金属巯蛋白在不同年龄大鼠肝脏中的表达
2014-08-09侯玮钰朱琼妮徐尚福陆远富
侯玮钰,张 丹,朱琼妮,徐尚福,吴 芹,陆远富,刘 杰,2
(1.遵义医学院 基础药理省部共建教育部重点实验室,贵州 遵义 563099 ; 2.University of Kansas Medical Center, Kansas City, KS 66160, USA)
1 Introduction
Metallothionein (MT) is a low-molecular-weight, cysteine-rich, metal-binding protein.MT genes are readily induced by various physiological and toxicological stimuli. MT is rich in cysteine residues, and plays important roles in the detoxification of heavy metals, in the homeostasis of essential metals, and in the scavenge of free radicals[1].There are four isoforms of MT in rodents, MT-1 and MT-2 are major isoforms in the liver and coordinately regulated[1].MT-3 mainly locates in brain and MT-4 in squamous tissues such as tong[1-2].MT is proposed to have a variety of biological functions, and implicated in liver diseases, ageing, and carcinogenesis[1-4].
Induction of MT protects against acute liver injury produced by cadmium, carbon tetrachloride, acetaminophen, and other toxicants[1-2].In chronic hepatitis C patients, hepatic MT levels are associated with the severity of HCV infection, and are linked to a better response to interferon therapy[3].MT gene therapy leads to resolution of liver fibrosis in mice[4], possibly by the inhibition of activated HSCs and increases in collagenases[5].Zinc deficiencies are observed in many types of liver disease, including alcoholic liver disease and viral liver diseases and MT is proposed to play important roles in maintaining zinc homeostasis. In addition, zinc therapy has replaced penicillamine as the first-line therapy for Wilson's disease, probably through induction of MT to sequester copper[6].Thus, liver is a major target of MT therapy[1-4].
Ageing is associated with gradual and spontaneous physiological changes and increased susceptibility to oxidative damage and various diseases[7].In ageing, the accumulation of damaged molecules provoked by oxidative stress and inflammation results in altered gene expressions and cellular dysfunction, the loss of MT is likely to be a major cause of ageing outcomes[8].MT protects against development of obesity in high-fat diet-fed mice and reduces high-fat diet-induced oxidative damage[9].Thus, MT is implicated in cellular senescence and is proposed to be a possible therapeutic target protecting against metabolic syndrome and the accumulation of dysfunctional senescent cells[8- 9].
The ontogeny of hepatic MT during the development was reported 30 years ago[10].Hepatic MT is high at the first week of birth and then decreased steadily to adult levels by Day 28 to 35[10- 11].Both hepatic MT-1 and MT-2 showed a similar ontogeny pattern[11].However, little is known about hepatic MT levels after 35 days, and no reports on hepatic MT levels during ageing is available.
Many physiological factors influence MT expression. For example, MT expression showed circadian variations, and sampling at the different times of the day significantly impacts the results[12], but little is known about other physiological factors such as development, ageing, pregnancy, and lactation on MT expression.
Because MT is an important biological molecule[1-4], to characterize the influencing factors are important in elucidating its biological function.This study was therefore aimed to make a systemic research of the expression of MT-1 and MT-2 mRNA in rat livers under different physiological conditions.
2 Materials and Methods
2.1AnimalsandsamplecollectionSprague Dawley (SD) rats (250~300 g) were purchased from the Experimental Animal Center of Third Military Medical College (Chongqing, China;.CXK2007-0005). Rats were kept in SPF-grade animal facilities (Certificate No. SYXK 2011-004) at Zunyi Medical College, withcontrolled environment (22±1 ℃,50 % humidity and a 12 h: 12 h light: dark cycle) and free access to purified water and standard laboratory chow. All animal care and experimental protocols are complied with the Animal Management Guidelines of the Chinese Ministry of Health and approved by Institutional Animal Use and Care Committee of Zunyi Medical College.
Rats were acclimatized for one week before timely-mating overnight. A vaginal plug in the next morning was designated as day (GD 0) of gestation. The livers were collected in development days (-2, 1, 7, 14, 21, 28, 35 and 60days) and ageing of days (28, 60, 180 ,540, and 800 days); For pregnant and lactating rats, maternal livers were collected on gestation days (GD) 10, 14 and 19, and on the postnatal days (PND) 1, 7,14 and 21. The age-matched virgin rats were used as controls. Livers were frozen in liquid nitrogen and stored at -80℃ prior to analysis.
2.2RNAIsolationandreal-timeRT-PCRanalysisApproximate 50~100 mg of liver tissues was homogenized in 1 ml TRIzol (TakaRa Biotechnology, Dalian, China).The quality and quantity of RNA were determined by the 260/280 ratio and gel-electrophoresis. Total RNA was reverse transcribed with the High Capacity Reverse Transcriptase Kit (Applied Biosystems, Foster City, CA, USA). The primers were designed with Primer3 software and listed in Table 1. iQTMSYBR Green Supermix (Bio-Rad Laboratories, Hercules , CA) was used for real-time RT-PCR analysis. Relative expression of genes was calculated by the 2-ΔΔCtmethod and normalizing to the house-keeping gene β-actin and G3PDH.
Table 1 Primer sequence for real-time RT-PCR analysis

GeneAccession#ForwardReverseG3PDHNM_017008AACTTTGGCATTGTGGAAGGGGGATGCAGGGATGATGTTCTβ-actinNM_031144AGCCATGTACGTAGCCATCCACCCTCATAGATGGGCACAGMT-1NM_013602CTCCGTAGCTCCAGCTTCACAGGAGCAGCAGCTCTTCTTGMT-2NM_008630CCGATCTCTCGTCGATCTTCAGGAGCAGCAGCTCTTCTTG
2.3StatisticanalysisThe SPSS16.0 software was used for statistical analysis. Results were described as Mean ± SEM. Differences between male and female rats were determined by two-tailed independent Student’sttest.Differences during pregnant and lactation were analyzed by the one-way ANOVA , followed by Dunnett’s test.P<0.05 was considered statistically significant.
3 Results
3.1AgeandsexvariationsofMT-1mRNAThe expression of MT-1 mRNA in rat livers at different ages is shown in Fig 1. MT-1 displayed a robust age-dependent variation.In male rats, MT-1 was high in fetal livers, slightly decreased at birth, quickly increased at 7 days, and then decreased at 14 days.At 21 days, MT-1 mRNA levels decreased from 11 000 to 1100 % of housekeeping genes, and continued to decreased at 800 days of age.The difference of MT-1 mRNA between 7 days and 800 days was 220-fold (PND7, 11 000 % vs 800d, 50% of housekeeping genes).In female rats, MT-1 mRNA expression followed the similar pattern as males, but at much lower levels at -2, 1, 7, and 14 days (P<0.05).The difference of MT-1 mRNA in females between 7 days and 800 days was 34-fold (PND7, 1700% vs 800 d, 50% of housekeeping genes).The expression of MT-1 was higher than that of housekeeping genes (β-actin and G3PDH), and is male-dominant (Fig 1).
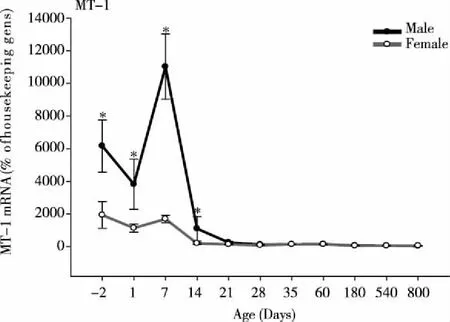
*Significantly different from female rats(P<0.05).Fig 1 Age and sex variations of MT-1 mRNA in male and female rat livers (n=6)
3.2AgeandsexvariationsofMT-2mRNAIn mammals, MT-1 and MT-2 are coordinately regulated, and have similar functions[1-3, 11].The expression of MT-2 mRNA was similar to MT-1, the highest expression occurred at 7 days of age and the lowest at 800 days of age.In contrast to MT-1, MT-2 mRNA increased at birth, reached a peak at 7 days of age, and then decreased thereafter.MT-2 remained low after weanling, but slightly increased at 60 days of age, and decreased again up to 800 days of age.In general, expression of MT-2 is lower than MT-1.In male rats, the difference of MT-2 expression between 7 days and 800 days of age was 20-fold (PND7, 945 % vs 800 d, 47 % of housekeeping genes).At 60 days of age the difference was 5.5 -fold (PND60, 270 % vs 800 d, 47% of housekeeping genes) in males and 5-fold in femalesIn female rats, MT-2 mRNA expression followed the same pattern as males, but at lower levels at -2, 1, 7, and 60 days (P<0.05,Fig 2) .
3.3VariationsofMT-1andMT-2mRNAinpregnantandlactationratsMT-1 mRNA was quantified in livers of pregnant rats from gestation days (GD) 10, 14, to GD19 and in livers of lactation rats (PND) 1, 7, 14, and PND 21.The expression of MT-1 mRNA decreased at GD14 and GD19, but increased in PND 1, then decreased again in PND 7, and 14 (Fig.3 left).
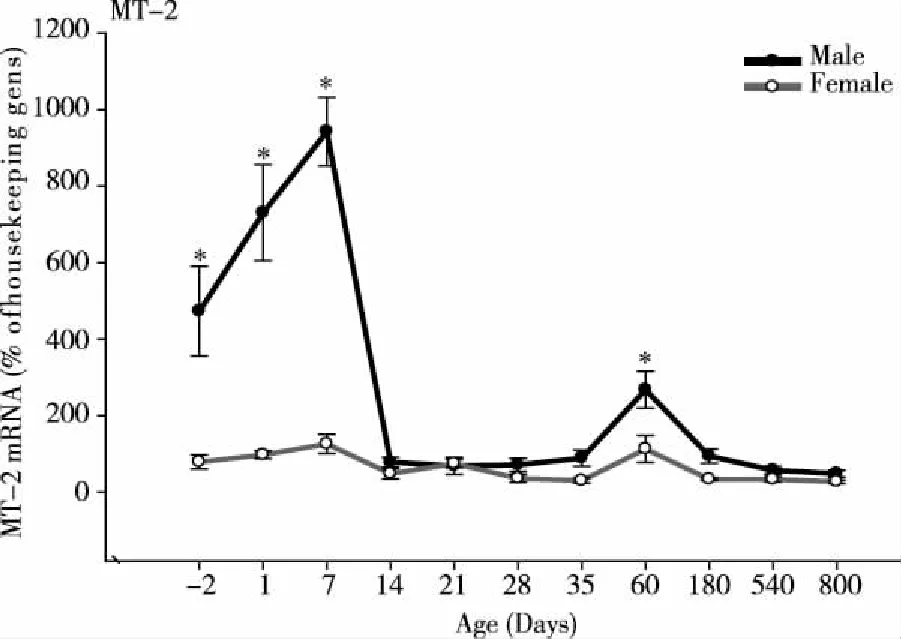
*Significantly different from female rats.P<0.05.Fig 2 Age and sex variations of MT-2 mRNA in male and female rat livers (n=6)
The expression of MT-2 mRNA decreased at later stages of pregnant (GD14 and GD19), then returned to control levels at PND 1, then decreased again at PND 7, 14 and 21 days (Fig 3 right )

*Significantly different from control rats. P<0.05.Fig 3 Variations of hepatic MT-1 and MT-2 mRNA in pregnant and lactation rats (n=6 for each time point)
We have also examined the expression of MT-3 and MT-4 mRNA , but the levels were too low to have significance (data not shown).The reason is that MT-3 mainly locates in brain and MT-4 in squamous tissues such as tong[1-3], not in the liver.
4 Discussion
MT is important in body defense against oxidative damage and is easily induced by toxic stimuli and physiological stress[1-4].Thus, to characterize physiological variations of MT is important to understand its regulation and function.The present study is among the first to characterize hepatic MT mRNA levels under different physiological conditions in rats, from the development, aging, sex, pregnancy and lactation.The information obtained together with our recent work on circadian variation of MT in mice[12]would be of significance in both basic and clinical sciences.
Newborn rats have high levels of hepatic MT, consistent with the literature[10-11]. The reasons for higher MT levels in fetal and neonatal livers could be multiple.One of the MT functions during development appears to protect against heavy metal toxicity, such as the toxicity of methylmercury[13]and cadmium[14].Indeed, neonatal mice deficient in MT are sensitive to Hg toxicity[15].Another reason could be related to zinc homeostasis, as MT plays important roles in providing zinc needs during development[16-17].Thus, the higher MT levels in the fetal and neonatal livers are important in protecting against toxic stimuli and maintaining zinc homeostasis.
Ageing is associated with biochemical and physiological changes and increased susceptibility to oxidative damage and various diseases including cancers[7-9].The accumulation of senescent cells and chronic inflammation/oxidative damage contributes to altered gene expressions and cellular dysfunction[8-9].MT has been proposed to modulate cellular senescent state and immune function, and is proposed to be a possible therapeutic target against ageing[7-9, 18].In the present study, we have provided the first evidence that MT mRNA levels decrease with ageing, and at 800 days of age, MT levels decreased many fold compared to young rats. Hepatic MT decreases in elderly people could be partially attributed to diminished cell proliferation and cellular defense[7-9]and diminished immune function[18], and could be a susceptible factor in hepatocarcinogenesis[19].
In hepatocellular carcinoma (HCC), hypermethylation of human MT isoforms and reduced MT gene expression are frequently observed[19-22].The suppressed MT gene expression is also observed in lung cancers[23].The expression of MT-1 and MT-2 was positive in the nucleus and cytoplasm of normal liver, but decreased in noncancerous livers and HCCs[19-20].Therefore, MT-1 and MT-2 are proposed to play a role in HCC differentiation and progression, and are proposed to be a biomarker to predict prognosis in patients with HCC[19-22].The down-regulated MT in HCC contributes to liver tumorigenesis, possibly mediated by increasing cellular NF-κB activity[21].Thus, the decreased MT expression in elderly is likely a risk factor inincreasing cancer incidence.
MT varies during different times of pregnancy and lactation.In pregnant and lactating women, lower expression of MT is associated with zinc and copper deficiency[24].In Cd-injected pregnant rats, hepatic MT decreased together in hepatic Cd accumulation but increases in renal Cd concentrations, making pregnant rats susceptible to Cd nephrotoxicity[25].In the present study, MTs decreased at later pregnancy and during lactation.The exact role of MT decreases during pregnancy and lactation remains unclear, and worth of further investigation.To understand the physiological changes of hepatic MT is important in examining MT function in liver diseases, cellular senescence, and HCC[7, 13 , 25].The potential use of MT gene therapy and MT modulation in liver fibrosis and ageing could be important in future therapeutics[4-5]. MT induction by traditional Chinese medicines could be another potential area of research, especially for liver diseases, HCC, and the elderly[1-2].
In conclusion, hepatic MT mRNA levels are influenced by the development, ageing, sex, pregnancy and lactation, as well as by circadian rhythm[12]. All of these could impact MT’s role in human liver diseases, and could be important to develop new therapeutic targets.
[References]
[1] Klaassen C D, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity[J].Annu Rev Pharmacol Toxicol, 1999,39:267-294.
[2] Liu J, Wu Q, Lu Y F,et al.New insights into generalized hepatoprotective effects of oleanolic acid: key roles of metallothionein and Nrf2 induction[J].Biochem Pharmacol, 2008,76(7):922-928.
[3] Nagamine T, Nakajima K. Significance of Metallothionein Expression in Liver Disease[J].Curr Pharm Biotechnol, 2013,Apr 11 (Epub ahead of print).
[4] Jiang Y, Kang Y J. Metallothionein gene therapy for chemical-induced liver fibrosis in mice[J].Mol Ther, 2004,10(6):1130-1139.
[5] Xu X, Shi F, Huang W, et al. Metallothionein gene transfection reverses the phenotype of activated human hepatic stellate cells[J].J Pharmacol Exp Ther, 2013,346(1):48-53.
[6] Hoogenraad T U. Paradigm shift in treatment of Wilson's disease: zinc therapy now treatment of choice[J].Brain Dev,2006,28(3):141-146.
[7] Swindell W R. Metallothionein and the biology of aging[J].Ageing Res Rev, 2011(1):132-145.
[8] Malavolta M, Cipriano C, Costarelli L, et al. Metallothionein downregulation in very old age: a phenomenon associated with cellular senescence[J]?Rejuvenation Res, 2008,11(2):455-459.
[9] Mocchegiani E, Costarelli L, Basso A, et al. Metallothioneins, ageing and cellular senescence: a future therapeutic target[J].Curr Pharm Des, 2013,19(9):1753-1764.
[10] Waalkes M P, Klaassen C D.Postnatal ontogeny of metallothionein in various organs of the rat[J].Toxicol Appl Pharmacol,1984,74(3):314-320
[11] Lehman-McKeeman L D, Andrews G K, Klaassen C D. Ontogeny and induction of hepatic isometallothioneins in immature rats[J].Toxicol Appl Pharmacol,1988,92(1):10-17.
[12] Zhang D, Jin T, Xu YQ, et al. Diurnal- and sex-related difference of metallothionein expression in mice[J].J Circadian Rhythms,2012,10:5.
[13] Peixoto N C, Serafim M A, Flores E M, et al. Metallothionein, zinc, and mercury levels in tissues of young rats exposed to zinc and subsequently to mercury[J].Life Sci, 2007,81(16):1264-1271.
[14] Goering P L, Klaassen C D. Resistance to cadmium-induced hepatotoxicity in immature rats[J].Toxicol Appl Pharmacol,1984,74(3):321-329.
[15] Yoshida M, Shimizu N, Suzuki M, et al. Emergence of delayed methylmercury toxicity after perinatal exposure in metallothionein-null and wild-type C57BL mice[J]Environ health perspect,2008,116(6):746-751.
[16] Mohammad M K, Zhou Z, Cave M, et al. Zinc and liver disease[J].Nutr Clin Pract, 2012,27(1):8-20.
[17] Maret W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc[J].Exp Gerontol,2008,43(5):363-369.
[18] Mocchegiani E, Malavolta M, Costarelli L, et al. Zinc, metallothioneins and immunosenescence[J].Proc Nutr Soc, 2010,69(3):290-299.
[19] Tao X, Zheng J M, Xu A M, et al. Downregulated expression of metallothionein and its clinicopathological significance in hepatocellularcarcinoma[J].Hepatol Res,2007,37(10):820-827.
[20] Park Y, Yu E. Expression of metallothionein-1 and metallothionein-2 as a prognostic marker in hepatocellular carcinoma[J].J Gastroenterol Hepatol,2013,28(9):1565-1572.
[21] Majumder S, Roy S, Kaffenberger T, et al.Loss of metallothionein predisposes mice to diethylnitrosamine-induced hepatocarcinogenesis by activating NF-kappaB target genes[J].Cancer Res, 2010, 7 (24) :10265-10276.
[22] Jacob S T, Majumder S, Ghoshal K. Suppression of metallothionein-I/IIexpression and its probable molecular mechanisms[J].Environ Health Perspect, 2002,110(Suppl 5):827-830.
[23] Liang G Y, Lu S X, Xu G, et al. Expression of metallothionein and Nrf2 pathway genes in lung cancer and cancer-surrounding tissues[J].World J Surg Oncol, 2013,11(1):199.
[24] Maia P A, Figueiredo R C, Anastácio A S, et al. Zinc and copper metabolism in pregnancy and lactation of adolescent women[J].Nutrition,2007,23(3):248-253.
[25] Chan H M, Cherian M G. Ontogenic changes in hepatic metallothionein isoforms in prenatal and newborn rats[J].Biochem Cell Biol, 1993,71(3-4):133-140.
