Adsorption Thermodynamics and Diffusion Kinetics of PX over NaY Zeolite Synthesized by In-Situ Crystallization from Kaolin Microsphere
2014-07-31ZhaoHuaSongLijuanQinYucaiDuanLinhaiSunZhaolin
Zhao Hua; Song Lijuan; Qin Yucai; Duan Linhai; Sun Zhaolin
(1. College of Chemistry & Chemical Engineering, China University of Petroleum, Qingdao 266555; 2. Key Laboratory of Petrochemical Engineering, Liaoning Shihua University, Fushun 113001)
Adsorption Thermodynamics and Diffusion Kinetics of PX over NaY Zeolite Synthesized by In-Situ Crystallization from Kaolin Microsphere
Zhao Hua1,2; Song Lijuan1,2; Qin Yucai1,2; Duan Linhai2; Sun Zhaolin1,2
(1. College of Chemistry & Chemical Engineering, China University of Petroleum, Qingdao 266555; 2. Key Laboratory of Petrochemical Engineering, Liaoning Shihua University, Fushun 113001)
Para-xylene was chosen as the probe molecule to study adsorption thermodynamics and diffusion kinetics on NaY zeolite and composite structured NaY zeolite synthesized by in-situ crystallization from kaolin microsphere (designated as NaY/kaolin composites ) separately, using a high precision intelligent gravimetric analyzer (IGA). The adsorption isotherms showed normal Langmuir type-I behaviors. The increased adsorption heat with an increasingp-xylene coverage supported a mechanism of phase transition, diffusion and re-arrangement ofp-xylene molecules during the adsorption process. The rearrangement seemed to be most pronounced at an adsorption loading of 2.13 and 2.29 mmol/g for NaY zeolite and NaY/kaolin composites respectively. Compared with NaY zeolite, a 2—3 times higher in the diffusion coefficient ofp-xylene was observed on NaY/kaolin composites when the pressure was more than 50 Pa. Temperature-programmed desorption (TPD) ofp-xylene on two samples from room temperature to 450 ℃at a special loading has also been investigated by IGA. Results showed only single desorption peak appeared for NaY zeolite, indicating that adsorption can only occur in the super-cage structure. Comparably, there were two different peaks for in-situ synthesized NaY zeolite, corresponding to the two thermo desorption processes in both super-cage structure and the channels provided by kaolin, respectively.
adsorption thermodynamics; diffusion kinetics; in-situ crystrallization; NaY zeolite; para-xylene
1 Introduction
NaY zeolite, as the faujasite (FAU) family with a framework consisting of sodalite cages which are connected through hexagonal prisms that generate supercages with an average pore diameter of 0.74 nm, has been extensively used as the active component of catalysts for fluid catalytic cracking (FCC)[1], hydrocracking and hydrofining[2], and adsorption[3-4]processes because of its unique catalytic properties[5]. However, with an ever increasing cost and difficulty of obtaining high-quality crude for FCC operations, many refiners are processing at least a portion of resid as the feedstock in the FCC units or even use 100% of resid as the feedstock, which lead to diffusion limitation when large molecules diffuse in the relatively narrow channels, such as the case with NaY zeolite. As a consequence, it is urgently needed to modify zeolite structure or to find new materials with a hierarchical architecture of porosity for increasing the zeolite ability to crack residues.
Several potential solutions have been proposed to overcome the diffusion limitation imposed by zeolitic structures, namely: synthesizing new zeolite with larger micropores[6], creating meso-micropores, micro-microporous composite zeolite[7-8]and modifying the pore structure of zeolite[9]. The diffusion capability of large molecules in zeolite channels can be improved by the introduction of mesopores, which have been indirectly proved by enhanced catalytic performance with model compounds. Among these, there is considerable interest in using kaolin as the aluminum source, because doping eliminates the need to form Na2SO4and simplifies the treatment of the mother liquor considerably. The kaolin of China wasfamous in the world for its good assortment, abundant reserves and high quality. Kaolin is extensively applied in petroleum and chemical engineering, paper making, functional filler and fire-proof materials production. It can be used as a support for the catalyst, and also as raw materials for synthesizing zeolite. Thus it is an ideal support of in-situ crystallized zeolitic molecular sieves. The in-situ synthesized zeolite has an ideal hydrothermal stability. In-situ synthesis of zeolite can mitigate the difficulty of filtration in the industry production and reduce the production cost. Attempts have been made repeatedly to use kaolin as the starting raw material for the synthesis of A, X and Y-type zeolites, such as NaY/kaolin composite zeolites[10-14], NaY-MCM41/kaolin composite catalytic material[15-17], Y/kaolin microsphere[18-19]and KS01/kaolin[20], etc. Characterization and results evaluation indicated kaolin is more suitable for cracking the residue feed because of its unique manufacturing process which provides this catalyst with appropriate pore structures by which large residue molecules can more easily enter the active sites[21]. However, the reported researches have focused on the synthesis and industrial applications of catalysts[22-24], investigation on the mechanism of adsorption and diffusion was seldom reported. The existing technical literature on adsorption and diffusion is also limited in the field of molecular simulation, FT-IR, XRD, TEOM[25]and ZLC techniques[26]. Furthermore, dynamic and thermodynamic data, such as the activation energy and adsorption heat, are not available for NaY/kaolin composite zeolite. Such data are helpful in understanding the diffusion process and the catalytic performance. Therefore, it has great practical value and significance to understand the theory in this area.
Herein, the adsorption and diffusion mechanism of the probe moleculep-xylene on NaY zeolite and in-situ crystallized NaY composites emanated from kaolin have been studied using a high precision intelligent gravimetric analyzer (IGA), a very accurate and completely computer-controlled gravimetric technique. The adsorption isotherms, adsorption thermodynamics and diffusion coefficient are also reported.
2 Experimental
The measurement ofp-xylene adsorption and diffusion in NaY zeolite and NaY/kaolin composites was carried out on a computer-controlled intelligent gravimetric analyzer (IGA, Hiden Analytical Ltd., Warrington, UK). A sensitive microbalance (with a resolution of 0.1 µg), which could automatically measure the mass of the sample as a function of time with the gas vapor pressure and sample temperature under computer control, was mounted in a thermostat enclosure to remove thermal coefficients of the weighing system and thus provide high stability and accuracy. The zeolite sample (about 126 mg, provide by the SINOPEC Fushun Research Institute of Petroleum and Petrochemicals) was degassed under a vacuum of less than 10-3Pa at 773 K for 10 h prior to the adsorption measurements. The sample temperature was regulated within 0.1 K by either a water bath or a furnace. The equilibrium pressures were determined by three high-accuracy, Baratron pressure transducers and maintained at the set-point by active computer control of the admittance/exhaust valve throughout the duration of the experiment. For each step, the amount of adsorbate introduced in the system was kept small enough to make the adsorption process isothermal. The increase of mass was continuously recorded.
TPD is not very accurate but is a rapid and successful method to obtain information about the adsorbateadsorbent interactions of various hydrocarbon/zeolite systems compared with isotherm determinations. Before starting the TPD run, the adsorbent was saturated with the adsorbate at room temperature under a specific pressure which was chosen from the isotherm to meet the required loading. The mass uptake was measured as a function of time and the approach to equilibrium was monitored in real time with a computer algorithm. The system was then heated at certain rate from room temperature to 400 ℃. The pressure was maintained constant during the heating process. The mass of the adsorbent was recorded as a function of temperature from which thermal gravimetry (TG) and differential thermal gravimetry (DTG) data could be derived.
The nitrogen adsorption of the sample was performed on a physical adsorption instrument ASAP2420 (Mike Company, USA) at the liquid nitrogen temperature (77 K) and the sample was degassed at 673 K for 4 h prior to adsorption analysis. N2-adsorption/desorption isothermswere recorded to determine the surface properties (surface area and pore volume distribution) according to the BET isothermal equation and the pore-volume by thet-plot method. The pore size was reported as the average pore diameter.
3 Results and Discussion
3.1 N2-adsorption/desorption
N2-adsorption and desorption isotherms at 77 K for NaY zeolite and NaY/kaolin composites are presented in Figure 1. Marked hysteresis loops could be seen in the isotherms at a high relative pressure(p/p0)of 0.5—1.0, indicating that mesopore distribution existed in both samples. It can be seen from the size of hysteresis loops that the NaY zeolite possessed a small amount of mesopores except for narrow micropores, which are less concentrated than the commercial NaY zeolite. Upon treating with kaolin, the zeolite structure showed a remarkably enhanced adsorption of N2accompanied by a sharper hysteresis loop indicative of extra meso-porosity and macroporosity.

Figure 1 N2-adsorption and desorption isotherms at 77K of NaY zeolite and NaY/kaolin composites
The detailed pore properties are listed in Table 1. The BET total surface area (SBET), the Langmuir surface area (SL), the total mesoporous surface area (Smeso), and the average pore diameter of NaY/kaolin composites were significantly different from those of the NaY zeolite. Remarkably, the mesoporous surface area of the composites, the average pore diameter, the BJH adsorption identified average pore diameter and cumulative volume of pores increased by 32.939 m2/g, 2.6 nm, 6.7 nm and 0.055 cm3/g respectively, as compared to the NaY zeolite. With the micropore properties retained, the microporous surface area decreased by only about 17 m2/g.
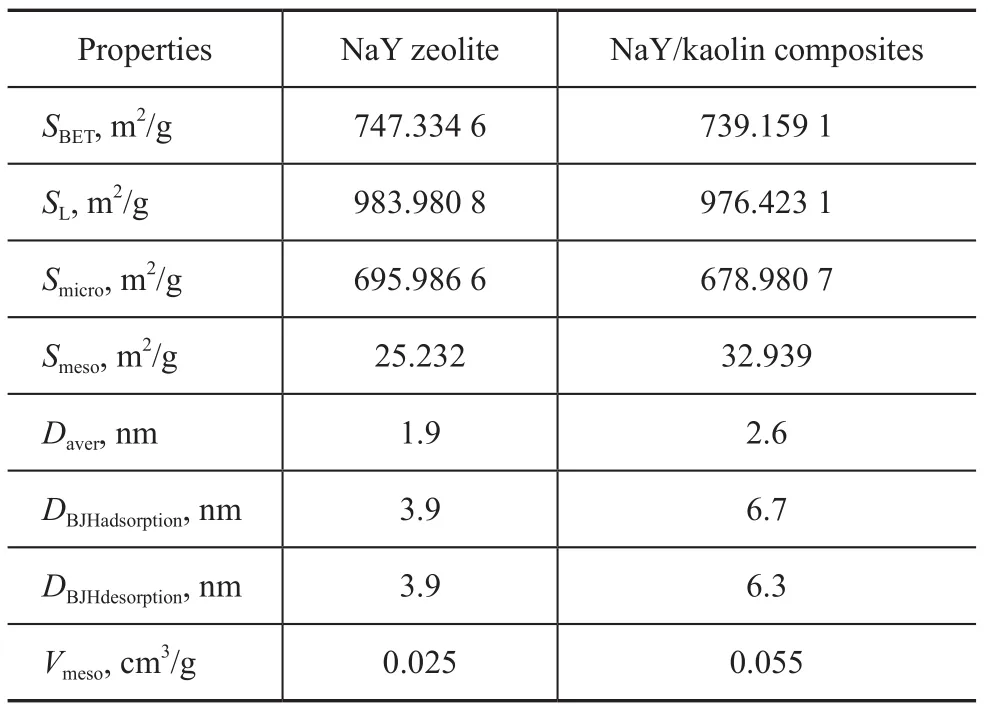
Table 1 The porosity properties of the NaY zeolite and NaY/kaolin composites
In summary, the above investigation showed that the kaolin-treatment afforded the NaY with mesoporous and macroporous structure while retaining a small amount of microporous structure. By using these two samples as adsorbents, the adsorption and diffusion capabilities ofpxylene were studied.
3.2 Para-xylene adsorption on the NaY zeolite and NaY/kaolin composites
3.2.1 Adsorption isotherms
The adsorption isotherms ofp-xylene measured using the IGA instrument at 303 K, 333 K and 373 K on the two samples are presented in Figure 2. The isotherms of zeolite NaY showed a typical Langmuir Type-I adsorption (according to the adsorption theory of Brunauer, Deming, Deming and Teller (BDDT)), which reached saturation at low pressure and then increased slightly with further increase of pressure, demonstrating a strong interactionbetween the zeolite andp-xylene. The isotherms for NaY/ kaolin composites were similar to that of NaY zeolite except for a small increment inp-xylene uptake (Figure 3). Because the adsorption inp-xylene/NaY andp-xylene/ NaY/kaolin systems is an exothermic process, the adsorption amount decreases with an increasing temperature.
It may be reasonable to understand that the combination of microporous, mesoporous and macroporous structure yielded by kaolin-treatment technique made thep-xylene diffusion and re-arrangement easier which was beneficial to the increase in adsorption capacity of a large molecule. Furthermore, during the calcination process, some of Al2O3species in kaolin microspheres lost the response in the crystallization, leaving SiO2highly activated to further increase the effective reaction of
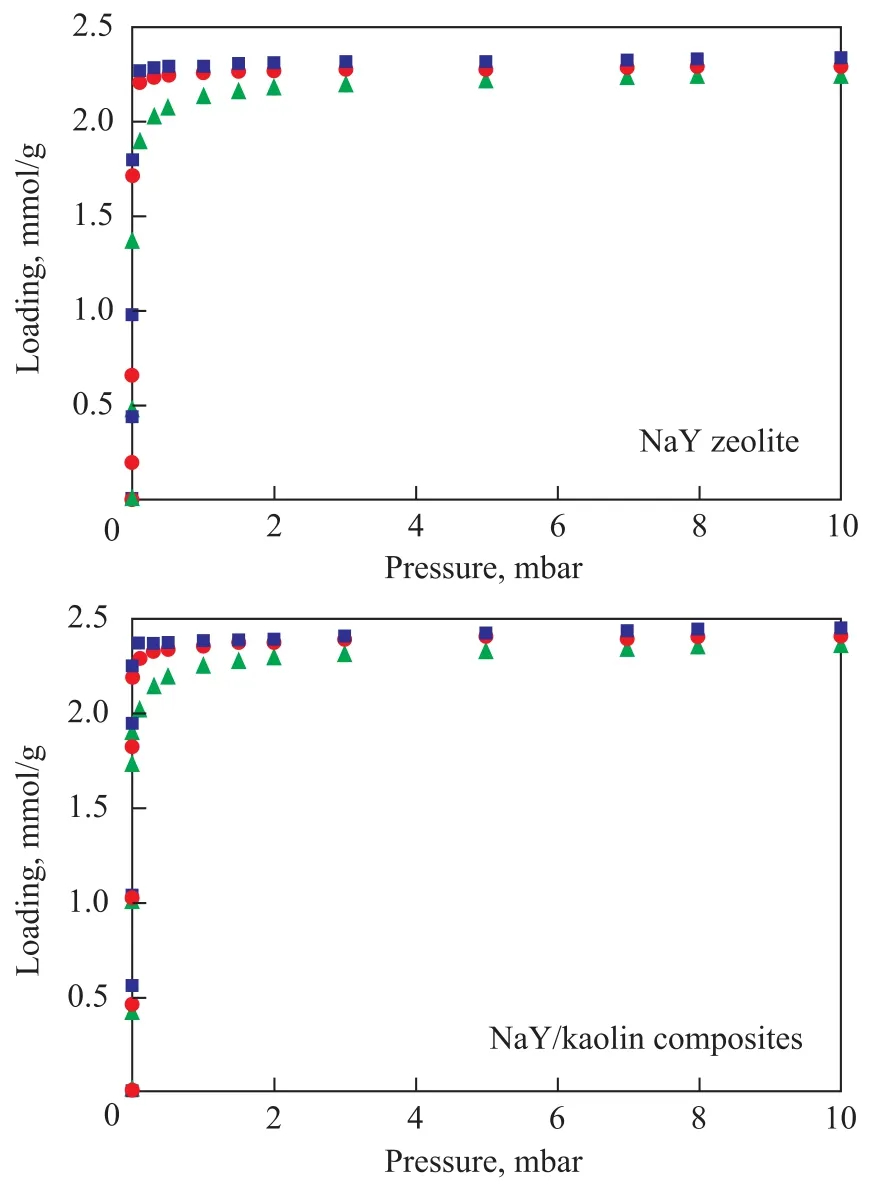
Figure 2 The adsorption isotherms of both zeolites by IGA at 303 K, 333 K and 373 K
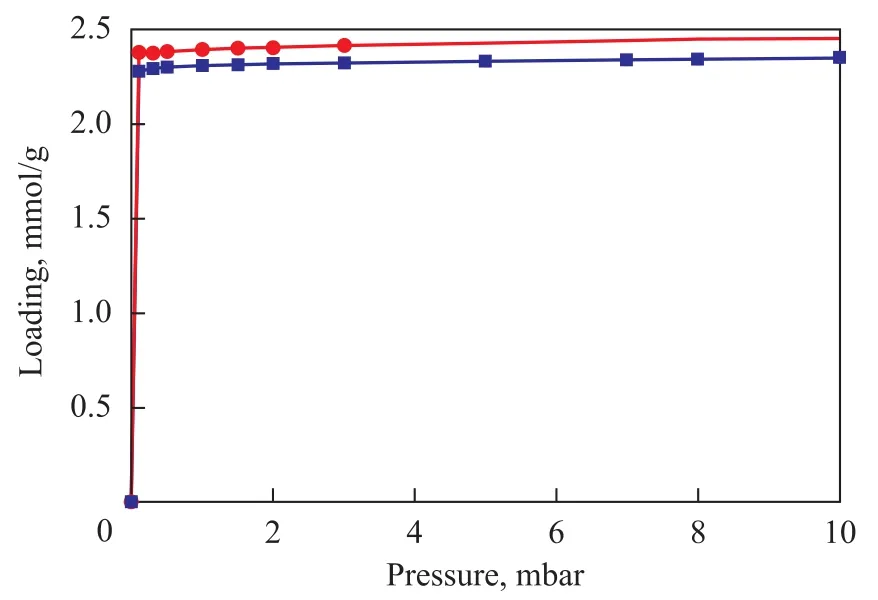
Figure 3 The adsorption amount of p-xylene on both zeolites versus pressure determined by IGA at 303 K
Figure 4 shows the adsorption isotherms ofp-xylene on kaolin measured by IGA at 333K. It can be seen that the isotherm was not reversible and displayed hysteresis loops, which were indicative of extra meso-porosity and macro-porosity. The characteristic shape of isotherm was attributed to the energetically heterogeneous surface of the adsorbents. Furthermore, the isotherm was similar to a straight line, suggesting a weak interaction betweenpxylene and kaolin.
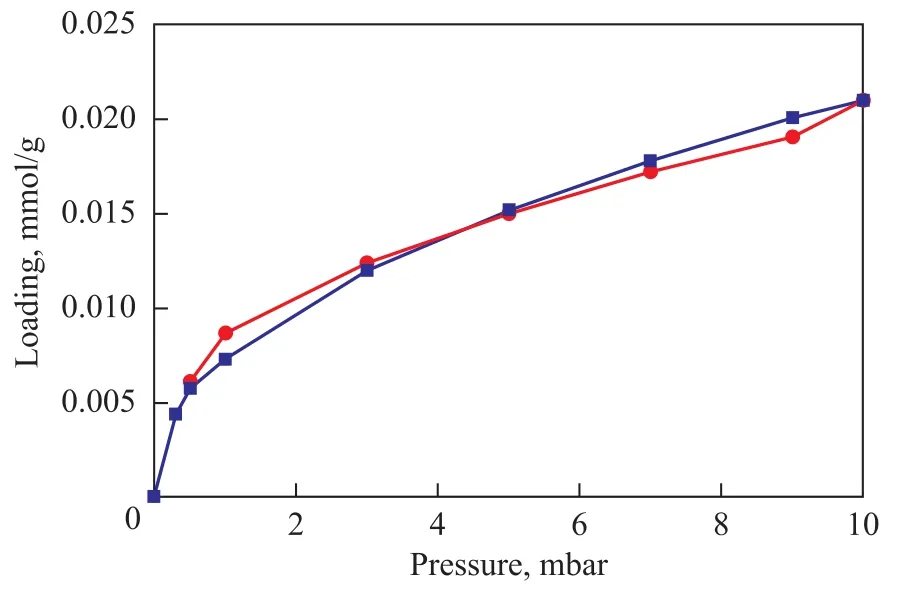
Figure 4 The adsorption isotherms of para-xylene on kaolin measured by IGA at 333 K
3.2.2 Isosteric heats of adsorption (Qst)
Isosteric heat of adsorption is also an important parameter for gas separation through physical adsorption. The isosteric heat of adsorption can be estimated from the Clausius-Clapeyron equation with the experimental data described by the Langmuir isotherm (Eq. 1):

whereP,TandCrepresent the equilibrium pressure (Pa), the temperature (K) and a constant, respectively.
Figure 5 shows that the isosteric heat of adsorptionQst, calculated by Eq. (1) increased in magnitude with an increasing adsorption loading ofp-xylene on NaY zeolites and NaY/kaolin composites, indicating to the results of adsorbate-adsorbent and adsorbate-adsorbate interactions as the loading increases. The heat of adsorption in NaY/ kaolin composites was relatively smaller than that in zeo-lite NaY, confirming the stronger interactions betweenp-xylene and zeolite NaY than those betweenp-xylene and NaY/kaolin composites. It was interesting to observe an inflection point at the adsorption capacity of 2.13 mmol/g and 2.29 mmol/g for NaY zeolite and NaY/kaolin composites, respectively. Jan pointed out that phase transition was a good approach to understand the inflection of adsorption isotherm and related energy changes[27]. As for NaY/kaolin composites, the steps appeared in Figure 6 might suggest that a phase transition and molecule rearrangement took place in the course ofp-xylene diffusion. A phase transition could occur, which was attributed to the formation of the complexes or the clustering of the aromatics. Following the adsorption equilibrium, thepxylene molecules commenced to diffuse into super-cages, mesopores or macropores introduced by kaolin and the intersections of the two channels, two different possible adsorption sites, via rearrangement. The step-like curve of NaY/kaolin composites exhibited energetically heterogeneous sorption sites. In the case of NaY zeolite, the molecules undergo condensation and a rearrangement at an adsorption loading of 2.13 mmol/g due to the presence of a small amount of mesopores. An increase in adsorption capacity may occur due to the introduction of kaolin making thep-xylene diffusion, phase transition, and rearrangement easier than those of NaY zeolite.

Figure 5 Isosteric heat of adsorption, Qst, of p-xylene on NaY zeolite and NaY/kaolin composites as a function of adsorption loading
3.2.3 Differential molar entropy of adsorption (ΔS0)
The differential molar entropy of adsorption at the standard pressurep0can be obtained from
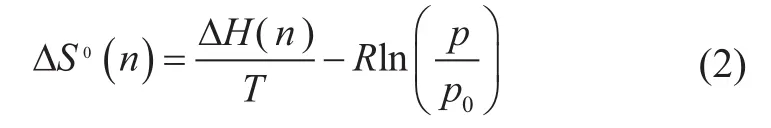
where ΔHis the differential enthalpy of adsorption.
Figs. 6 and 7 show the differential molar entropies of adsorption at 0.1 MPa and 303 K, 333 K and 373 K for NaY zeolite and NaY/kaolin composites, respectively, as calculated by Eq. (2). Similar toQst,ΔS0changed with the adsorption capacity, and there was an inflection point suggesting a small but sudden increase in loss of freedom at an adsorption capacity of 2.13 mmol/g and 2.29 mmol/g for NaY zeolite and NaY/kaolin composites, respectively. ΔS0changed little with temperature at a constant coverage in the temperature range and decreased slightly with an increasing temperature.
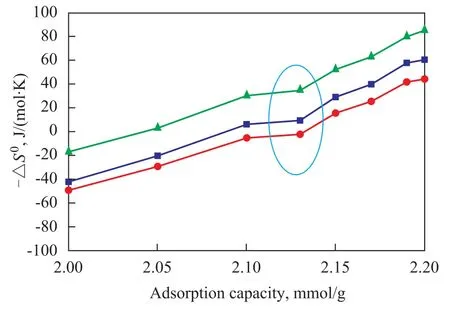
Figure 6 Change of differential molar entropy of adsorption of p-xylene on NaY zeolite as a function of adsorption loading

Figure 7 Change of differential molar entropy of adsorption of p-xylene on NaY /kaolin composites as a function of adsorption loading
3.3 Diffusion coefficients
According to Fick’s law, the mathematical solution forthe transient diffusion equation for a spherical particle assumes the following well-known form:
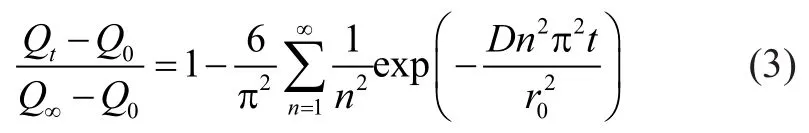
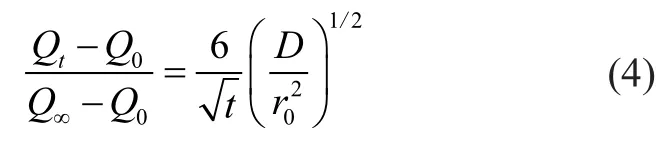
It can be seen from Equation (4) that the plot ofversus the square root of time should be linear in the initial part of the curve and the slop yields the diffusion time constantIn the present study, the diffusion coefficient ofp-xylene was compared according to the slop of the plot. Table 2 listed the diffusion coefficients ofp-xylene on the two samples at various pressures at 303 K. At low pressure (<0.5 mbar), the diffusion coefficient of NaY zeolite were slightly higher than that of NaY/kaolin composites thanks to the large amount of regular microporous structure in NaY zeolite. And then, however, the diffusion coefficient of NaY/kaolin composites was 2—3 times higher than NaY zeolite. These data indicated that the introduced mesoporosity and macroporosity significantly improved the diffusion rate. The improvement in diffusion ability was expected to greatly benefit the cracking capability of these types of large molecules. It was also observed from the slope that thep-xylene diffusivity in two samples decreased slightly with an increase inp-xylene pressure. This was probably attributed to the fact that the adsorbent pores were partially saturated and blocked at higher adsorption loading in the higher pressure range giving rise to sluggish kinetics.
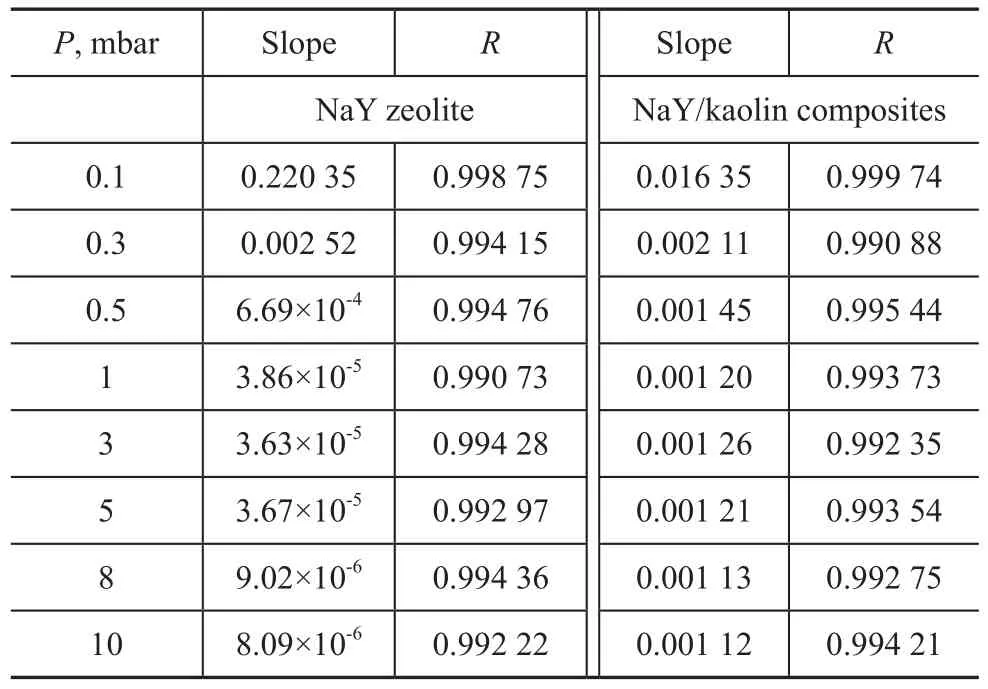
Table 2 The diffusion coefficient ofp-xylene on two zeolites at 303 K
3.4 TPD
TPD ofp-xylene in NaY zeolite or NaY/kaolin composites from room temperature to 450 ℃ at a loading of 2.13 mmol/g (NaY) or 2.29 mmol/g (NaY/kaolin composites) at a heating rate of 20 ℃/min had been investigated by IGA (Figure 8). There were two different peaks in TPD profiles of NaY/kaolin composites, which corresponded to the two thermo-desorption processes that were consistent with two-site adsorption mechanism reported by Ban, et al.[28]In fact, the desorption of adsorbate molecules should take place along the easiest path available. It was possible that desorption occurred simultaneously from both the super-cages in NaY zeolite and the channels provided by kaolin. However, the NaY zeolites produced only single desorption peak profiles, supporting the onesite adsorption mechanism.
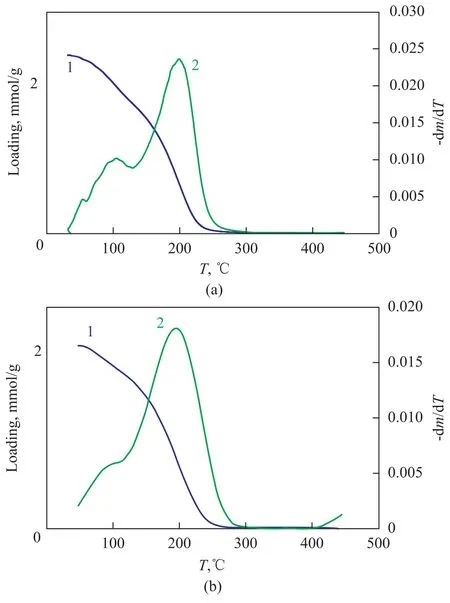
Figure 8 The TPD profiles for (a) NaY/kaolin composites and (b) NaY zeolite
4 Conclusions
The adsorption isotherms showed a normal Langmuir Type-I behavior. The adsorption and diffusion data ofp-xylene increased in both the adsorption amount and the diffusion coefficient on kaolin-treated NaY, as compared to untreated NaY zeolite. The diffusion coefficients increased by 2—3 times those of NaY zeolites after the introduction of kaolin, which were ascribed to improvements in diffusion. Data of enthalpy and entropy of adsorption reflected the packing differences ofp-xylene molecules on two samples, and the interactions betweenp-xylene molecules were also different. It was found out that there was a process of diffusion, phase transition, and re-arrangement ofp-xylene molecules during the adsorption process. The rearrangement seemed to be most pronounced at an adsorption loading of 2.13 mmol/g and 2.29 mmol/g for NaY zeolite and NaY/kaolin composites, respectively. The TPD profiles showed that there was a single adsorption site for NaY zeolite, while two different adsorption sites for NaY/kaolin composites were identified.
Acknowledgements:We would like to thank Dr. Song for numerous discussions during the course of this research work and the financial support from the National Natural Science Foundation of China (20976077, 21076100) and the National 973 Foundation of China (2007CB216403).
[1] Wei Guoyou, Zhou Jihong, Chen Zhenyu, et al. Industrial synthesis and catalytic properties of Y-zeolite-containing composite material with micro/mesoporous structure[J]. Petroleum Processing and Petrochemicals, 2013, 44(4): 59-63 (in Chinese)
[2] Yang Junjie, Fan Hongfei, Zhao Chongqing, et al. Hydrocracking performance of small crystal size zeolite Y catalyst[J]. Petroleum Processing and Petrochemicals, 2012, 43(8): 59-62 (in Chinese)
[3] Dong Shiwei, Qin Yucai, Wang Yuan, et al. Study on the removal of sulfur compounds in gasoline by selective adsorptive desulfurization process[J]. Petroleum Processing and Petrochemicals, 2013, 44(3): 26-31 (in Chinese)
[4] Myroslav S, Maya L, Altar P T, et a1. Ammonium sorption from aqueous solutions by the natural zeolite transcarpathian clinoptilolite studied under dynamic conditions[J]. Journal of Colloid and Interface Science,2005, 284: 408-415
[5] Yan J, Yu D H, Sun P, et al. Catalytic dehydration of lactic acid to acrylic acid over NaY zeolites modified with metallic ions[J]. Petrochemical Technology, 2011, 40(5): 476-481 (in Chinese)
[6] Corma A, Diaz-Cabanas M J, Martinez-Triguero J, et al. A large cavity zeolite with wide pore windows and potential as an oil refining catalyst[J]. Nature, 2002, 418(5): 514-517
[7] Huang L M, Guo W P, Deng P. Investigation of synthesizing MCM-41/ZSM-5 composites[J]. J Phys Chem B, 2000, 104(13): 2817-2823
[8] Guo W P, Huang L M, Deng P. Characterization of Beta/ MCM-41 composite molecular sieve compared with the mechanical mixture[J]. Micropor Mesopor Mater, 2001, 44: 427-434
[9] Tao Y, Kanoh H, Aneko K K. Developments and structures of mesopores in alkaline-treated ZSM-5 zeolites[J]. Adsorption, 2006, 12(5/6): 309-316
[10] Zheng S Q, Dou X H, Duan C Y, et al. Synthesis and characterization of composite catalyst based on in situ crystallization on kaolin[J]. China Non- metallic Mining Industry Herald, 2004, 42(4): 32-33 (in Chinese)
[11] Yi H H, Zhang Y M, Yao B Y. Progress in in-situ crystallization synthesis of Y zeolite with kaolin[J]. Speciality Petrochemicals, 2009, 26(1): 77-82(in Chinese)
[12] Wu J X, Liu H, Zhou Z H, et al. Synthesis of NaY zeolite with high crystallinity from coal-series kaolin[J]. Non-Metallic Mines, 2014, 37(1): 36-39 (in Chinese)
[13] Liu X M, Wang H P, Lou Y T. In-situ synthesis of NaY zeolite with coal-based kaolin[J]. Journal of Natural Gas Chemistry, 2003, 12(1): 63-70 (in Chinese)
[14] Zhou Jihong, Luo Yibin, Zong Baoning. Formation mechanism of porous molecular sieve Y composite[J]. Petroleum Processing and Petrochemicals, 2012, 43(1): 26-31 (in Chinese)
[15] Liu H T, Bao X J, Wei W S. Synthesis and characterization of kaolin/NaY/MCM-41 composites[J]. Micropor Mesopor Mater, 2003, 66(1): 117-125
[16] Liu H T, Bao X J, Wei W S, et al. Synthesis of NaYMCM-41/kaolin composite by in situ crystallization[J]. Journal of Molecular Catalysis, 2003, 17(6): 241-246
[17] Li Q, Zhang Y, Cao Z J, et al. Influence of synthesis parameters on the crystallinity and Si/Al ratio of NaY zeolite synthesized from kaolin[J]. Pet Sci, 2010, 7(3): 403-409
[18] Xu M C, Cheng M J, Bao X H. Growth of ultrafine zeolite Y crystals on metakaolin microspheres[J]. Chem Commun, 2000, 12(3): 1873-1874
[19] Hong H L, Jian T M, Gao X H. Synthesis, characterization and evaluation of a novel resid FCC catalyst based on in site synthesis on kaolin microspheres[J]. Catalysis Letters, 2006, 110(3/4): 229-234
[20] Xu M C, Cheng M J, Tan D L, et al. Growth of zeolite on kaolin microspheres[J]. Chinese Journal of Catalysis, 2001, 22(1): 31-34
[21] Wang Mingjin, Xu Mingde, Zhu Yuxia. Development and application of CDOS series catalysts for bottoms cracking[J]. China Petroleum Processing and Petrochemical Technology, 2013, 15(4): 9-13
[22] Shao H Q, Li Y F, Gao X L. Preparation and catalytic performance of silica-supported Cr(acac)3/PNP for ethylene tetramerization[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(1): 45-51
[23] Ooi Y S, Zakaria R, Mohamed A R. Synthesis of composite material MCM-41/Beta and its catalytic performance in waste used palm oil cracking[J] Appl Catal A: Gen, 2004, 274(1/2): 15-23
[24] Zhang Z D, Liu Z Y, Yan Z F, et al. Application of new heavy metals resistant porous binder material used in fluid catalytic cracking reaction[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(1): 35-39
[25] Lee C K, Ashtekar S, Gladden L F, et al. Adsorption and adsorptions kinetics of hydrocarbons in FCC catalysts studied using a tapered element oscillating microbalance (TEOM): Part 1: Experimental measurements[J]. Chem Eng Sci, 2004, 59(1): 307-314
[26] Lose A C, Rodrigues A E. Sorption and Diffusion of n-Pentane in Pellets of 5A Zeolite[J]. Ind Eng Chem Res, 1997, 36(2): 493-500
[27] Jan T D, Mirosaw K, Janina M D. Computer modeling and analysis of heterogeneous structures of microporous carbonaceous materials[J]. J Mol Model, 2005, 11: 416-430
[28] Ban H Y, Gui J Z, Zhang X T, et al. TPD of cyclic hydrocarbons in silicalite-1 studied by intelligent gravimetry[J]. Thermochimica Acta, 2005, 439(1): 121-126
Received date: 2014-04-09; Accepted date: 2014-08-25.
Professor Song Lijuan, Telephone: +86-413-56650568; E-mail: lsong56@263.net; zh.113@126. com.
杂志排行
中国炼油与石油化工的其它文章
- Comparative Studies on Low Noise Greases Operating under High Temperature Oxidation Conditions
- A Method for Crude Oil Selection and Blending Optimization Based on Improved Cuckoo Search Algorithm
- Experimental Research on Pore Structure and Gas Adsorption Characteristic of Deformed Coal
- Mathematical Model of Natural Gas Desulfurization Based on Membrane Absorption
- Ni2P-MoS2/γ-Al2O3Catalyst for Deep Hydrodesulfurization via the Hydrogenation Reaction Pathway
- Effects of Airflow Field on Droplets Diameter inside the Corrugated Packing of a Rotating Packed Bed
