Catalytic Performance of MFI/MFI Core-Shell Zeolites in Benzene Methylation
2014-07-31ZhangBaozhongLiuXiaopeng
Zhang Baozhong; Liu Xiaopeng
(1. General Research Institute for Nonferrous Metals, Beijing 100088; 2. SINOPEC Tianjin Company, Tianjin 300271)
Catalytic Performance of MFI/MFI Core-Shell Zeolites in Benzene Methylation
Zhang Baozhong1,2; Liu Xiaopeng1
(1. General Research Institute for Nonferrous Metals, Beijing 100088; 2. SINOPEC Tianjin Company, Tianjin 300271)
Thanks to its 10-membered-ring structure with a three-dimensional uniform pore system and acid distribution, ZSM-5 zeolite is a key catalytic material for benzene methylation with methanol. After epitaxial inter-growth of a dense layer of shell consisting of nano-particles on the conventional ZSM-5 crystal surface, the MFI/MFI core-shell zeolite not only has much more active surface area, but also can enrich the diffusion and reaction pore system at the same time, which can significantly improve its catalytic performance. In contrast to the performance of conventional ZSM-5 catalyst, an indepth investigation on the reaction parameters of benzene methylation over the core-shell structured zeolite is of great significance.
core-shell zeolite, methanol, benzene methylation, process parameters
1 Introduction
As the world economy grows, there is a growing demand for xylene—one of the most important basic chemical materials. Large-scale industrial production of aromatics mainly relies on integrated aromatics units, which use such technologies as catalytic reforming, toluene disproportionation, transalkylation and aromatics separation processes[1-2]. With the decrease in oil resources and restriction of the total supply of naphtha, the shortage of raw materials for aromatics production will be persisting for a long period, so it is important to find an economical and feasible way for the sustainable production of aromatics. The SINOPEC Corporation has successfully combined coal chemical and petrochemical technologies to realize alkylation of toluene with methanol (toluene methylation), and effectively increased the production of xylene[3]. In view of the relatively excessive supply of benzene and methanol, the direct synthesis of toluene and xylene by means of alkylation of benzene with methanol will solve the supply-demand imbalance in the benzene, methanol and aromatics market, improve the product structure, optimize the allocation and utilization of resources, and promote product upgrading to promise a good prospect for industrial development[4]. The main catalysts used for alkylation of benzene with methanol at home and abroad are based on the ZSM-5 zeolite, which has advantage in terms of both catalytic performance and product distribution[5-7]. Hu Huimin[8]found out that as the Si/Al ratio increases, the pore size and specific surface area of ZSM-5 zeolite and the acidity strength increase, but the total acid amount decreases; and the catalytic activity is the highest when the Si/Al ratio is 399. Lu Lu[9]discovered that, compared with ordinary ZSM-5 zeolite, ZSM-5 zeolite with a hierarchical porous structure has a better activity and selectivity in catalyzing the alkylation of benzene with methanol due to its large number of mesopores, and it is more suitable for operation at a high weight hourly space velocity (WHSV). Hu Hualei, et al.[10]recently discovered that the promoter Pt has a significant inhibiting effect on the production of ethylbenzene. Through optimization of the synthesis scheme, Deng Wei, et al.[11]found out that the xylene yield reached 34.9% on the hierarchically porous ZSM-5 zeolite with a Si/Al ratio of 180. According to literature information, there is hardly any study on the process parameters of benzene alkylation with methanol, except for the research of Fu Peng[12], who pointed out that temperature has a significant effect on benzene alkyl-ation reaction, but the effect of WHSV is negligible. Using the MFI/MFI core-shell zeolite (abbreviated as BMZ hereinafter) as the catalyst and cylindrical ZSM-5 (abbreviated as BM, having a Si/Al ratio of 180) as a reference sample, the catalytic mechanism of benzene methylation over the core-shell catalyst and the relevant process parameters are discussed in this paper.
2 Experimental
2.1 Synthesis of zeolite
Synthesis of ZSM-5 zeolite was carried out as follows. Water glass (containing 28% SiO2, manufactured by the Sinopec Catalyst Co., Ltd), aluminum sulfate (AR, provided by the Sinopharm Group Company), ethylamine (CP, provided by the Sinopharm Group Company) and water was mixed according to a molar ratio of SiO2: Al2O3:C2H5NH2:H2O=180:1:25:3033 to generate gel, which was then hydrothermally crystallized at 150 ℃for 2 days. The product obtained thereby was calcined at 540 ℃ for 2 h, then was mixed and stirred with a 0.1 mol/L ammonium nitrate solution at 95 ℃ for 3 h prior to filtration and drying. The stirring, filtering and drying procedure was repeated for three times, and then the product was calcined at 540 ℃ for 4 h to obtain the H-ZSM-5 zeolite.
Synthesis of the core-shell zeolite was carried out as follows. TEOS (tetraethyl orthosilicate, AR, provided by the Sinopharm Group Company), TPAOH (tetrapropylammonium hydroxide 25%, manufactured by the Yixing Dahua Chemicals Company), EtOH (ethanol, AR, provided by the Sinopharm Group Company) and H2O was mixed according to a molar ratio of SiO2:EtOH: TPAOH:H2O=12:16:3.7:800, and stirred continuously at room temperature for 24 h. The above-mentioned ZSM-5 sample which was dried but not calcined was added into the solution, and the mixture was stirred for 1 h and then was transferred into a TFE-lined stainless steel crystallization kettle to crystallize at 180 ℃ for 1 day to obtain the target product.
2.2 Characterization of zeolite
The powder XRD characterization of samples was carried out by a Bruker X-ray diffractometer using CuKα radiation equipped with a graphite monochromator at a tube voltage of 40 kV, a tube current of 40 mA, and under a 10°—30° scanning range with a scanning speed of 0.02 (°)/s. A Philips XL30E scanning electron microscope (SEM) was used to analyze the crystal morphology and the shell growth of the composite material.
2.3 Catalyst shaping
The zeolite, binder and extrusion promoter were mixed homogeneously at fixed proportions, and then the mixture was kneaded, extruded, cured, dried and baked to obtain the shaped catalyst.
2.4 Evaluation of catalyst
The catalyst performance for benzene methylation was evaluated in a fixed bed reactor. The reactor was Φ25 mm×1 200 mm in size, had a catalyst loading of 20.0 g and was purged with a hydrogen stream with a purity of higher than 99%. Unless otherwise stated, the catalyst evaluation was conducted at: a reaction temperature of 400 ℃, a reaction pressure of 1.0 MPa, a benzene WHSV of 2.0 h-1, a hydrogen to hydrocarbon molar ratio (H/C) of 5:1, and a benzene to methanol molar ratio (B/M) of 1:1 (with benzene and methanol mixed evenly using an electromagnetic mixer).
The product analyses involved liquid product analysis and tail gas analysis. The feedstock toluene and liquid reaction products were analyzed using an HP-5890 gas chromatograph, which was equipped with a hydrogen flame ionization detector and a polyethyleneglycol capillary column, and the material composition was quantified by the corrected area normalization method. The non-aromatic hydrocarbons (C1—C5) in tail gas were also analyzed using an HP-7890 gas chromatograph, which was equipped with a hydrogen flame ionization detector and an aluminum oxide capillary column, and the composition of tail gas was quantified by the external standard method.
3 Results and Discussions
3.1 Physical and chemical properties of zeolite samples
Figure 1 shows the XRD patterns of the ZSM-5 zeolite and the MFI/MFI core-shell zeolite samples. It can be seen that the two characteristic diffraction peaks nearly overlap; in comparison with the standard spectral dia-gram, both ZSM-5 zeolite and the MFI/MFI core-shell zeolite samples possess the MFI phase structure with good crystal form free from visible mixed crystals. The full width at half diffraction peak of the MFI/MFI coreshell zeolite samples is obviously wider than that of ZSM-5 zeolite, especially in areas with large diffraction angles. This fact indicates that the MFI/MFI core-shell zeolite samples have a smaller average grain size.
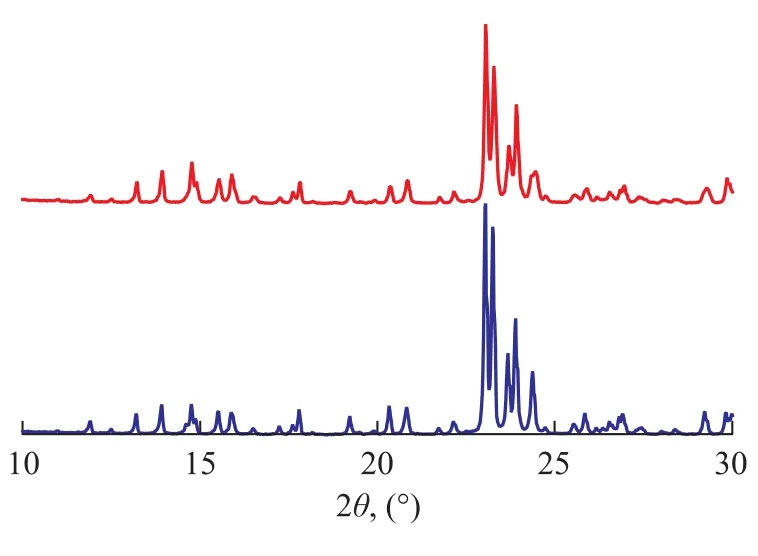
Figure 1 XRD patterns of ZSM-5 and MFI/MFI coreshell samples
Figure 2 shows the SEM photographs of the zeolite samples. It can be seen from Figure 2 that the crystal grains of both zeolite samples are composed of regular circular cylinders with similar sizes. The difference is that the MFI/MFI core-shell zeolite sample forms dense shell nanocrystals on the surface, and the shell crystal grains are rather uniform and the average grain size decreases substantially during the formation of the shell. This outcome agrees well with the result of XRD characterization. According to the XRD data, it can be inferred that the MFI/MFI core-shell zeolite sample possesses a core-shell structure.
3.2 Catalytic performance in benzene methylation
The performance data of BM and BMZ catalysts in alkylation of benzene with methanol are listed in Table 1. As a result of the formation of a shell on the outside surface of the MFI/MFI core-shell zeolite, the Si/Al ratio increases on the whole, thus the total acid amount decreases. Because the proportion of shell is relatively low and the increase of Si/Al ratio improves the gross acid strength, the benzene conversion rate decreases slightly. However, the selectivity of products on BM and BMZ samples in catalytic alkylation of benzene with methanol is significantly different, and the catalytic product toluene formed on BMZ decreases slightly; in comparison with the conversion rates, it can be known that with the increase of acid strength the benzene rings are more likely to be further methylated. Therefore, the catalytic products formed on BMZ contain more xylene (ΣX) and C9. Meanwhile, it is found that the core-shell zeolite has certain reaction selectivity, and would not likely to generate more tail gas to initiate side reactions to produce ethylbenzene and non-aromatic hydrocarbons, or to undergo the alkylation of reactants excessively to generate C10+ products. In addition, because of its unique shell, the core-shell zeolite has certain shape selectivity on products, thus the proportion of para-isomeride PX in xylene mixture increases from 17.8% to 35.9%.

Figure 2 SEM photographs of samples
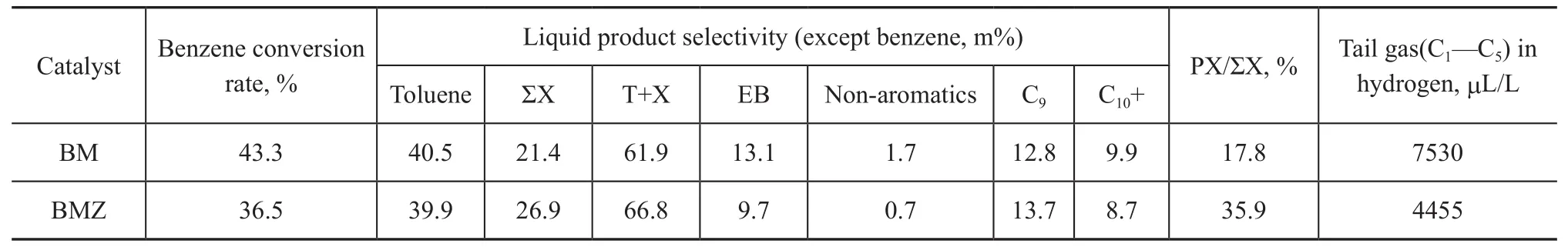
Table 1 Catalytic performance of BM and BMZ catalysts
3.3 Selection of reaction parameters for benzene methylation over BMZ catalyst
The methylation of benzene with methanol is an electrophilic substitution process in the benzene ring following the carbenium ion mechanism[13-14]; specifically, the feedstock methanol is activated by the Bronsted acid sites on catalyst surface, and its methyl carbocation species attack the weakly-absorbed benzene to generate toluene and xylene as well as H2O. Side reactions during the methylation process are more complicated because they involve a variety of aromatic reactions such as disproportionation and transalkylation, and side reactions of methanol such as etherification, alkylation, aromatization and decomposition, as well as side reactions between aromatics and methanol such as aromatic ethylation, propylation and further methylation could take place.
As shown in Table 2, the benzene conversion rate increases slightly with an increasing reaction temperature within the experimental range; the proportions of toluene, xylene and ethylbenzene in liquid products decrease, while the proportions of liquid non-aromatics, C9, C10+ and tail gas increase. In general, 400 ℃ is a suitable temperature for the benzene methylation reaction on BMZ.
The results obtained at different B/M ratios in Table 3 show that in comparison with the results of reaction conducted at B/M=1:1, the excessive benzene in feedstock directly leads to the decrease of benzene conversion rate and the decrease of C8+ products. Although excessive methanol in feedstock can increase benzene conversion rate, methanol on zeolite is more likely to undergo MTH (methanol to hydrocarbons) side reaction solely to generate hydrocarbon compounds, leading to the decrease of selectivity of toluene and ΣX, the increase of tail gas and liquid non-aromatics, as well as the production of C9and C10+ heavy aromatics due to excessive alkylation. By comparison, the molar ratio of benzene to methanol of 1:1 is a suitable feedstock proportion for benzene alkylation. The experimental results measured at different H/C ratios in Table 4 show that the selectivity of product toluene increases slightly with an increasing H/C ratio; in comparison with the results obtained at H/C=5, neither too high nor too low H/C ratios are beneficial for achieving high conversion of benzene, and the proportion of ΣX in products reduces, while other liquid by-products and tail gas would increase to some extent, which is adverse to the selectivity of products.The experiment results obtained at different reaction pressures in Table 5 show that the reaction pressure has no significant effect on the conversion rate of benzene; with the increase in reaction pressure, the total proportion of T+X in liquid products reduces; if the pressure is too high or too low, it is likely to lead to the generation of by-products like ethylbenzene and non-aromatics. Although high pressure can reduce the yield of tail gas, it also increases the yield of C9by-products and signif icantly decreases the proportion of PX in xylene. After comprehensive consideration of product selectivity, 1.0 MPa is regarded as the suitable reaction pressure for benzene methylation.
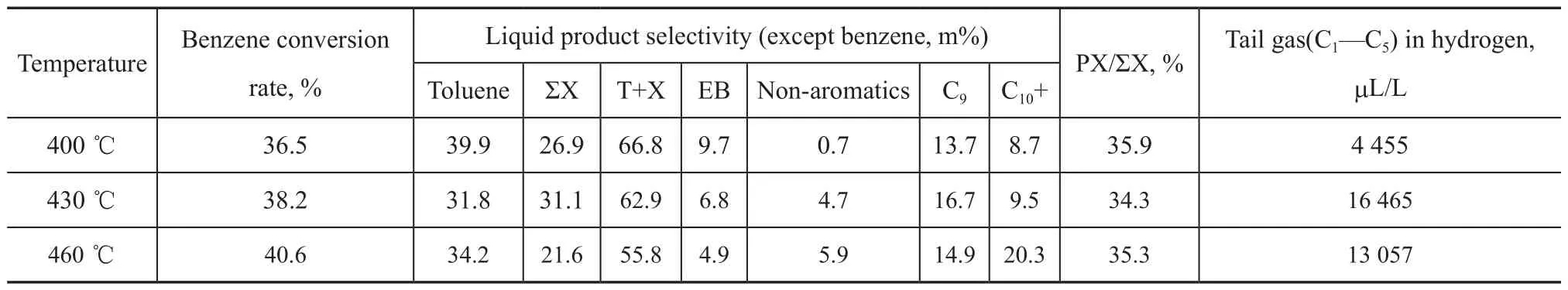
Table 2 Performance of BMZ measured at different reaction temperatures

Table 3 Performance of BMZ measured at different benzene/methanol ratios
The experimental results obtained at different WHSV values of benzene in Table 6 show that the benzene conversion rate decreases with an increasing feedstock WHSV value. Upon comparing the results obtained at a benzene WHSV of 2.0 h-1, the selectivity of both toluene and ΣX decreases to varying degrees when the WHSV value is 4.0 h-1. Although the ethylbenzene selectivity decreases, the yields of other by-products like liquid non-aromatics, C9, C10+ and tail gas increase significantly. After comprehensive consideration, 2.0 h-1is a suitable WHSV parameter for the reaction.
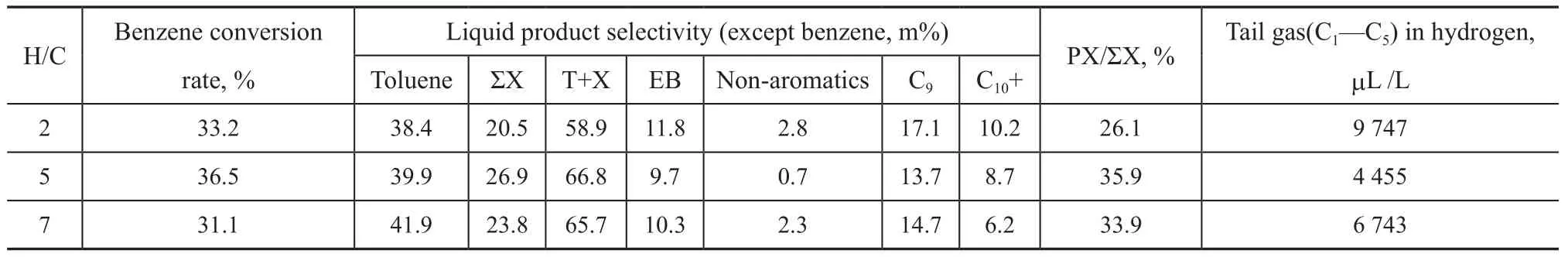
Table 4 Performance of BMZ measured at different hydrogen to hydrocarbon molar ratio
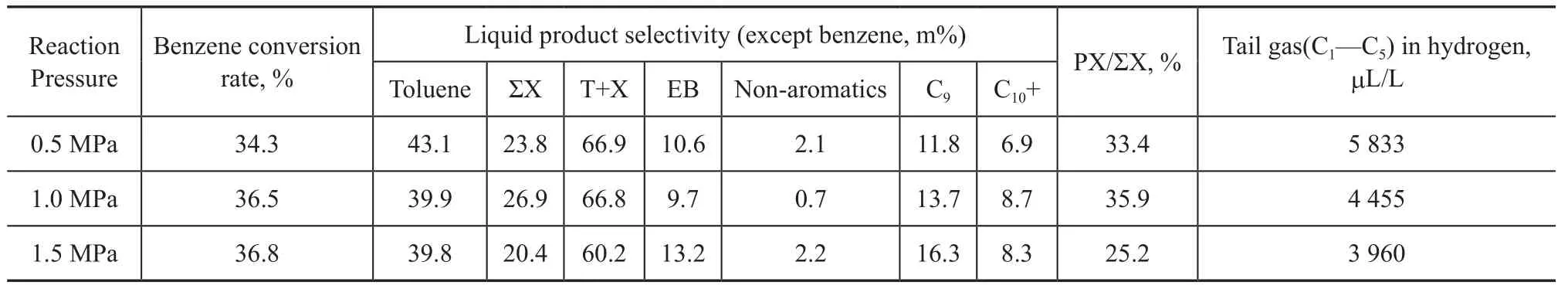
Table 5 Performance on BMZ measured at different reaction pressures
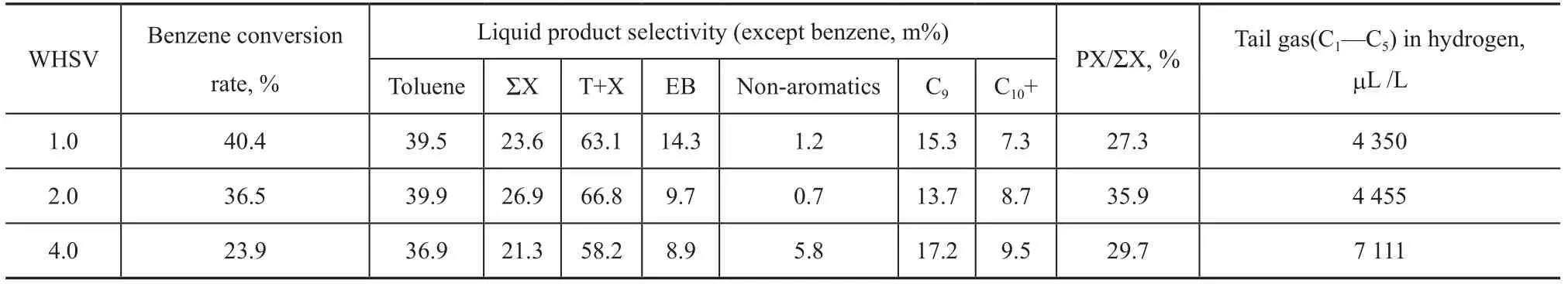
Table 6 Performance on BMZ measured at different WHSV values of benzene
4 Conclusions
The analysis of core-shell structure of ZSM-5 zeolite—the MFI/MFI core-shell zeolite shows good reaction selectivity and product selectivity in the methylation of benzene with methanol. The further research on reaction parameters of BMZ catalyst in benzene alkylation has shown that the suitable parameters for benzene alkylation with methanol involve: a reaction temperature of 400 ℃, a benzene/methanol molar ratio of 1:1, a benzene WHSV value of 2.0 h-1, a reaction pressure of 1.0 MPa, and a hydrogen/hydrocarbon molar ratio of 5:1. Subsequent research on the stability of the catalyst will be helpful forfurther exploring the potential application of MFI/MFI core-shell zeolite in place of the ZSM-5 zeolite for benzene alkylation with methanol, as well as the structurefunction relationship of the MFI/MFI core-shell zeolite.
[1] Kong D, Yang W. Advance in technology for production of aromatic hydrocarbons[J]. Chemical Industry and Engineering Progress, 2011, 30(1): 16-25 (in Chinese)
[2] Dai H. Outlook of aromatics production technology[J]. Petroleum Processing and Petrochemicals, 2013, 44(1): 2-9 (in Chinese)
[3] Kong D, Qi X, Zheng J, et al. Novel catalytic materials towards industrial production of aromatics[J]. Chemical Reaction Engineering and Technology, 2013, 29(5): 449-455 (in Chinese)
[4] Svelle S, Visur M, Olsbye U, et al. Mechanistic aspects of the zeolite catalyzed methylation of alkenes and aromatics with methanol: A review[J]. Top Catal, 2011, 54: 897-906
[5] Van der Mynsbrugge J, Van Speybroeck V, Svelle S, et al. Methylation of benzene by methanol: Single-site kinetics over H-ZSM-5 and H-beta zeolite catalysts[J]. Journal of Catalysis, 2012, 292: 201-212
[6] Bleken F, Skistad W, Barbera K, et al. Conversion of methanol over 10-ring zeolites with differing volumes at channel intersections: comparison of TNU-9, IM-5, ZSM-11 and ZSM-5[J]. Phys Chem Chem Phys, 2011, 13(7): 2539-2549
[7] Li Y. Catalysts Research for Alkylation of Benzene with Methanol[D]. Shanghai: East China University of Science & Technology, 2011 (in Chinese)
[8] Hu H. Methylation of Benzene with Methanol over a Series of ZSM-5 Catalysts[D]. Hunan: Hunan Normal University, 2007 (in Chinese)
[9] Lu L. Study on the Properties of ZSM-5 Catalysts in Benzene Alkylation with Methanol[D]. Shanghai: East China University of Science & Technology, 2012 (in Chinese)
[10] Hu Hualei, Zhang Qunfeng, Cen Jie, et al. High suppression of the formation of ethylbenzene in benzene alkylation with methanol over ZSM-5 catalyst modified by platinum[J]. Catalysis Communications, 2014, 57: 129-133.
[11] Deng W, He X, Zhu K, et al. Promoting xylene production in benzylation using hierarchically porous ZSM-5 derived from a modified dry-gel route[J]. Chin J Chem Eng, 2014, 22(8): 921-929
[12] Fu P. Catalysts Research of Benzene Alkylation with Methanol for Toluene and Xylene Production[D] . Shanghai: East China University of Science & Technology, 2010 (in Chinese)
[13] Moors S L, De Wispelaere K, Van der Mynsbrugge J, et al. Molecular dynamics kinetic study on the zeolite-catalyzed benzylation in ZSM-5[J]. ACS Catal, 2013(3): 2556-2567
[14] Hill I M, Malek A, Bhan A. Kinetics and mechanism of benzene, toluene, and xylene methylation over H-MFI[J]. ACS Catal, 2013(3): 1992-2001
Received date: 2014-10-15; Accepted date: 2014-11-14.
Zhang Baozhong, Telephone: +86-22-63805188; E-mail: zhangbaozhong.tjsh@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Comparative Studies on Low Noise Greases Operating under High Temperature Oxidation Conditions
- A Method for Crude Oil Selection and Blending Optimization Based on Improved Cuckoo Search Algorithm
- Experimental Research on Pore Structure and Gas Adsorption Characteristic of Deformed Coal
- Mathematical Model of Natural Gas Desulfurization Based on Membrane Absorption
- Ni2P-MoS2/γ-Al2O3Catalyst for Deep Hydrodesulfurization via the Hydrogenation Reaction Pathway
- Effects of Airflow Field on Droplets Diameter inside the Corrugated Packing of a Rotating Packed Bed
