Hydrothermal Synthesis of MoO2and Supported MoO2Catalysts for Oxidative Desulfurization of Dibenzothiophene
2014-07-31WangDanhongZhangJianyongLiuNiZhaoXinZhangMinghui
Wang Danhong; Zhang Jianyong; Liu Ni; Zhao Xin; Zhang Minghui
(Key Laboratory of Advanced Energy Materials Chemistry (MOE), College of Chemistry, Nankai University, Tianjin 300071)
Hydrothermal Synthesis of MoO2and Supported MoO2Catalysts for Oxidative Desulfurization of Dibenzothiophene
Wang Danhong; Zhang Jianyong; Liu Ni; Zhao Xin; Zhang Minghui
(Key Laboratory of Advanced Energy Materials Chemistry (MOE), College of Chemistry, Nankai University, Tianjin 300071)
A novel method for obtaining spherical MoO2nanoparticles and SiO2-Al2O3supported MoO2by hydrothermal reduction of Mo (VI) species was studied. The obtained MoO2catalysts show very high catalytic activity in the oxidative desulfurization (ODS) process. The effect of hydrothermal temperature and crystallization temperature on ODS activity was investigated. The ODS activity of supported MoO2catalysts with various MoO2contents were also investigated. The mechanism for formation of MoO2involving oxalic acid was proposed.
MoO2; hydrothermal reduction; oxidative desulfurization; nanoparticle
1 Introduction
Much interest has been shown over the last decade on the application of oxidative desulfurization (ODS) for producing liquid fuels[1-6]. This process has been intensively investigated because of the very stringent environmental regulations that have limited the level of sulfur content in diesel to less than 15 μg/g since 2006 in the US, less than 10 μg/g since 2005 in Europe, and less than 10 μg/g starting 2018 in China. Hydrodesulfurization (HDS) is highly efficient for removal of thiols, sulfides, and disulfides. However, it is difficult to reduce more stable sulfur compounds such as dibenzothiophene (DBT) and its alkysubstituted derivatives in particular 4,6-dimethydibenzothiophene (4,6-DMDBT) to an ultra low level using only conventional HDS process[7-8]due to the steric hindrance of their alky chains.
ODS has been considered to be one of the most promising methods for ultra-deep desulfurization of fuel oil due to its advantages compared with HDS, namely: (i) mild reaction conditions at low temperature (<100 ℃) and under atmospheric pressure; (ii) no need of using the expensive hydrogen; and (iii) higher reactivity for converting stable sulfur compounds.
In our previous study[9], MoO3/Al2O3catalyst was found to show high activity for ODS of DBT. The mechanism of MoO3/Al2O3catalyst activity in ODS is inferred that the coordination of hydroperoxide to Mo—O is prompted by the polarization of Moδ+—Oδ-bond when MoO3is dispersed on Al2O3. Through the formation of a fivemembered ring, the electrophilicity of peroxy oxygen is prompted, and therefore a high oxidative activity can be obtained.
Molybdenum forms two simple binary oxides, namely, MoO3and MoO2. MoO2is less important in technological applications than MoO3, but it has been used as a catalyst for alkane isomerization[10]or oxidation[11]reactions. Molybdenum dioxide (MoO2) exhibits an unusual metallic electrical conductivity, which is not a common characteristic of metal oxides. This is attributed to its relatively high density of state observed in the valence band energy region. The existence of these free electrons is considered to enhance the catalytic activity of Mo4+ions in MoO2, unlike Mo6+ions in MoO3, where all the valence electrons of the metal are bonded to neighboring oxygen atoms[12-14]. Although MoO3has been widely used as the ODS catalyst, little research has been focused on MoO2catalyst for ODS reaction. Li, et al.[15]reported the molybdenum-dioxide-based oxidative desulfurization catalyst. The MoO2-based catalyst showed higher oxidative desulfurization activity than the conventional MoO3-based catalyst. Li-ang, et al.[16-17]reported a hydrothermal method for preparation of nano-sized MoO2powders. In this paper, novel hydrothermal methods using hydrazine hydrate as the reducing agent and oxalic acid as the complexing reagent for preparation of MoO2and supported MoO2catalysts are provided. The ODS activities on synthesized MoO2catalysts are investigated in a fixed-bed reactor using tertbutyl hydroperoxide (TBHP) as the oxidant.
2 Experimental
2.1 Materials
0.828 g of (NH4)6Mo7O24·4H2O and 1.182 g of H2C2O4·2H2O (n(Mo):n(H2C2O4·2H2O)=1:2) were added to 12 mL of deionized water. The solution was stirred at 70 ℃ for 3 h. After the solution was cooled down to room temperature, 9.4 mL of 80% N2H4·H2O (n(Mo):n(N2H4·H2O)=1:40) was added. The solution was transferred into a teflon-lined autoclave. The autoclave was sealed and heated to 180 ℃ for 12 hours. Then the autoclave was cooled down and the precipitate was separated by filtration, and then washed by deionized water and ethanol. The product was dried in a vacuum oven at 60 ℃ for 3 h, and crystallized at 400 ℃ in the argon atmosphere. The unsupported MoO2catalyst was obtained with its XRD pattern shown in Figure 1.

Figure 1 XRD pattern of synthesized MoO2catalyst
The SiO2-Al2O3supported MoO2catalyst was synthesized using the same hydrothermal method as described above. 2.4 g of SiO2-Al2O3support were added to the solution before the mixture was transferred into a teflon-lined autoclave. After the procedure the SiO2-Al2O3supported catalyst containing 20% of MoO2was obtained.
2.2 Catalytic performance test
For the unsupported MoO2catalyst, a mixture of 0.20 g of synthesized MoO2and 0.80 g of macroporous silica gel was ground, pressed and crushed, and then sieved to 40—60 mesh. For the supported MoO2catalyst, one gram of synthesized MoO2/SiO2-Al2O3was ground, pressed and crushed, and then sieved to 40—60 mesh.
A typical oxidative desulfurization process was carried out in a temperature-controlled stainless steel fixed-bed reactor (with an i. d. of 3 mm). 0.10 g of sieved catalyst was put into the reactor and then preheated to a specified temperature. Then the feed (500 μg/g of DBT in decalin) was introduced by a peristaltic pump at atmospheric pressure with a WHSV of 40 h-1. After the effluent has flowed out for one hour at each temperature, the reaction solution at that temperature was collected within half an hour and analyzed by a gas chromatograph (GC-FID).
2.3 Characterization methods
Powder X-ray diffraction (XRD) patterns were recorded on a Rigaku D MAX diffractometer using CuKα radiation (40 kV, 100 mA), with the scanning angle ranging from 10° to 80° (2θ) at a scanning rate of 12(°)/min. Scanning electron microscopy (SEM) images were collected by employing a Quanta 200 scanning electron microscope operated at 30 kV. The oxidation products were analyzed by a gas chromatograph, which was equipped with a SGE AC10 capillary column, 0.25 mm in diameter and 30 m in length. The products were identified by checking the retention time in comparison with standard materials.
3 Results and Discussion
3.1 Synthesis of unsupported MoO2catalysts
The ODS activity of unsupported MoO2catalysts synthesized at different hydrothermal temperatures is shown in Figure 2 (I). The results show that a highest ODS activity was obtained over the MoO2catalyst synthesized at a hydrothermal temperature of 180 ℃. All catalysts had the same XRD pattern of MoO2as shown in Figure 1. Figure 2 (II) shows corresponding SEM images of MoO2catalyst synthesized at different hydrothermal temperatures. MoO2synthesized at 180 ℃ demonstrated a most regularspheroidicity, which might be the reason leading to its highest ODS activity among the synthesized MoO2catalysts.
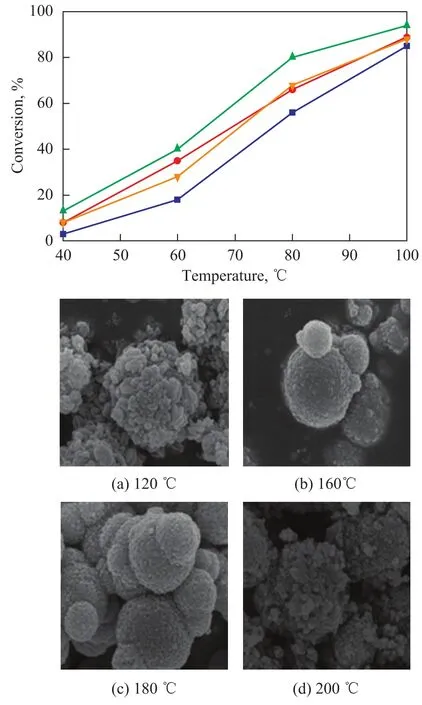
Figure 2 ODS activity (I) and SEM images (II) of MoO2catalyst synthesized at different hydrothermal temperatures
3.2 Synthesis of SiO2-Al2O3supported MoO2catalysts
Figure 3 shows the ODS activity (I) and XRD patterns (II) of the SiO2-Al2O3supported MoO2catalysts with different MoO2contents. The ODS activity increased with an increasing MoO2content. The XRD results show that the MoO2crystalline phase appeared when 15% of MoO2was supported on SiO2-Al2O3, indicating that an amorphous MoO2phase existed in the SiO2-Al2O3support when MoO2content was less than 15%. Figure 4 shows the ODS activity on 10% MoO2/SiO2-Al2O3crystallized at different temperatures. The results show that a highest ODS activity was obtained when the catalyst was crystallized at 400 ℃. Figure 5 shows the ODS activity of the SiO2-Al2O3supported 10% MoO2, which was synthesized with or without the addition of oxalic acid. The results show that after the addition of oxalic acid the ODS activity of the catalyst was enhanced. Oxalic acid could promote the reduction of Mo (VI) species during the hydrothermal process.
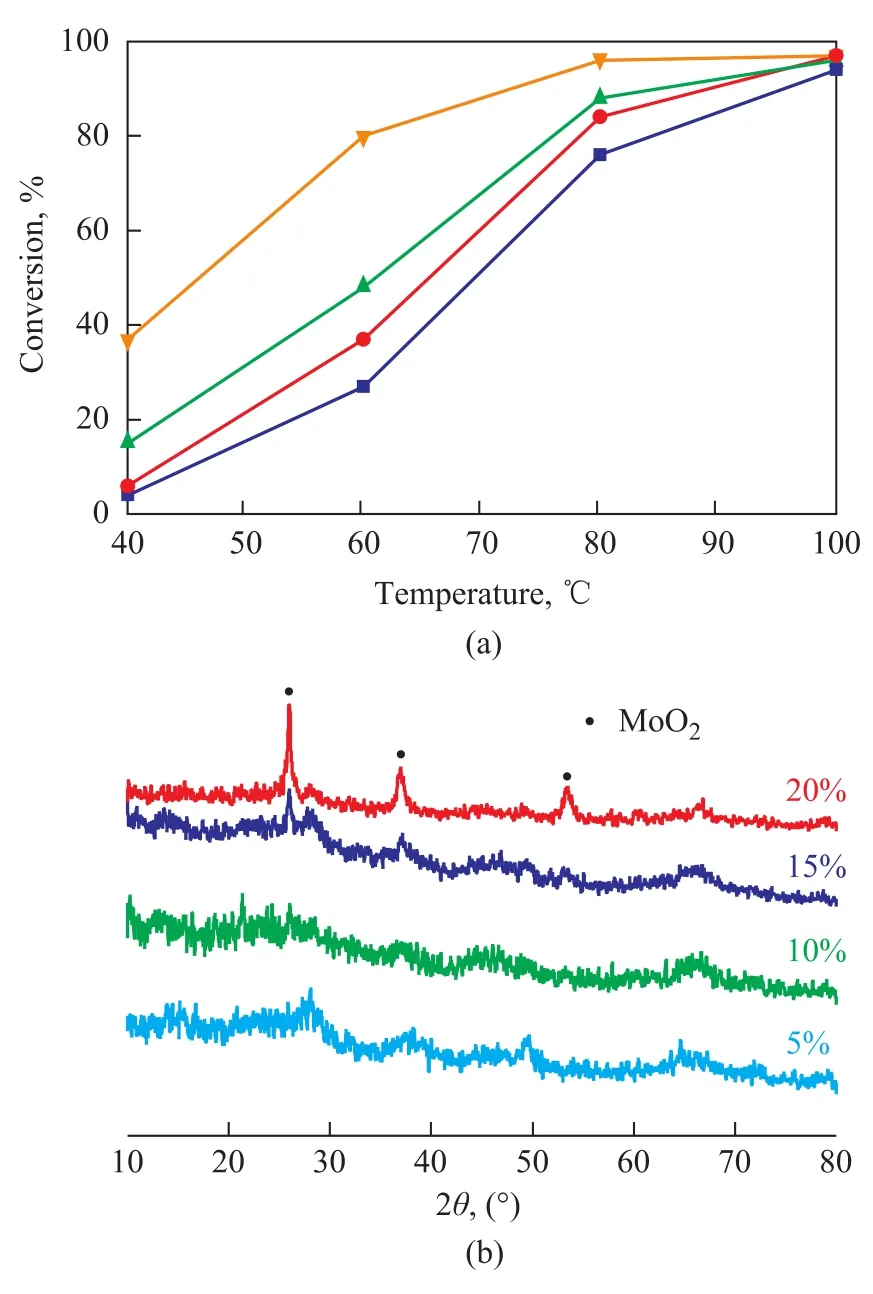
Figure 3 ODS activities (I) and XRD patterns (II) of SiO2-Al2O3supported MoO2catalysts with different MoO2content
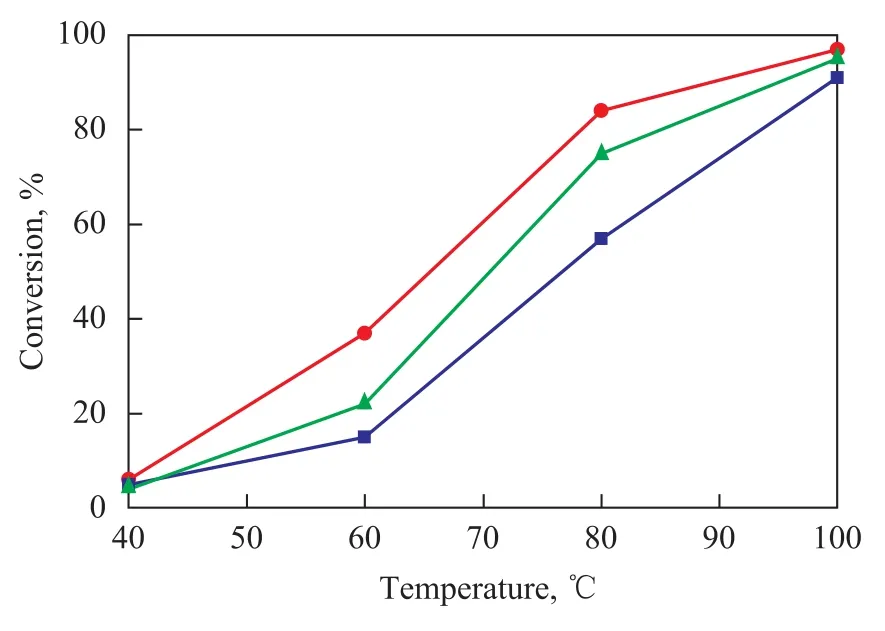
Figure 4 ODS activities on 10% MoO2/SiO2-Al2O3crystallized at different temperatures
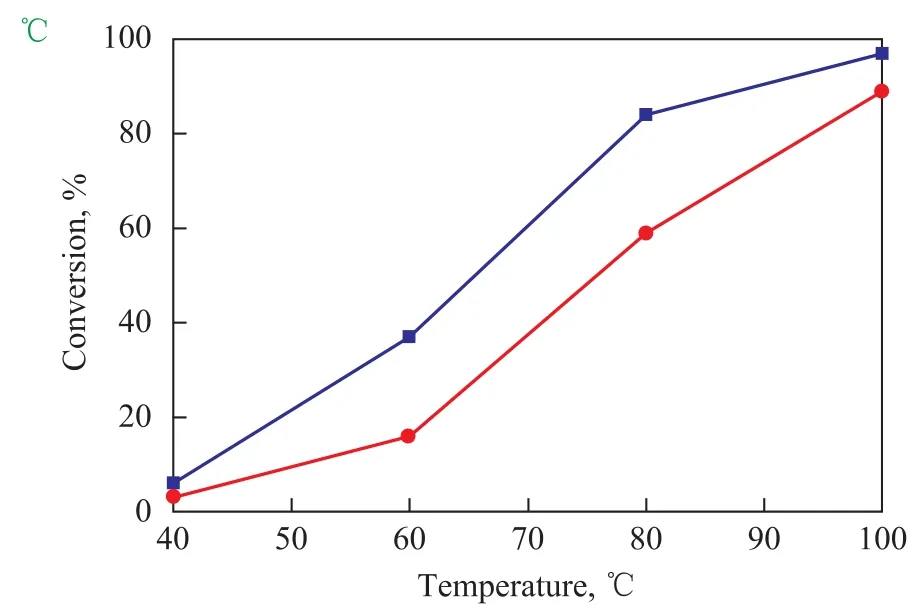
Figure 5 ODS activity of 10% MoO2/SiO2-Al2O3catalysts synthesized with or without the addition of oxalic acid
3.3 Reaction mechanism of MoO2catalyst
The chemical reactions involving oxalic acid are briefly proposed as follows:

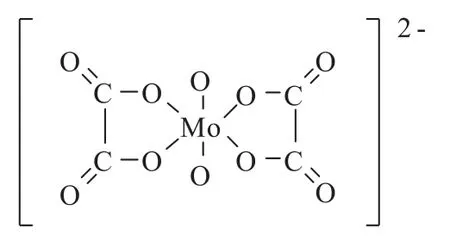
Scheme 1 Structure of[MoO2(C2O4)2]2-anion
4 Conclusions
Spherical MoO2nanoparticles and SiO2-Al2O3supported MoO2catalysts were successfully synthesized by hydrothermal reduction of Mo (VI) species. MoO2synthesized at a hydrothermal temperature of 180 ℃ demonstrated a most regular spheroidicity and possessed the highest ODS activity among the synthesized MoO2catalysts. The highest ODS activity for SiO2-Al2O3supported MoO2catalysts was obtained when the catalyst was crystallized at 400 ℃. The ODS activity increased with an increasing MoO2content in the SiO2-Al2O3supported MoO2catalysts. With the addition of oxalic acid the ODS activity of the SiO2-Al2O3supported MoO2catalyst was enhanced. Oxalic acid might help to promote the reduction of Mo (VI) species during the hydrothermal process.
Acknowledgments:This work was partly supported by the National Nature Science Foundation of China (NSFC grant No. 21303088), the Natural Science Foundation of Tianjin (14JCYBJC20000), and the MOE Innovation Team (IRT13022 and IRT13R30) of China.
[1] Wang Guangjian, Liu Yinghuan, Zeng Na, et al. Preparation of Ti-HMS molecular sieves and study on their catalytic oxidtive desulfurization preformance[J]. Petroleum Processing and Petrochemicals, 2013, 44(2): 18-21 (in Chinese)
[2] Zhang J, Wang A, Li X, et al. Oxidative desulfurization of dibenzothiophene and diesel over[Bmim]3PMo12O40[J]. J Catal, 2011, 279(2): 269-275
[3] Ishihara A, Wang D H, Dumeignil F, et al. Oxidative desulfurization and denitrogenation of a light gas oil using an oxidation/adsorption continuous flow process[J]. Appl Catal A, 2005, 279(1/2): 279-287
[4] Chica A, Corma A, Domine M E. Catalytic oxidative desulfurization (ODS) of diesel fuel on a continuous fixed-bed reactor[J]. J Catal, 2006, 224(2): 299-308
[5] Prassad V V D N, Jeong K, Chae H, et al. Oxidative desulfurization of 4,6-dimethyl dibenzothiophene and light cycle oil over supported molybdenum oxide catalysts[J]. Catal Commun, 2008, 9(10): 1966-1969
[6] Zhao Rongxiang, Li Xiuping. Hydrothermal synthesis of WO3nanorods and its catalytic performance of removing dibenzothiophene[J]. Petroleum Processing and Petrochemicals, 2012, 43(11): 47-50 (in Chinese)
[7] Rothlisberger A, Prins R. Intermediates in the hydrodesulfurization of 4,6-dimethyl-dibenzothiophene over Pd/gamma-Al2O3[J]. J Catal, 2005, 235(1): 229-240
[8] Oyama S T, Zhao H, Freund H J, et al. Unprecedented selectivity to the direct desulfurization (DDS) pathway in a highly active FeNi bimetallic phosphide catalyst[J]. J Catal, 2012, 285(1): 1-5
[9] Wang D H, Qian E W H, Amano H, et al. Oxidative desulfurization of fuel oil - Part I. Oxidation of dibenzothiophenes using tert-butyl hydroperoxide[J]. Appl Catal A, 2003, 253(1): 91-99
[10] Katrib A, Leflaive P, Hilarie L. Molybdenum based catalysts: I. MoO2as the active species in the reforming of hydrocarbons[J]. Catal Lett, 1996, 38 (1/2): 95-99
[11] Marin-Flores O G, Ha S. Activity and stability studies of MoO2catalyst for the partial oxidation of gasoline[J]. Applied Catalysis A: General, 2009, 352 (1/2): 124-132
[12] Al-Kandari H, Al-KhorafiF, Belatel H, et al. The bifunctional catalytic properties of a partially H2-reduced MoO3[J]. Catal Commun, 2004, 5 (5): 225-229
[13] Choi J G, Thompson L T. XPS study of as-prepared and reduced molybdenum oxides[J]. Applied Surface Science, 1996, 93 (2): 143-149
[14] Lalik E. Kinetic analysis of reduction of MoO3to MoO2[J]. Catal Today, 2011, 169 (1): 85-92
[15] Li X, Yang H B, Wang A J, et al. Molybdenum-dioxidebased oxidative desulfurization catalyst: China, CN 201310176425[P]. 2013-05-12 (in Chinese)
[16] Liang Y, Yi Z, Lei X, et al. A novel route to prepare nanosized MoO2powders in various dimensions[J]. Journal of Alloys and Compounds, 2006, 421 (1/2): 133-135
[17] Liang Y, Yang S, Yi Z, et al. Low temperature synthesis of a stable MoO2as suitable anode materials for lithium batteries[J]. Materials Science and Engineering B, 2005, 121 (1/2): 152-155
[18] Liu G, Zhang S W. Synthesis and structure of binuclear molybdenum oxalate[J]. Acta Chimica Sinica, 2000, 58 (7): 912-916 (in Chinese)
[19] Li Xiaolin, Liu Junfeng, Li Yadong. Low-temperature conversion synthesis of M(OH)2(M=Ni, Co, Fe) nanoflakes and nanorods[J]. Materials Chemistry and Physics, 2003, 80(1): 222-227
Received date: 2014-06-10; Accepted date: 2014-09-04.
Wang Danhong, Telephone: +86-22-23507730; E-mail: dhwang@nankai.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Comparative Studies on Low Noise Greases Operating under High Temperature Oxidation Conditions
- A Method for Crude Oil Selection and Blending Optimization Based on Improved Cuckoo Search Algorithm
- Experimental Research on Pore Structure and Gas Adsorption Characteristic of Deformed Coal
- Mathematical Model of Natural Gas Desulfurization Based on Membrane Absorption
- Ni2P-MoS2/γ-Al2O3Catalyst for Deep Hydrodesulfurization via the Hydrogenation Reaction Pathway
- Effects of Airflow Field on Droplets Diameter inside the Corrugated Packing of a Rotating Packed Bed
