马来海松酸二酰亚胺的合成及其构象*
2014-09-05王恒山李剑飞覃坚妹朱永涛叶鳗仪李亚军姚贵阳
王恒山,李剑飞,覃坚妹,朱永涛,叶鳗仪,李亚军,姚贵阳
(广西师范大学化学与药学学院,药用资源化学与药物分子工程重点实验室,广西 桂林 541004)
马来海松酸二酰亚胺的合成及其构象*
王恒山,李剑飞,覃坚妹,朱永涛,叶鳗仪,李亚军,姚贵阳
(广西师范大学化学与药学学院,药用资源化学与药物分子工程重点实验室,广西 桂林 541004)
以一级松香与马来酸酐的Diels-Alder加成产物马来海松酸及其进一步甲酯化产物马来海松酸甲酯为反应原料,制备了1个新型N-芳香基马来海松酸二酰亚胺.化合物的结构均经元素分析、NMR和MS表征,用COSY,HMQC和HMBC进一步对新型N-芳香基马来海松酸二酰亚胺进行结构分析,首次发现松香背景的衍生物在溶剂中能够进行构型的动力学翻转,并利用2D NMR确定了其trans和cis在溶液中的构象.
位阻异构;马来海松酸二酰亚胺;合成;构象
现代医药产业深深地受到手性的影响:已经证实,对映异构体在药代动力学、生物活性和毒性等方面存在很大的差别.例如反应停[1-4]和心舒宁[5]的案例,它们不同的对映异构体在实效和代谢特性上存在截然不同的药效作用.因此,在药物开发上解决立体化学的问题成为药物研究的重点[6-8].
脱氢松香酸是可再生资源松香的众多异构体成分之一.在脱氢松香酸骨架上引入和建构杂环衍生物,合成具有药理活性和特殊生物特性的化合物是目前重要的研究方向[9-10].现代研究已表明,脱氢松香酸、松脂有许多生物和药理作用,包括抗肿瘤[9]、抗过敏[10]、抗炎[11]、杀虫[12]、抗惊厥[13]、抗菌性能[14],以及降低胆固醇[15]和抑制ATP酶的活性.目前,在脱氢松香酸骨架上引入和建构杂环衍生物,合成具有药理活性和特殊生物特性的化合物成为重要的研究方向.酰亚胺类化合物具有多样的生物活性[16],马来海松酸二酰亚胺的生物活性也有报道,但是未见对于研究脱氢松香酸类的位阻异构的相关报道.笔者在前期对N-芳环取代的马来海松酸二酰亚胺生物抗肿瘤活性研究过程中,发现新型N-芳香基马来海松酸二酰亚胺具有位阻异构现象,并利用2D NMR确定了其trans和cis在溶液中的构象.

图1 N-(5-异喹啉基)-甲酯化马来海松酸二酰亚胺的合成路线
1 实验部分
N-(5-异喹啉基)-甲酯化马来海松酸二酰亚胺的合成路线如图1所示.
1.1试剂和仪器
试剂:实验中使用的一级松香购自广西梧州松脂厂;除特别说明之外,固体试剂购回后未经处理就直接使用;液体试剂均为市售分析纯,必要时进行干燥及纯化处理.
仪器:BRUKER AVANCE 500 MHz超导核磁共振仪(瑞士,布鲁克公司);ESQUIRE HCT 型质谱仪(美国,布鲁克·道尔顿公司);Nicolet ESP 360 FT-IR型傅立叶变换红外光谱仪(美国,溴化钾压片);WZZ-2B型自动旋光测定仪(上海精密科学仪器有限公司);WRS-1A数字熔点仪(上海精密科学仪器公司);DLSB-5/20型低温冷却液循环浆(郑州长城科工贸有限公司).
1.2化合物3和4的合成


4II型:1H NMR (500 MHz,CDCl3):δ9.29 (s,1H,C1’-H),8.52 (d,1H,J=6.0 Hz,C3’-H),8.05 (d,1H,J=7.0 Hz,C8’-H),7.67 (t,1H,J=8.0 Hz,C7’-H),7.48 (d,1H,J=7.5Hz,C6’-H),7.25 (m,1H,J=7.0 Hz,C4’-H),5.79 (s,1H,C14-H),3.68 (s,3H,COOCH3-),3.27 (s,1H,C12-H),3.14 (dd,J=8.5,3.0 Hz,C21-H),2.74 (d,1H,J=8.5 Hz,C22-H),2.56 (d,1H,J=10.5 Hz,C7-Ha),2.37 (m,1H,J=7.0 Hz,C15-H),1.70~1.86 (m,4H,C7-Hax,C5-H,C11-Hax,C3-Hax),1.44~1.63 (m,6H,C9-H,C3-Heq,C6-Hax,C2-H,C1-Heq),1.31~1.38 (ddd,1H,J=13,5.4,3.0 Hz,C11-Heq),1.21~1.28 (m,1H,C6-Heq),1.19 (s,3H,C4-CH3),1.03~1.10 (m,6H,J=7.0 Hz,C15-2CH3),1.01 (dt,1H,J=13,5 Hz,C1-Hax),0.68 (s,3H,C10-CH3);13C NMR (CDCl3,125 MHz):δ179.33 (C20),177.75 (C24),176.45 (C23),153.10 (C1’),148.05 (C13),143.75 (C3’),132.37 (C5’),130.69 (C9’),130.60 (C6’),129.48 (C8’),128.45 (C10’),127.09 (C7’),125.20 (C14),115.88 (C4’),54.92 (C9),53.04 (C22),52.18 (CO2CH3),49.74 (C5),47.34 (C4),46.37 (C21),41.41 (C8),38.34 (C10),38.00 (C1),36.96 (C3),35.70 (C12),35.38 (C7),32.80 (C15),27.72 (C11),21.97 (C6),20.53 (C16),19.80 (C17),17.23 (C2),16.93 (C19),15.93 (C18);MS (APCI)m/z:541 (M+H+).
2 结果与讨论
用一级松香与马来酸酐的D-A加成反应制得马来海松酸(化合物2),进一步制得其甲酯化产物(化合物3),并以此为原料,通过在冰醋酸中回流,制得并纯化得到光学纯的1个新型N-芳香基马来海松酸二酰亚胺(化合物4),发现它在溶剂中能够进行构型的动力学翻转,并利用2D NMR确定了trans和cis在溶液中的构象(图1,4I和4II).


图2 化合物4I的HMBC相关(H→C)
2.2化合物4的1HNMR
测定化合物4的1H NMR,其中,具有分子间电子效应的N-(5-异喹啉基)-甲酯化马来海松酸二酰亚胺(化合物4),平衡态trans型和cis型比例及其化学位移与其他萘胺类比较接近.化合物4的局部1H NMR谱图(于CDCl3)见图3,清晰地显示了溶液状态时异物体的存在.
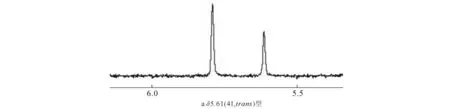

图3 化合物4的局部1H NMR谱图
2.3化合物4的COSY,HMQC和HMBC解析
氮杂萘系列二酰亚胺由于有电子效应,因此在平衡点trans和cis构型比例不同.利用COSY,HMQC和HMBC分别对化合物4进行结构分析,发现在室温在CDCl3中达到平衡态时,化合物4的trans型和cis型比例为37.3∶62.7(见图4—6),结果表明化合物4在溶剂中能够进行构型的动力学翻转.

图4 化合物4的 图5 化合物4的 HMQC 图6 化合物4的 HMBC
3 结语
以一级松香与马来酸酐的D-A加成反应、酯化和酰胺反应获得新型化合物4.对化合物4的生成机理进行讨论,且用COSY,HMQC和HMBC对化合物4进行结构分析,得知化合物4在溶剂中能够进行构型的动力学翻转,确定了其trans和cis在溶液中的构象.这些研究无疑给位阻异构研究增添了新的元素,在一定程度上给立体化学的研究提供了新的实验依据,并将给予不对称合成及催化剂研究以新的启示.
[1] BLASCHKE G,KRAFT H P,FICKENTSCHER K,et al.Chromatographic Separation of Racemic Thalidomide and Teratogenic Activity of Its Enantiomers[J].Arzneimittel Forschung,1979,29(10):1 640.
[2] FABRO S,SMITH R L,WILLIAMS R T.Toxicity and Teratogenicity of Optical Isomers of Thalidomide[J].Nature,1967,215:296-296.
[3] REIST M,CARRUPT P A,FRANCOTTE E,et al.Chiral Inversion and Hydrolysis of Thalidomide:Mechanisms and Catalysis by Bases and Serum Albumin and Chiral Stability of Teratogenic Metabolites[J].Chem. Res. Toxicol.,1998,11:1 521-1 528.
[4] ERIKSSON T,BJORKMAN S,ROTH B,et al.Enantiomers of Thalidomide:Blood Distribution and the Influence of Serum Albumin on Chiral Inversion and Hydrolysis[J].Chirality,1998,10:223-228.
[5] ROUHI A M.Chirality at Work:Drug Developers Can Learn Much from Recent Successful and Failed Chiral Switches[J].Chem. Eng. News,2003,81(18):56-61.
[6] BRINGMANN G,MORTIMER A J P,KELLER P A,et al.Atroposelective Synthesis of Axially Chiral Biaryl Compounds[J].Angew. Chem. Int. Ed.,2005,44(34):5 384-5 427.
[7] DOS SANTOS A R,PINHEIRO C,SODERO A C R,et al.Atropisomerism:The Effect of the Axial Chirality in Bioactive Compounds[J].Quim. Nova.,2007,30:125-137.
[8] CLAYDEN J,MORAN W J,EDWARDS P J,et al.The Challenge of Atropisomerism in Drug Discovery[J].Angew. Chem. Int. Ed.,2009,48:6 398-6 401.
[9] YANG Nianyun,LIU Li,TAO Weiwei,et al.Diterpenoids from Pinus Massoniana Resin and Their Cytotoxicity Against A431 and A549 Cells[J].Phytochemistry,2010,71:1 528-1 533.
[10] SU Shulan,WANG TuanJie,CHEN Ting,et al.Cytotoxicity Activity of Extracts and Compounds from Commiphora Myrrha Resin Against Human Gynecologic Cancer Cells[J].Med. Plant. Res.,2011,5:1 382-1 389.
[11] ULUSU N N,ERCIL D,SAKAR M K,et al.Abietic Acid Inhibits Lipoxygenase Activity[J].Phytother. Res.,2002,16:88-90.
[12] TAKAHASHI N,KAWADA T,GOTO T,et al.Abietic Acid Activates Peroxisome Proliferator-Activated Receptor-Q(PPARQ) in RAW264.7 Macrophages and 3T3-L1 Adipocytes to Regulate Gene Expression Involved in Infammation and Lipid Metabolism[J].FEBS Lett.,2003,550:190-194.
[13] YAO Guiyang,YE Manyi,HUANG Rizhen,et al.Synthesis and Antitumor Activity Evaluation of Maleopimaric Acid N-Aryl Imide Atropisomers[J].Bioorg. & Med. Chem. Lett.,2013,23(24):6 755-6 758.
[14] TALEVI A,CRAVERO M S,CASTRO E A,et al.Discovery of Anticonvulsant Activity of Abietic Acid Through Application of Linear Discriminant Analysis[J].Medicinal Chemistry Letters,2007,17:1 684-1 690.
[15] DEGTYARENKO A S,PEHK T,MAKHNACH S A.Salts of 3-Amino-2-Hydroxypropyl Pimarate and Their Biological Activity [J].Vestsi Akad.Navuk BSSR Ser. Khim. Navuk.,1987,5:44-48.
[16] 薛 伟,龚华玉,赵洪菊,等.含苯并噻唑基双酰胺衍生物的合成与生物活性[J].吉首大学学报:自然科学版,2012,33(5):92-97.
[17] PAN Yingming,YANG Lin,WANG Hengshan,et al.Maleopimaric Acid Trimethyl Ester[J].Acta Cryst. Section E,2006,E62:o5 701-o5 703.
(责任编辑 易必武)
SynthesisandConformationofMaleopimaricAcidDiimide
WANG Hengshan,LI Jianfei,QIN Jianmei,ZHU Yongtao,YE Manyi,LI Yajun,YAO Guiyang
(Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources,School of Chemistry & Pharmacy of Guangxi Normal University,Guilin 541004,Guangxi China)
Maleopimaric acid (compound 2) was formed by Diels-Alder addition of maleic acid anhydride to resin acid,then maleopimaric acid methyl ester (compound 3) was prepared by esterification of dimethyl sulfate.After maleopimaric acid methyl ester interacting with 5-Aminoisoquinoline,the N-aryl methyl maleopimaric acid diimides (compound 4) was obtained.The structures of all compounds were characterized by elemental analyses,NMR and MS.Atropisomerism was first found in compound 4 in CDCl3,and the proportion of different atropisomer(trans:cis) was calculated by 2D NMR.
atropisomerism;maleopimaric acid diimide;synthesis;conformation
1007-2985(2014)01-0063-05
2013-08-21
国家自然科学基金资助项目(81260472,21101035);973计划前期研究专项(2011CB512005);广西自然科学基金资助项目(2011GXNSFD018010,2010GXNSFF013001)
王恒山(1965-),男,浙江温州人,广西师范大学化学与药学学院教授,博士,主要从事手性药物抗肿瘤活性研究.
O643.1
A
10.3969/j.issn.1007-2985.2014.01.015
