小,同时国家的资金支持力度也比较大,因而对于自身的竞争优势以及经济收益问题并不重视[1]。然而随着市场经济的不断发展,军工企业要想生存并长期发展下去,就必须要尊重市场规律,参与到市场竞争中来,不断提升自身的核心竞争力,而市场导向下的军民融合发展策略则恰恰能够帮助军工企业实现这一目标。因此,军工企业的军民融合发展,在很大程度上也是为了满足市场经济环境下国防科技工业的进一步发展需求。
In order to further elucidate the regulation mechanism of BnWRI1,functional elements in the promoters of the putative WRI1 target genes in Arabidopsis were screened using the PLACE program(Higo et al.1998).The putative target genes were selected out based on their differential expressions between wild-type and wri1 mutant by microarray(Ruuska et al.2002).Both Arabidopsis WRI1 and BnWRI1 contain two putative AP2/EREBP DNA-binding domains(Figure S1),which usually bind specifically to GCC-boxes(Shigyo et al.2006).However,only promoters of nine downregulated genes and two upregulated genes contained at least one GCC-box(Figure S3).Among which,several have proved to be directly involved in FA biosynthesis,including the dominant chloroplast plastidic pyruvate kinase beta subunit 1(At5g52920),pyruvate dehydrogenase E1a-like subunit(At1g01090),and the putative pyruvate dehydrogenase beta subunit E1b (At1g30120)(Figure S3).What is noteworthy is that three photosynthetic genes LHB1B2(At2g34420),CAB3(At1g29910),and CAB1(At1g29930)were also included(Figure S3).The results supported the hypothesis that Arabidopsis WRI1 and BnWRI1 act as transcriptional regulators of GCC-box-mediated gene expression.In contrast to GCC-box,almost all of those target promoters of WRI1 contained at least one GT1-element(data not shown),a motif associated with transcriptional control of light-responsive genes(Green et al.1988).
Therefore,yeast one hybrid assays were performed to determine which cis-element of GCC-box and GT1-element was essential for BnWRI1 transcription activation activity.Yeast reporter plasmids carrying either the GT1-element(GT1::HIS3)or the GCC-box(GCC::HIS3)upstream of the HIS3 reporter gene were generated.The select medium of SD/-Leu/-Trp/-His supplemented with 30 mmol/L 3-AT,a concentration to completely eliminate the leaky HIS3 expression of GT1::HIS3 and GCC::HIS3,was used to test the interaction.As shown in Figure 5(B),yeast strains(Y187)co-transformed with either GCC::HIS3 or GT1::HIS3 and pGAD-BnWRI1 expressing the BnWRI1-GAL4 AD fusion protein could grow well on the select medium,indicating that BnWRI1 could bind to both elements.Additionally,in vivo activation effect of BnWRI1 on reporters containing the quintuplicate of either GCC-box or GT1-element was examined using the well-established transient expression assay method in N.benthamiana leaves(Yang et al.2000).Each quintuplicate was fused with the–49 minimal 35S promoter to drive the GUS reporter gene.When the GT1::GUS or GCC::GUS reporter alone was infiltrated into N.benthamiana,GUS activity was detected at a relatively low level(Figure 5C).While,coexpression of either GT1::GUS or GCC::GUS with 35SPr::BnWRI1 led to obvious increased GUS activities(Figure 5C).These results demonstrated that BnWRI1 directly activates genes containing GT1-element and/or GCC-box within their promoters in planta.

Figure 4.BnWRI1 is nucleus localized and acts as a transcriptional activator(A)Targeting of the BnWRI1 protein to the nuclei of plant cells.Green fluorescent protein(GFP)fluorescence in Nicotiana benthamiana epidermal cells with 35SPr::eGFP(1–3)or 35SPr::BnWRI1-eGFP(4–6)were examined at 2 d after infiltration by confocal microscopy.(B)Determination of the transcriptional activation domains in yeast cells.The β-galactosidase(β-gal)activities of transformants harboring different BnWRI1 deletions were determined on SD/Trp.The β-gal activity of cells carrying pBD-BnWRI1 with the entire coding sequence of BnWRI1 was set as 100.The empty vector pGBKT7 was used as a negative control.All data are presented as means±SD of three replicates.Significant differences were determined by one-way ANOVA test(*P<0.05;***P<0.001).
DISCUSSION
In this study,the function of BnWRI1 in coordinating both FA biosynthesis and photosynthesis pathways during seed development was characterized in detail.Previous studies have demonstrated that constitutive overexpression of WRI1(Cernac and Benning 2004)or BnWRI1(Liu et al.2010)in Arabidopsis leads to increased seed oil content,but with some negative pleiotropic phenotypes when seedlings were grown on sugar containing medium(Cernac et al.2006).Because oil content in the major rapeseed cultivars seeds nowadays is generally much higher than that in Arabidopsis seeds,it makes more sense to investigate whether upregulation of BnWRI1 can promote oil deposition in the oilseed crops with an already high oil genetic background.Here,transgenic rapeseed harboring seed-specific overexpression of BnWRI1 driven by the napin promoter had been generated.Transgenic plants had increased oil contents with augmented oil bodies and seed weight in the high oil rapeseed seeds(Figure 1).However,it was also found that improved oil content in the transgenic rapeseed seeds was accompanied by increased seed mass(Figure 1)and Chl content(Figure 3),which indicated that BnWRI1 I possibly involved in regulating seed photosynthesis to provide more carbon flux towards seed oil biosynthesis.

Figure 5.BnWRI1 binding activities in yeast and in planta(A)BnWRI1 bound to the promoters of BCCP2 and LHB1B2.The pAD-BnWRI1 plasmid and the reporter construct pBCCP or pLHB1B2 were co-transformed into yeast strain Y187.The co-transformation of the empty vector pHIS2 with pAD-BnWRI1 was used as a negative control.(B)BnWRI1 bound to the GCC-box and GT1-element by yeast one hybrid assay.The pAD-BnWRI1 plasmid and the reporter construct GCC::HIS3 or GT1::HIS3 were co-transformed into yeast strain Y187.(C)BnWRI1 activated the transcriptions of GCC-box and GT1-element reporter genes in planta.Transient assays of Nicotiana benthamiana leaves were conducted by cotransformed 35SPr::BnWRI1 with GCC::GUS or GT1::GUS(containing quintuple tandem repeated GCC-box or GT1-element,respectively)via agroinfiltration.GUS activities were monitored at 48 h after agroinfiltration by histochemical staining.The images of tobacco leaves showed GUS activities stained at 37°C for 3 h.Arrows indicate the infiltration locations.
Oil bodies are unique intracellular organelles of storage oil deposition(He and Wu 2009).The relationship between seed oil content and number,size,and morphology of oil bodies is intricate.The Arabidopsis dgat1 null mutants display decreased seed oil content and smaller oil bodies(Zhang et al.2009),while oleosin suppression resulted in significant decrease in seed oil content accompanied by unusually large oil bodies(Siloto et al.2006).In rapeseed,the ratio of sum areas of oil bodies per cotyledon cell is significantly positively correlated with seed oil content(Dong et al.2009;Hu et al.2013).Our TEM observation results showed that the seed of NAPINPr::BnWRI1 transgenic rapeseed exhibited an increase in oil body to cotyledon cell area ratio compared with the control(Figure 1D),in line with the increased oil content in the BnWRI1 overexpressed B.napus matured seed,as a consequence of the elevated FA biosynthesis and photosynthesis levels during seed development.
It is well known that de novo FA synthesis in source leaf chloroplasts is light dependent.Photosynthesis not only ensures nicotinamide adenine dinucleotide phosphate and ATP for energetically expensive FAs but also provides intermediates for the metabolism(Ruuska et al.2004;Goffman et al.2005).However,though seeds of several major oilseed crops including rapeseed(Figure 2D),soybean,and cotton are green during the course of seed development(Ruuska et al.2004),the situation is more complex in photosynthetic oilseeds partially because they are predominantly sink tissues and in a low-light environment inside the siliques.Light can partially filter through the silique wall and be captured by the developing green embryos and then drives the generation of intermediates,reductants,and ATP for FA synthesis(Asokanthan et al.1997;Ruuska et al.2002;Schwender and Ohlrogge 2002;Schwender et al.2003).Conversely,inhibition of PSII significantly decreases the conversion efficiency of carbon sources into storage products and consequently reduces biomass and oil synthesis(Ruuska et al.2004;Goffman et al.2005).Transcripts of photosynthesisrelated genes reach a maximum during the period of oil biosynthesis in the developing seeds of both Arabidopsis and rapeseed(Ruuska et al.2002,2004;Hajduch et al.2006;Niu et al.2009).All of these supported the opinion that light stimulates FA biosynthesis in developing green seeds.However,the regulatory factors involved in controlling co-expression of FA biosynthesis and photosynthesis pathways are rarely reported in a special focus on seeds.
In this study,the molecular mechanism how BnWRI1 mediated the cross-talk between FA biosynthesis and photosynthesis pathways during seed development was further unveiled.It was found that maximal Chl content in developing seeds of rapeseed occurred at the very time when oil rapidly accumulated(Figure 2).This kind of photosynthetic apparatus was consistent with the temporal expression patterns in developing seeds of BnCAB,which plays an important role in the light reactions by binding Chl a/b,and BnBCCP,which is involved in de novo FA biosynthesis(Figure 2A).Above all,BnWRI1 resembled those expression patterns and expressions of BnWRI1,BnBCCP,BnrbcS,and BnCAB were all obviously inhibited by dark treatment(Figure 2).Considering BnWRI1 and its homologs belong to the AP2/EREB transcription factor subfamily(Cernac et al.2006;Qu et al.2012),BnWRI1 was,therefore,emerging as a possible regulator of both FA biosynthesis and photosynthesis pathways.Furthermore,BnWRI1 overexpression led to significant upregulation of BnBCCP and BnCAB expression and hence the Chl contents in seeds of NAPINPr::BnWRI1 transgenic rapeseeds were increased(Figure 3),which indicated that BnWRI1 overexpression stimulates photosynthetic capacities of the transgenic seeds and in turn contributes to more oil accumulation.Together with the microarray analysis that genes related to FA biosynthesis and photosynthesis,such as genes encoding biotin carboxyl carrier protein(BCCP2),KAS I,and PSII type I Chl a/b-binding protein were downregulated in the Arabidopsis wri1 mutant(Ruuska et al.2002),these results suggest BnWRI1 serves as a central transcriptional regulator in the cross-talk between the major FA biosynthesis and photosynthesis pathways.
Previous studies have revealed that WRI1 and ZmWRI1 bind to the conserved AW-box present in the upstream regulating regions of genes involved in FA biosynthesis,which were also predicted to be putative target genes based on microarray analysis(Maeo et al.2009;Pouvreau et al.2011).Our results showed that BnWRI1 could function as a transcription activator like its Arabidopsis homolog:BnWRI1 protein localized to the nucleus(Figure 4),it possesses transcriptional activation domains at the C-terminal(Figure 4),and it can directly bind to the promoter regions of BCCP2 and LHB1B2(Figure 5).As lots of reported AP2/EREBP proteins,BnWRI1 could bind to GCC-box(Figure 5).However,in silico analysis of functional elements in the promoters of the putative WRI1 target genes showed that only several promoters contain at least one GCC-box(Figure S3),but almost all promoters have at least one copy of a light response GT1-element(data not shown),suggests that the GT1-element may be a more widespread binding site than the GCC-box for WRI1-like proteins.In fact,BnWRI1 displayed tight binding to the GT1-element in vivo(Figure 5C).Moreover,overexpression of BnWRI1 obviously promoted GUS activities driven by quintuplicate copies of either the GCC-box or GT1-element fused with the–49 minimal 35S promoter(Figure 5C).Together with the deep analysis of the putative target genes of WRI1 characterized by the GCC-box in their promoters included at least two genes encoding FA synthesisrelated proteins,the pyruvate dehydrogenase E1 alpha subunit and the putative pyruvate dehydrogenase E1 beta subunit,and the three genes encoding light-harvesting Chl a/b-binding proteins,LHB1B2,CAB1,and CAB3(Figure S3);the results,therefore,indicate that BnWRI1(WRI1)directly binds to the GT1-elements and GCC-boxes in the upstream regions of its target genes,and thereby coordinates FA biosynthesis and photosynthetic pathway.
In summary,we have provided new insights into the functions of BnWRI1.Except being a key regulator of oil accumulation in rapeseed,BnWRI1 is also involved in the crosstalk of FA synthesis and photosynthesis pathways during the course of seed development and oil accumulation.The possible underpinning mechanism is through regulating GT1-element and/or GCC-box-mediated gene expression.Therefore,seed-specific overexpression of BnWRI1 in rapeseed promotes increased seed oil accumulation and seed mass.
MATERIALS AND METHODS
Plant materials and growth conditions
Rapeseed(Brassica napus cv.ZGY2)plants were grown in the greenhouse with a 14:10 h light:dark cycle.To harvest seeds at different developmental stages,flowers on the main axis were tagged on the day of anthesis.The seeds were collected at nine different development stages from 8 to 32 DAA with an interval of 2 d.For dark treatment,28 DAA siliques on the main stems of ZGY2 plants were wrapped with aluminum foil alternately after 4 h light and harvested 0,4,8,or 24 h after dark treatment.Samples without dark treatment were collected at the indicated points as corresponding controls for light treatment.The developing seeds were separated from siliques.Seeds from unwrapped siliques nearest the wrapped ones were used as controls to dark treatment.All collected plant tissues were frozen in liquid nitrogen and stored at-80°C until use.
Isolation of the BnWRI1 gene from rapeseed
Seed-specific overexpression of BnWRI1 in rapeseed
For seed-specific overexpression of BnWRI1,the 35S promoter and other regions between the EcoRI and BamHI sites of pFGC5941 were replaced by a 1,144 bp napinA promoter(GenBank no.AF420598),generating the pFGC-NAPIN plasmid.The open reading frame of BnWRI1 was digested by BamHI and EcoRV from the pMD-BnWRI1 plasmid and inserted into the pFGC-NAPIN cut by BamHI and SmaI.The resulting NAPINPr::BnWRI1 construct was introduced into rapeseed as described previously(Zou et al.1997).Homozygous T3 rapeseed transgenic plants were used for further analysis.
Subcellular localization of BnWRI1-eGFP fusion protein
The eGFP reporter gene was amplified with primer pairs eGFPFXhoI-NcoI and eGFPR-BamHI,digested by restriction enzymes XhoI and BamHI and ligated into the plasmid pFGC5941 to form a pFGC-eGFP structure.Then,the BnWRI1 cDNA fragment without the stop codon amplified using the Pyrobest DNA Polymerase(TaKaRa Bio)with primer pairs BnWRI1-SalI and BnWRI1-NcoI was subcloned upstream of the start codon of the eGFP coding sequence into the pFGC–eGFP plasmid,resulting in a final chimeric 35SPr::BnWRI1-eGFP-TOCSconstruct,abbreviated as BnWRI1-eGFP.The chimeric constructs of BnWRI1-eGFP and the eGFP control plasmid were transiently expressed,respectively,in N.benthamiana leaves as previously described by Yang et al.(2000).Images were collected on a Leica TCS SP5 confocal microscope(Leica Microsystems,Wetzlar,Germany)within a 48 h period after agroinfiltration.
Yeast two hybrid assay and analysis of β-gal activity
The full open reading frame and a series of different portions of BnWRI1 with suitable restriction sites were obtained by PCR using primers listed in Table S3.The PCR products were digested and then fused in-frame with the GAL4 DNA-binding domain in the pGBKT7 vector.Constructs used were transferred into yeast strain AH109 and then streaked on SD/–Trp and SD/–Trp Ade-His-medium.The transcription activity of each fusion construct was evaluated according to its growth status after incubation at 30°C for 3 d.Relative transcriptional activations of the BD-BnWRI1 derivatives were measured by liquid β-gal assay.The pGBKT7 was used as a negative control.The β-gal activity of cells carrying pBDBnWRI1 was set to 100.Yeast transformation and the calculation of relative β-gal activities were performed according to the manufacturer’s instructions(Yeast Protocols Handbook;Clontech,Mountain View,CA,USA).
Yeast one hybrid assays
Yeast one hybrid assays were performed using the BD Matchmaker library construction and screening kits(Clontech).The reporter constructs were made in the plasmid pHIS2.The bait constructs GCC::HIS3 and GT1::HIS3 contained triple tandem repeats of the GCC-box(CAT AAG AGC CGC CAC T)or GT1-element(GTG TGT GGT TAA TAT G),respectively.These bait constructs were transformed into yeast strain Y187.The background level of HIS was determined by culturing the yeast strains on the SD/–His medium containing various concentrations of 3-AT.For construction of the GAD-BnWRI1 fusions,the BnWRI1 CDS was ligated in-frame to the GAL4 activation domain in the pGADT7-Rec2 vector,forming pGAD-BnWRI1.The pGAD-BnWRI1 plasmid was then co-transformed with GCC::HIS3 or GT1::HIS3 into Y187.Yeast transformants were tested on SD/-Leu/-His/-Trp medium containing an optimal concentration of 3-AT.
A similar procedure was adopted to test the binding ability of BnWRI1 to the promoters of BCCP2 and LHB1B2.An 180 bp fragment of the BCCP2 promoter according to Baud et al.(2009)and a fragment corresponding to the region-1,614 to-1,226 relative to the LHB1B2 translational start codon were amplified and inserted into the pHIS2 vector between the EcoRI and SacI sites.The corresponding reporter plasmids pBCCP2 and pLHB1B2 were co-transformed with the pGAD–BnWRI1 plasmid into Y187 and selected on appropriate media.
Transactivation of the GCC-box and GT1-element reporter gene in N.benthamiana leaves
The GCC-box or GT1-element was multimerized five times and placed upstream of the minimal 35S promoter in the–49-35SPr::GUS construct.The resulting reporter constructs were designated as GCC::GUS and GT1::GUS,respectively.The BnWRI1 effector construct was 35SPr::BnWRI1.Transient transformations were conducted by co-transforming the reporter/effector constructs GCC::GUS/35SPr::BnWRI1,GT1::GUS/35SPr::BnWRI1,or–49-35SPr:GUS/35SPr::BnWRI1 by Agrobacterium-mediated infiltration into the abaxial surface of N.benthamiana leaves as described(Yang et al.2000).GCC::GUS or GT1::GUS constructs were used as control.GUS activities in the agroinfiltrated leaves within a 48 h period were histochemically stained for 3 h.
Quantitative RT-PCR analysis of gene expression
Total RNA was isolated using the TRIzol reagent(Invitrogen,San Diego,CA,USA)according to the manufacturer’s protocol and treated with RQ1 RNase-free DNaseI(Promega,Madison,WI,USA)to remove any contaminating DNA.First-strand cDNA was synthesized with 2 μg total RNA,oligo-d(T)18 primer,and M-MLV reverse transcriptase(Promega).Quantitative RT-PCR analyses were performed to examine gene expression with the ABI SYBR Green PCR Master Mix(Applied Biosystems,Foster City,CA,USA)on an ABI PRISM 7000(Applied Biosystems).BnACT was used as an internal control in rapeseed.
Seed mass and oil content determination
Average seed mass of each transgenic rapeseed line was determined by weighing three batches of 200 mature dry seeds.Seed oil content of rapeseed was analyzed by Soxhlet extraction method(Mu et al.2008).Fatty acid composition was analyzed with a Shimadzu GC-14B(Shimadzu Seisakusho,Kyoto,Japan)gas chromatograph.For each analysis,seeds were collected from at least 10 plants of ZGY2 or the transgenic plants per set.Three biological replicates were performed.
Ultrastructural observations of cotyledon cells in rapeseed mature seeds
For TEM observation,mature seeds were fixed,ultrathin sectioned,and stained according to He and Wu(2009).Sections were examined by transmission electron microscope(JSM-6360LV;JEOL).
Analysis of Chl content
Rapeseed seeds at different developmental stages(200 μg per batch)were used and Chl content was determined with a GBC Cintra 10e ultraviolet-visible spectrometer(GBC,Sydney,NSW,Australia)according to the method of Wintermans and de Mots(1965).Three biology replicates and duplicate experiments were performed.
Cis-element analysis
Up to the 3 000 bp upstream region corresponding to ATG for each selected gene was extracted from the Bulk Data Retrieval and Analysis database at the Arabidopsis information resource(http://www.arabidopsis.org/tools/bulk/sequences/index.jsp).Cis-elements were identified with the PLACE program(http://www.dna.affrc.go.jp/PLACE)(Higo et al.1999).
Accession numbers
Sequences in this article can be found in the GenBank/European Molecular Biology Laboratory data libraries with the following accession numbers:BnWRI1(DQ370141,DQ402050),BnCAB(DQ645428),BnBCCP(X90730),BnrbcS(DQ242646),BnACT(DQ370142),BCCP2(AT5G15530),CAB1(AT1G29930),and LHB1B2(AT2G34420).
ACKNOWLEDGEMENTS
We thank Hong-Wei Xue(Institute of Plant Physiology and Ecology,the Chinese Academy of Sciences)for helpful discussions for the research and critical comments on the article.We thank Mei-Qing Xing(Institute of Plant Physiology and Ecology,the Chinese Academy of Sciences),and Xu Wang(Institute of Virology and Biotechnology,the Zhejiang Academy of Agricultural Sciences)for technical assistance.This work was supported by grants from the National High Technology Research and Development Program of China(2008AA02Z103),the Program of NSFC(30671332),the Key Program of Zhejiang Provincial Natural Science Foundation(Z304430),and the Zhejiang Province Community Technology Research Projects(2012C22037).
Asokanthan PS,Johnson RW,Griffith M,Krol M(1997)The photosynthetic potential of canola embryos.Physiol Plant 101:353–360
Baud S,Mendoza MS,To A,Harscoet E,Lepiniec L,Dubreucq B(2007)WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis.Plant J 50:825–838
Baud S,Wuilleme S,To A,Rochat C,Lepiniec L(2009)Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in Arabidopsis.Plant J 60:933–947
Cernac A,Benning C(2004)WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis.Plant J 40:575–585
Cernac A,Andre C,Hoffmann-Benning S,Benning C(2006)WRI1 is required for seed germination and seedling establishment.Plant Physiol 141:745–757
Dong J,Shi D,Gao J,Li C,Liu J,Qi C,Yang W(2009)Correlation between the quantity and the sum of areas of oil bodies and oil content in rapeseed(Brassica napus).Chin Bull Bot 44:79–85
Eastmond P,Kolacna L,Rawsthorne S(1996)Photosynthesis by developing embryos of oilseed rape(Brassica napus L.).J Exp Bot 47:1763–1769
Focks N,Benning C(1998)wrinkled1:A novel,low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism.Plant Physiol 118:91–101
Goffman FD,Alonso AP,Schwender J,Shachar-Hill Y,Ohlrogge JB(2005)Light enables a very high efficiency of carbon storage in developing embryos of rapeseed.Plant Physiol 138:2269–2279
Green PJ,Yong MH,Cuozzo M,Kano-Murakami Y,Silverstein P,Chua NH(1988)Binding site requirements for pea nuclear protein factor GT-1 correlate with sequences required for light-dependent transcriptional activation of the rbcS-3A gene.EMBO J 7:4035–4044
Hajduch M,Casteel JE,Hurrelmeyer KE,Song Z,Agrawal GK,Thelen JJ(2006)Proteomic analysis of seed filling in Brassica napus.Developmental characterization of metabolic isozymes using high-resolution two-dimensional gel electrophoresis.Plant Physiol 141:32–46
Han XX,Yin LL,Xue HW(2012)Co-expression analysis identifies CRC and AP1 the regulator of Arabidopsis fatty acid biosynthesis.J Integr Plant Biol 54:486–499
Hay J,Schwender J(2011)Computational analysis of storage synthesis in developing Brassica napus L.(oilseed rape)embryos:Flux variability analysis in relation to13C metabolic flux analysis.Plant J 67:513–525
He YQ,Wu Y(2009)Oil body biogenesis during Brassica napus embryogenesis.J Integr Plant Biol 51:792–799
Higo K,Ugawa Y,Iwamoto M,Higo H(1998)PLACE:A database of plant cis-acting regulatory DNA elements.Nucleic Acids Res 26:358–359
Higo K,Ugawa Y,Iwamoto M,Korenaga T(1999)Plant cis-acting regulatory DNA elements(PLACE)database:1999.Nucleic Acids Res 27:297–300
Hu ZY,Hua W,Zhang L,Deng LB,Wang XF,Liu GH,Hao WJ,Wang HZ(2013)Seed structure characteristics to form ultrahigh oil content in rapeseed.PLoS ONE 8:e62099
Jiang J,Shao Y,Li A,Lu C,Zhang Y,Wang Y(2013)Phenolic composition analysis and gene expression in developing seeds of yellow-and black-seeded Brassica napus.J Integr Plant Biol 55:537–551
Li Y,Beisson F,Pollard M,Ohlrogge J(2006)Oil content of Arabidopsis seeds:The influence of seed anatomy,light and plant-to-plant variation.Phytochemistry 67:904–915
Liu J,Hua W,Zhan G,Wei F,Wang X,Liu G,Wang H(2010)Increasing seed mass and oil content in transgenic Arabidopsis by the overexpression of wri1-like gene from Brassica napus.Plant Physiol Biochem 48:9–15
Liu J,Hua W,Yang HL,Zhan GM,Li RJ,Deng LB,Wang XF,Liu GH,Wang HZ(2012)The BnGRF2 gene(GRF2-like gene from Brassica napus)enhances seed oil production through regulating cell number and plant photosynthesis.J Exp Bot 63:3727–3740
Ma W,Kong Q,Arondel V,Kilaru A,Bates PD,Thrower NA,Benning C,Ohlrogge JB(2013)WRINKLED1,a ubiquitous regulator in oil accumulating tissues from Arabidopsis embryos to oil palm mesocarp.PLoS ONE 8:e68887
Maeo K,Tokuda T,Ayame A,Mitsui N,Kawai T,Tsukagoshi H,Ishiguro S,Nakamura K(2009)An AP2-type transcription factor,WRINKLED1,of Arabidopsis thaliana binds to the AW-box sequence conserved among proximal upstream regions of genes involved in fatty acid synthesis.Plant J 60:476–487
Meng XB,Zhao WS,Lin RM,Wang M,Peng YL(2005)Identification of a novel rice bZIP-type transcription factor gene,OsbZIP1,involved in response to infection of Magnaporthe grisea.Plant Mol Biol Rep 23:301–302
Mu J,Tan H,Zheng Q,Fu F,Liang Y,Zhang J,Yang X,Wang T,Chong K,Wang XJ,Zuo J(2008)LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis.Plant Physiol 148:1042–1054
Niu Y,Wu GZ,Ye R,Lin WH,Shi QM,Xue LJ,Xu XD,Li Y,Du YG,Xue HW(2009)Global analysis of gene expression profiles in Brassica napus developing seeds reveals a conserved lipid metabolism regulation with Arabidopsis thaliana.Mol Plant 2:1107–1122
Pouvreau B,Baud S,Vernoud V,Morin V,Py C,Gendrot G,Pichon JP,Rouster J,Paul W,Rogowsky PM(2011)Duplicate maize Wrinkled1 transcription factors activate target genes involved in seed oil biosynthesis.Plant Physiol 156:674–686
Qu J,Ye J,Geng YF,Sun YW,Gao SQ,Zhang BP,Chen W,Chua NH(2012)Dissecting functions of KATANIN and WRINKLED1 in cotton fiber development by virus-induced gene silencing.Plant Physiol 160:738–748
Ruuska SA,Girke T,Benning C,Ohlrogge JB(2002)Contrapuntal networks of gene expression during Arabidopsis seed filling.Plant Cell 14:1191–1206
Ruuska SA,Schwender J,Ohlrogge JB(2004)The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes.Plant Physiol 136:2700–2709
Sanjaya,Durrett TP,Weise SE,Benning C(2011)Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis.Plant Biotechnol J 9:874–883
Schwender J,Ohlrogge JB(2002)Probing in vivo metabolism by stable isotope labeling of storage lipids and proteins in developing Brassica napus embryos.Plant Physiol 130:347–361
Schwender J,Ohlrogge JB,Shachar-Hill Y(2003)A flux model of glycolysis and the oxidative pentosephosphate pathway in developing Brassica napus embryos.J Biol Chem 278:29442–29453
Schwender J,Goffman F,Ohlrogge JB,Shachar-Hill Y(2004)Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds.Nature 432:779–782
Shigyo M,Hasebe M,Ito M(2006)Molecular evolution of the AP2 subfamily.Gene 366:256–265
Siloto RM,Findlay K,Lopez-Villalobos A,Yeung EC,Nykiforuk CL,Moloney MM(2006)The accumulation of oleosins determines the size of seed oil bodies in Arabidopsis.Plant Cell 18:1961–1974
Sullivan JA,Deng XW(2003)From seed to seed:The role of photoreceptors in Arabidopsis development.Dev Biol 260:289–297
To A,Joubes J,Barthole G,Lecureuil A,Scagnelli A,Jasinski S,Lepiniec L,Baud S(2012)WRINKLED transcription factors orchestrate tissue-specific regulation of fatty acid biosynthesis in Arabidopsis.Plant Cell 24:5007–5023
Wang X,Zhang C,Li L,Fritsche S,Endrigkeit J,Zhang W,Long Y,Jung C,Meng J(2012)Unraveling the genetic basis of seed tocopherol content and composition in rapeseed(Brassica napus L.).PLoS ONE 7:e50038
Wintermans JF,de Mots A(1965)Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol.Biochim Biophys Acta 109:448–453
Yang Y,Li R,Qi M(2000)In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves.Plant J 22:543–551
Zhang M,Fan J,Taylor DC,Ohlrogge JB(2009)DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development.Plant Cell 21:3885–3901
Zou J,Katavic V,Giblin EM,Barton DL,MacKenzie SL,Keller WA,Hu X,Taylor DC(1997)Modification of seed oil content and acyl composition in the brassicaceae by expression of a yeast sn-2 acyltransferase gene.Plant Cell 9:909–923
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article:
Figure S1.Sequence analysis of BnWRI1
Figure S2.Determination of the transcriptional activation domain of BnWRI1 in yeast cells
Figure S3.Schematic representation of the GCC-box mapped to the promoters regions of 11 WRI1 putative target genes
Table S1.Characterization of the NAPINPr::BnWRI1 rapeseed lines
Table S2.Fatty acid compositions in seeds of untransformed rapeseed and NAPINPr::BnWRI1 transgenic rapeseed
Table S3.Primers used in this study
猜你喜欢
杂志排行
Journal of Integrative Plant Biology的其它文章
- Genetic analysis of biomass and photosynthetic parameters in wheat grown in different light intensities
- Molecular characterization and expression analysis of Triticum aestivum squamosa-promoter binding protein-box genes involved in ear development
- Rice MtN3/saliva/SWEET gene family:Evolution,expression profiling,and sugar transport
- Polycomb-group histone methyltransferase CLF is required for proper somatic recombination in Arabidopsis
- A new loss-of-function allele 28y reveals a role of ARGONAUTE1 in limiting asymmetric division of stomatal lineage ground cell
- A step-by-step protocol for formaldehyde-assisted isolation of regulatory elements from Arabidopsis thaliana
