铁铜双金属催化剂选择性催化氧化氨为氮气
2014-09-17孙萌萌房志涛陈耀强
孙萌萌 曹 毅 兰 丽 邹 莎 房志涛 陈耀强
(四川大学化学学院,教育部绿色化学重点实验室,成都610064)
1 Introduction
NH3is seen as a toxic gas with a pungent odor,and shows potential damage to public safety,1which is used as reactants in a lot of chemical processes such as nitric acid production and urea manufacturing.As a byproduct,it is produced in many reactions such as de-nitration process.Thus environmental problems induced by ammonia emission become more severe.Selective catalytic oxidation(SCO)of ammonia to molecular nitrogen and water is proposed as an efficient and promising method to solve problems of ammonia pollution,and it has become increasingly important in recent years.
Thecatalyticoxidationofammoniacanproceedthreereactions:2,3

Except that the reaction(1)mentioned above is promoted,reactions(2)and(3)should be prevented due to the production of toxic nitrogen oxide.
To achieve such goal,various catalysts(e.g.,noble metal catalysts,transition metal catalysts)for oxidizing NH3have been developed.The noble metals(e.g.,Pt,Pd,Rh,Ag)are active for ammonia oxidation reaction,4-9but the selectivity of N2is low.The transition metal catalysts such as V2O5,Fe2O3,CuO,MnO2,TiO2are also employed for the ammonia oxidation reaction.Among them Fe2O3and CuO have attracted much attention.10,11Such as high activity of ammonia oxidation at high temperatures via Febased catalysts is well studied by Yang et al.12-14However,the activity over Fe-based catalysts is very low at low temperatures.For copper-based catalysts,they behave quite opposite to Febased catalysts,i.e.,better activity is observed at low temperatures for copper-based catalysts.15-19Thus it is necessary to prepare the catalysts with both Cu and Fe,which can improve the activity at low temperatures as well as the N2selectivity.In this study,copper and iron catalysts were loaded into the routine support materials(ZSM-5)with different Cu/Fe mass ratios,in order for developing a better catalyst to improve the activity and N2selectivity for the ammonia oxidation reaction.
2 Experimental
2.1 Preparation of catalysts
The samples were synthesized by an impregnation method using FeCl2·4H2O(A.R.)and Cu(NO3)2·3H2O(A.R.)as the metal precursors.ZSM-5(SiO2/Al2O3molar ratio=25)was purchased in Nankai University Catalyst Factory as the support.The total content of copper and iron was maintained at 4%(mass fraction,without cordierite).Three different catalysts with different Cu/Fe mass ratios were prepared,namely Fe0.25Cu0.75/ZSM-5,Fe0.50Cu0.50/ZSM-5,and Fe0.75Cu0.25/ZSM-5.After impregnation,the samples were dried in the oven.And then the sample was calcined at 300°C for 2 h and 550°C for 3 h.After that,the catalyst powders with certain distilled water were ball-milled to form homogeneous slurry.The slurry was coated on a honeycomb cordierite(5.2 cm3,Corning,America)with the coating amount of 160 g·L-1.Fe1-xCux/ZSM-5(x=0.75,0.50,0.25)with cordierites was used to evaluate the performance of catalysts.
2.2 Characterization of catalysts
N2adsorption-desorption isotherms were measured at-196°C on a QUANTACHROME SI automated surface area&pore size Analyzer.Before each measurement,the catalyst powders(without cordierite)were degassed at 300°C for 3 h under vacuum.The specific surface area and the pore size distribution of the catalyst powders were calculated using the Brunauer-Emmett-Teller(BET)and Barrett-Joyner-Halenda(BJH)methods,respectively.
H2temperature-programmed reduction(H2-TPR)was carried out in a quartz tubular micro-reactor equipped with a thermal conductivity detector(TCD).Prior to the analysis,0.10 g of sample(without cordierite)was loaded into the quartz tubular micro-reactor andthenpretreatedwith a flowof N2for 1 hat 450°C.Afterwards,the sample was cooled to room temperature in N2.The measurement was performed from room temperature to 750°C in a flow of 5%H2/N2at a heating rate of 8 °C·min-1.
NH3temperature-programmed desorption(NH3-TPD)was detected by a TCD.0.10 g of the catalyst powders(without cordierite)was first pretreated at 450°C for 1 h.Subsequently,the sample was cooled to 60°C.After that,ammonia was adsorbed at 60°C for 1 h,and the excess NH3was purged using He,then the temperature was increased linearly to 600°C with flowing He at a heating rate of 10 °C·min-1.
The powder X-ray diffraction(XRD)patterns were recorded on a D/max-rAdiffractometer(RIGAKU Corp.)with Cu Kαradiation(40 kV,25 mA,λ=0.15406 nm).During analysis,the sample(without cordierite)was scanned from 5°to 60°.The compound was identified by comparison with reference data from the International Centre for Diffraction Data(ICDD).
X-ray photoelectron spectra of samples were performed using anAXIS Ultra DLD(KRATOS)spectrometer withAl Kαradiation(1486.6 eV).X-ray radiation was operated at 300 W by setting the electron energy analyzer.
2.3 Performance evaluation of catalysts
The catalytic reaction for ammonia selective catalytic oxidation was carried out under atmospheric pressure in a temperatureprogrammed reaction fixed-bed quartz-glass reactor.The inlet and outlet gas compositions were analyzed by an on-line ANTARIS IGSAnalyzer.The reactant gas composition was as follows:2×10-8(volume fraction)NH3,10%O2,8%CO2,and balance N2.The gas hourly space velocity(GHSV)was 100000 h-1.Before running an experiment,the catalyst(with cordierite)was pretreated under the reaction atmosphere at 500°C for 1 h to make the property of catalyst stable.Then the temperature was decreased to the reaction temperature.Data were collected in a steady state.The NH3conversion and N2O selectivity were defined as:
NH3conversion=([NH3]in-[NH3]out)/[NH3]in×100%
N2O selectivity=2[N2O]out/([NH3]in-[NH3]out)×100%
It was impossible to directly measure N2by Fourier transform infrared(FTIR)spectrometry.N2selectivity was calculated based on the following formula:

3 Results and discussion
3.1 Property of catalysts
The ammonia oxidation reaction was studied on the Fe1-xCux/ZSM-5(x=0.25,0.50,0.75)catalysts(with cordierite).Fig.1 showed the results of NH3conversion in ammonia oxidation reaction.We could clearly see that the activity increased by increasing temperature for all the catalysts.When the appropriate amount of copper and iron was mixed,a significant influence on catalytic activity in low temperature range was observed.We found that the activity increased by increasing the mass ratio of Cu/Fe at 225-400°C.For Fe0.75Cu0.25/ZSM-5 catalyst,the NH3conversion was only 84%at 500°C.However,90%NH3conversion was achieved for Fe0.50Cu0.50/ZSM-5 and Fe0.25Cu0.75/ZSM-5 catalysts at about 390 and 327°C,respectively.The results of activity suggested that copper was more active than the same amount of iron at low temperatures.
Fig.2a displayed the N2selectivity on Fe1-xCux/ZSM-5 catalysts.All the catalysts exhibited high selectivity to N2,in which the N2selectivity increased by increasing the mass ratio of Cu/Fe at lower temperatures(<400°C),while the selectivity of N2increased by decreasing the mass ratio of Cu/Fe at higher temperatures(>400°C).For example,the N2selectivity approached 97%for Fe0.25Cu0.75/ZSM-5 catalyst at 300°C and decreased by increasing the temperature,even the N2selectivity was still 88%at 500°C.The N2selectivity of Fe0.50Cu0.50/ZSM-5 behaved much better than Fe0.75Cu0.25/ZSM-5 due to the increasing mass ratio of Cu/Fe below 400°C.However,the N2selectivity was close,regardless of the Cu/Fe mass ratio in the temperature range from 400 to 500°C.In details,the Fe0.75Cu0.25/ZSM-5 catalyst with the highest iron loading exhibited the best selectivity of N2(98%)at 500°C.

Fig.1 NH3conversion over Fe0.25Cu0.75/ZSM-5,Fe0.5Cu0.5/ZSM-5,and Fe0.75Cu0.25/ZSM-5 catalysts

Fig.2 N2selectivity(a)and N2O selectivity(b)over Fe1-xCux/ZSM-5 catalysts
From Fig.2b,we could clearly see that the selectivity of N2O was relatively low(<5%)for all catalysts.Moreover,Cu contributed to the low N2O selectivity at relative low temperatures while Fe replaced the role of Cu at high temperatures.

Fig.3 NH3conversion over Fe0.25Cu0.75/ZSM-5 catalyst at 350°C
Fig.3 showed the NH3conversion over Fe0.25Cu0.75/ZSM-5 sample at 350°C.97%NH3conversion was observed under the condition of 2×10-8(volume fraction)NH3,10%O2,8%CO2,balanced with N2and GHSV=100000 h-1.When 5×10-7(volume fraction)SO2was added into the reaction,NH3conversion sharply decreased to 38%in 0.5 h,then slightly went down to 23%in 2 h.However,NH3conversion increased to 65%after turning off the SO2.Fig.4 showed the NH3conversion over Fe0.25Cu0.75/ZSM-5 sample after regeneration at 550°C for 1 h.The NH3conversion was increased by increasing the temperature.The NH3conversion exhibited some decrease compared with the fresh catalyst below 350°C,but the high NH3conversion(>93%)was still obtained from 350 to 500°C.The results indicated that SO2tolerance of the catalyst was not very good,but the activity was mostly recovered after regeneration.
Fig.5 exhibited the NH3conversion and N2selectivity of Fe0.25Cu0.75/ZSM-5 catalyst after severely aging at 800°C for 10 h in air with 5%H2O.NH3conversion increased with increasing the temperature.In addition,90%NH3conversion was observed at 390°C and the highest NH3conversion(about 95%)was obtained at 450°C.We also found that Fe0.25Cu0.75/ZSM-5 catalyst had 76%N2selectivity at 300°C.Furthermore,the selectivity of N2went up to 82%at 400°C.The lowest N2selectivity was still 58%at 500°C.
3.2 Textural properties of catalysts

Fig.4 NH3conversion over Fe0.25Cu0.75/ZSM-5 catalyst after regeneration at 550°C for 1 h
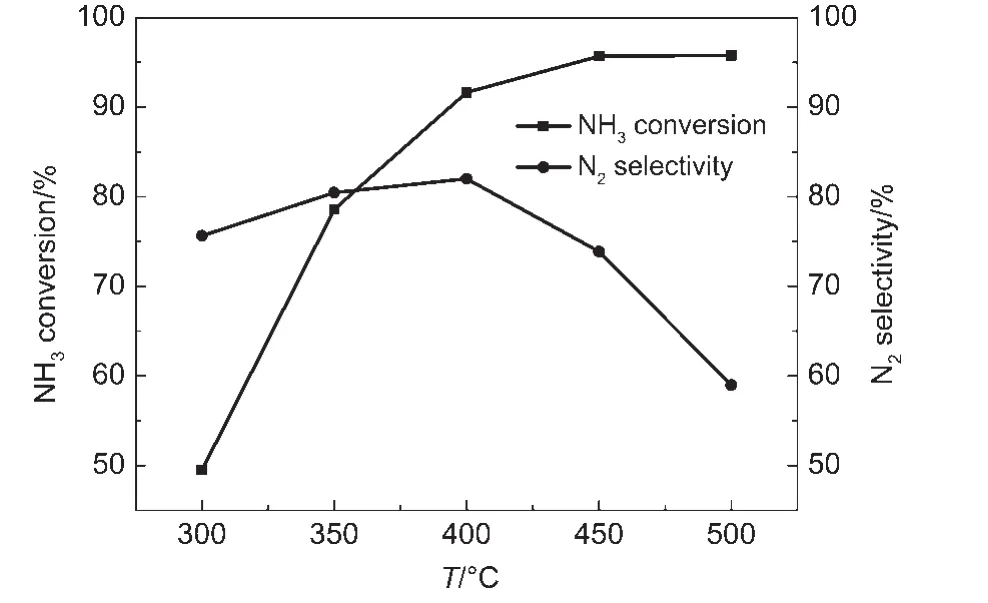
Fig.5 NH3conversion and N2selectivity of the Fe0.25Cu0.75/ZSM-5 catalyst after aging at 800°C for 10 h in air with 5%H2O

Table 1 Textural parameters of Fe1-xCux/ZSM-5 catalysts
Table 1 listed the textural parameters of Fe1-xCux/ZSM-5 catalysts.The adsorption-desorption isotherm shape of Fe1-xCux/ZSM-5 catalysts remained unchanged compared with ZSM-5 zeolite.All the catalysts had an obvious hysteresis loop,which was H4 type(did not show in this article).However,after the copper and iron were incorporated through impregnation in the ZSM-5 zeolite,the surface areas and pore volumes decreased compared with those of ZSM-5 zeolite.For example,the values of Fe0.25Cu0.75/ZSM-5 decreased from 335.1 m2·g-1and 0.19 cm3·g-1to 298.0 m2·g-1and 0.18 cm3·g-1.The surface area of ZSM-5 support was obtained after thermal treatment.So the reason for the loss of surface area was more likely to be the covering of the external surface of ZSM-5 by copper and iron species and blocking of the pores of ZSM-5.After the mass ratio of Cu/Fe was decreased,the surface areas and pore volumes of the catalysts still kept unchanged compared with those of Fe0.25Cu0.75/ZSM-5 catalyst.Thus it could be concluded that the textural properties of catalysts made little contribution to the variation of reaction activity.
3.3 XRD measurements
Fig.6 showed the XRD patterns of Fe1-xCux/ZSM-5 catalysts.All the catalysts exhibited typical peaks of the ZSM-5(PDF No.39-0225)zeolite,indicating that the structure of ZSM-5 zeolite kept intact after impregnation.The intensities of the principle diffraction peaks of the Fe1-xCux/ZSM-5 catalysts decreased compared with those of the ZSM-5,because the copper and iron had higher absorption coefficient for the XRD or the lower content of zeolite.20For Fe1-xCux/ZSM-5 catalysts,the peaks shifted to lower angles than the peaks of ZSM-5,while the values remained the same among Fe1-xCux/ZSM-5 catalysts.That was to say,the addition of copper and iron could enlarge crystallite di-mension of the pure ZSM-5,but the crystallite dimension remained unchanged with adjusting the mass ratio of Cu/Fe.It indicated that the crystallite dimension was not the main factor which influenced the activity.For Fe0.25Cu0.75/ZSM-5 catalyst,no peaks associated with iron or copper compounds were found.It may be due to the interaction between copper and iron species or the low content of copper and iron species.It was in favor of the activity that Cu2+and Fe3+were completely incorporated into the crystal lattice of ZSM-5 zeolite.

Fig.6 XRD patterns of different samples with Cu Kαradiation(40 kV,25 mA,λ=0.15406 nm)
But peaks(at 33.2°,35.6°,40.9°,49.5°,and 54.1°)ascribed to Fe2O3(PDF No.33-0664)were detected for Fe0.50Cu0.50/ZSM-5 catalyst when the iron loading was further increased.Combining with the result of activity and N2selectivity,it can be concluded that the Fe2O3species may facilitate the improvement of selectivity of N2at higher temperatures,but did not contribute to the activity at low temperatures.12
3.4 XPS results
The XPS technique was used to understand the states of Fe and Cu species.Fig.7a showed the results of Cu 2p3/2of Fe1-xCux/ZSM-5.Two peaks appeared at 933.1 and 934.8 eV,which were assigned to CuO species and Cu2+in zeolite.21In addition,we found that the amounts of CuO species and Cu2+in zeolite increased by increasing the mass ratio of Cu/Fe,which was consistent with the activity of the catalysts.We could conclude that CuO species and Cu2+in zeolite contributed to good activity at low temperatures.The selective catalytic oxidation of ammonia to nitrogen was a two-step route12,13that involved the oxidation of ammonia to NOxat CuO sites and the selective catalytic reduction of NOxto N2at Cu2+sites.22Table 2 listed the XPS results of Cu 2p3/2and Fe 2p3/2for the samples.From Table 2,the atomic percentage of Cu2+in CuO increased by increasing the mass ratio of Cu/Fe.It suggested that CuO species was a key factor to determine the activity at low temperatures.It might be due to the fact that the reaction of ammonia oxidation to NOxwas the rate determine step.23

Fig.7 XPS results of Cu 2p3/2(a)and Fe 2p3/2(b)in Fe1-xCux/ZSM-5(x=0.75,0.50,0.25)
Fig.7b exhibited the spectra of Fe 2p3/2of Fe1-xCux/ZSM-5(x=0.75,0.50,0.25)catalysts.Two peaks of Fe 2p3/2were located at 711.1 and 713.2 eV,higher than the binding energy(710.7 eV)of Fe2O3,24which strongly suggested that the iron was present as Fe3+species.25The peak at 711.1 eV was assigned to the Fe2O3species and the peak at 713.2 eV was the Fe3+in the zeolite.From Fig.7b and Table 2,we could see that the peak area of Fe3+in the zeolite kept unchanged,while the atomic percentage of Fe3+in Fe2O3was decreased by increasing the mass ratio of Cu/Fe.Combining with the activity and N2selectivity,we could find out that Fe2O3species contributed little to the SCO activity at low temperatures,which was consistent with the result of XRD.However,Fe2O3species might improve the selectivity of N2at high temperatures because Fe0.75Cu0.25/ZSM-5 catalyst with the largest amounts of Fe2O3species had the best N2selectivity.
3.5 H2-TPR result
To understand the redox properties of the catalysts,the temperature-programmed reduction measurement was performed.H2-TPR of the pure ZSM-5 was also performed,and no signal peaks of H2consumption were found(did not show in this article).Therefore,the peaks for the catalysts were attributed to the reduction of copper and iron species.For Fe1-xCux/ZSM-5 catalysts,the peaks α1and α2were attributed to the reduction of copper species,which were assigned to the reduction of Cu2+to Cu+and Cu+to Cu0,respectively.26Moreover,the H2consumption ratio of α1/α2was around 1,which indicted that the copper species were mostly present in the form of Cu2+.This result was consistent with the spectra of Cu 2p3/2.On the other hand,the H2consumptions of peaks β1
-β3at higher temperatures were assigned to the reduction of iron species.The peak β1was due to the reduction of Fe3+in molecular sieves and the peak β2was ascribed to the reduction of Fe2O3to Fe2+,the peak β3was attributed to the reduction of Fe2+species to Fe0.In addition,we also found that the strength ratio of(β1+β2)/β3was about 1/2,which exhibited that the iron species were mostly present in the form of Fe3+.Although FeCl2·4H2O was the metal precursor,most iron species were present as Fe3+after calcination,which was also proved by XPS.

Table 2 XPS results of Cu 2p3/2and Fe 2p3/2for the samples
Table 3 showed H2consumption of catalysts.From Table 3,the amount of H2consumption for peak α1increased by increasing the mass ratio of Cu/Fe at the low temperatures.This result was consistent with the activity of catalysts,larger amount of Cu2+species contributed to better activity at low temperatures.The amount of H2consumption for peak β1of Fe1-xCux/ZSM-5(x=0.25,0.50,0.75)slightly increased from 37.5 to 48.0 μmol·g-1with increasing the amount of Fe.The amount of H2consumption for peak β2of Fe0.25Cu0.75/ZSM-5 was 42.2 μmol·g-1,when increased Fe loading,the value of Fe0.75Cu0.25/ZSM-5 increased to 117.8 μmol·g-1.However,the activity did not get better with decreasing the mass ratio of Cu/Fe.It suggested that Fe2O3contributed little to good activity at low temperatures.
In addition,the selectivity of N2was significantly influenced by the reduction ability of the catalysts.The amount of H2consumption for copper species at low temperatures increased with increasing the mass ratio of Cu/Fe.This result was consistent with the selectivity of the catalysts,and the amount of H2consumption for iron species at high temperatures increased with decreasing the mass ratio of Cu/Fe,indicating that the catalysts with larger amount of iron had excellent reduction ability at high temperatures owe to the increasing of the amount of Fe2O3,it may be one reason for better N2selectivity.
From the results of XPS and TPR,we can conclude that Cu2+was a main factor to improve the activity and N2selectivity.Furthermore,the rate-determine step(NH3+O2→NOx+H2O)happened at CuO sites then selective catalytic reduction of NOxto N2at Cu2+sites.In addition,Fe3+could play an important role in increasing the N2selectivity above 400°C.
Moreover,we also studied the reduction of iron species of Fe1/ZSM-5.The H2consumption peaks β1-β3appeared at 448,568,and 646°C.From Fig.8 we found that the reduction temperaturesof copper species moved to lower values with decreasing loading amount of copper,and the reduction temperatures of iron species shifted to lower values as the amount of iron decreased,the gradual shifts of reduction temperature may be due to the interaction between copper and iron species.21The interaction was beneficial to improve the reduction ability of the active species,which was considered as one reason for good activity.

Table 3 H2consumption of catalysts

Fig.8 TPR profiles of Fe0.25Cu0.75/ZSM-5(a),Fe0.50Cu0.50/ZSM-5(b),Fe0.75Cu0.25/ZSM-5(c),and Fe1/ZSM-5(d)
3.6 NH3-TPD result
In order to understand the strength and amount of different acid sites,temperature-programmed desorption of ammonia was carried out.Fig.9 showed the NH3-TPD profiles on ZSM-5 and Fe1-xCux/ZSM-5 catalysts.
Two main desorption peaks were found at about 211 and 436°C for parent ZSM-5,corresponding to the weak and the strong acid sites,respectively.The desorption peak at the lower temperatures had been proven to generate from the physically adsorbed NH3or ammonium species,27probably hydrogen bonded,but not the ammonia species adsorbed on acid sites and the peak at higher temperatures was ascribed to NH3strongly adsorbed which could determine the property of acid sites.

Fig.9 TPD profiles of pure ZSM-5(a),Fe0.25Cu0.75/ZSM-5(b),Fe0.50Cu0.50/ZSM-5(c),and Fe0.75Cu0.25/ZSM-5(d)
The peak β for the Fe0.25Cu0.75/ZSM-5 at about 365 °C moved to a lower temperature than parent ZSM-5,which implied that the strength of the peak β was decreased.In addition,the peak β shifted to a higher temperature with increasing the amount of iron,which may be caused by the interaction between copper and iron.It could explain that the activity turned worse at lower temperatures after increasing the iron content.
From the literature,12the FTIR result showed that NH3was adsorbed on the acid sites and the intensity of adsorbed ammonia species decreased with the temperature increasing during researching the IR spectra in flowing NH3and O2.Accordingly,we could deduce that larger number of acid sites was conducive to better activity.Moreover,from Fig.9 we also found that the area of peak β of Fe1-xCux/ZSM-5 catalysts increased with increasing the mass ratio of Cu/Fe,and Fe0.25Cu0.75/ZSM-5 catalyst with the largest mass ratio of Cu/Fe in this study had the best activity,indicating that the increase of the number of acid sites was corresponded to the activity date,we could deduce that the activity of the catalysts had a close relationship with the acid amounts.In other words,the conversion of ammonia increased with increasing the acid amounts.
4 Conclusions
In summary,the iron and copper bimetallic catalysts demonstrated high activity and excellent selectivity of N2.The activity and N2selectivity in low temperature range were increased by increasing the mass ratio of Cu/Fe.In contrast,the higher N2selectivity was achieved with larger amount of iron at higher temperatures.Fe0.25Cu0.75/ZSM-5 had the best activity and high N2selectivity.The characterization results indicated that strong interaction between copper and iron species was obtained.And the Cu and Fe species were mostly presented as Cu2+and Fe3+,respectively.The activity increased with the increase in the amount of the Cu active species at low temperatures.Larger amount of Fe2O3was a probable reason for better N2selectivity at high temperatures.In addition,the acid content,strength of catalysts,and the redox property of catalysts also played important roles in the ammonia oxidation reaction.
(1) Krupa,S.V.Environ.Pollut.2003,124,179.doi:10.1016/S0269-7491(02)00434-7
(2) Hung,C.M.Powder Technol.2009,196,56.doi:10.1016/j.powtec.2009.07.001
(3)Akah,A.;Cundy,C.;Garforth,A.Appl.Catal.B:Environ.2005,59,221.doi:10.1016/j.apcatb.2004.10.020
(4) Sobczyk,D.P.;Hensen,E.J.M.;Jong,A.M.D.;Santen,R.A.V.Top.Catal.2003,23,1.doi:10.1023/A:1024834800948
(5) Hung,C.M.Powder Technol.2010,200,78.doi:10.1016/j.powtec.2010.02.014
(6) Broek,A.C.M.V.D.;Grondelle,J.V.;Santen,R.A.V.J.Catal.1999,185,297.doi:10.1006/jcat.1999.2506
(7) Gang,L.;Anderson,B.G.;Grondelle,J.V.;Santen,R.A.V.Appl.Catal.B:Environ.2003,40,101.doi:10.1016/S0926-3373(02)00129-7
(8) Zhang,L.;Zhang,C.B.;He,H.J.Catal.2009,261,101.doi:10.1016/j.jcat.2008.11.004
(9) Zhang,L.;He,H.J.Catal.2009,268,18.doi:10.1016/j.jcat.2009.08.011
(10) Long,R.Q.;Yang,R.T.J.Catal.2002,207,158.doi:10.1006/jcat.2002.3545
(11) Cui,X.Z.;Zhou,J.;Ye,Z.Q.;Chen,H.R.;Li,L.;Ruan,M.L.;Shi,J.L.J.Catal.2010,270,310.doi:10.1016/j.jcat.2010.01.005
(12) Qi,G.S.;Yang,R.T.Appl.Catal.A:Gen.2005,287,25.doi:10.1016/j.apcata.2005.03.006
(13) Qi,G.S.;Gatt,J.E.;Yang,R.T.J.Catal.2004,226,120.doi:10.1016/j.jcat.2004.05.023
(14) Long,R.Q.;Yang,R.T.J.Catal.2001,201,145.doi:10.1006/jcat.2001.3234
(15) Gang,L.;Grondelle,J.V.;Anderson,B.G.;Santen,R.A.V.J.Catal.1999,186,100.doi:10.1006/jcat.1999.2524
(16) Metkar,P.S.;Harold,M.P.;Balakotaiah,V.Appl.Catal.B:Environ.2012,111,67.
(17) Song,S.Q.;Jiang,S.J.Appl.Catal.B:Environ.2012,117,346.
(18)Liang,C.X.;Li,X.Y.;Qu,Z.P.;Tade,M.;Liu,S.M.Appl.Surf.Sci.2012,258,3738.doi:10.1016/j.apsusc.2011.12.017
(19)Shi,L.;Yu,T.;Wang,X.Q.;Wang,J.;Shen,M.Q.Acta Phys.-Chim.Sin.2013,29,1550.[石 琳,于 铁,王欣全,王 军,沈美庆.物理化学学报,2013,29,1550.]doi:10.3866/PKU.WHXB201304283
(20)Rauscher,M.;Kesore,K.;Mönnig,R.;Schwieger,W.;Tißler,A.;Turek,T.Appl.Catal.A:Gen.1999,184,249.doi:10.1016/S0926-860X(99)00088-5
(21)Zhang,T.;Liu,J.;Wang,D.X.;Zhao,Z.;Wei,Y.C.;Cheng,K.;Jiang,G.Y.;Duan,A.J.Appl.Catal.B:Environ.2014,148,520.
(22)Wang,J.;Huang,Y.;Yu,T.;Zhu,S.C.;Shen,M.Q.;Li,W.;Wang,J.Q.Catal.Sci.Technol.2014,4,3004.doi:10.1039/c4cy00451e
(23) Brüggemann,T.C.;Keil,F.J.J.Phys.Chem.C 2009,113,13860.
(24)Wagner,C.D.;Riggs,W.M.;Davis,L.E.;Moulder,J.F.Handbook of X-ray Photoelectron Spectroscopy,1st ed.;Muilenberg,G.E.Ed.Perkin-Elmer Corporation:Eden Prairie,Minnesota,USA,1979.
(25) Gurgul,J.;Ła˛tka,K.;Hnat,I.;Rynkowski,J.;Dzwigaj,S.Microporous Mesoporous Mat.2013,168,1.doi:10.1016/j.micromeso.2012.09.015
(26) Lisi,L.;Pirone,R.;Russo,G.;Stanzione,V.Chem.Eng.J.2009,154,341.doi:10.1016/j.cej.2009.04.025
(27) Long,R.Q.;Yang,R.T.J.Catal.2001,198,20.doi:10.1006/jcat.2000.3118
