Application of Modified Attapulgite Clay as the Adsorbent in Gasoline Desulfurization
2014-07-25XiXinguoZhangJilingJiangRuiyuXuQi
Xi Xinguo; Zhang Jiling; Jiang Ruiyu; Xu Qi
(1. School of Materials Engineering, Yancheng Institute of Technology, Yancheng 224051; 2. East-China Design Branch, China Petroleum Engineering Construction Company, Qingdao 266071; 3. Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province, Yancheng Institute of Technology, Yancheng 224051; 4. School of Chemical and Biological Engineering, Yancheng Institute of Technology, Yancheng 224051)
Application of Modified Attapulgite Clay as the Adsorbent in Gasoline Desulfurization
Xi Xinguo1; Zhang Jiling2; Jiang Ruiyu3; Xu Qi4
(1. School of Materials Engineering, Yancheng Institute of Technology, Yancheng 224051; 2. East-China Design Branch, China Petroleum Engineering Construction Company, Qingdao 266071; 3. Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province, Yancheng Institute of Technology, Yancheng 224051; 4. School of Chemical and Biological Engineering, Yancheng Institute of Technology, Yancheng 224051)
Attapulgite clay is a kind of special silicate mineral with high adsorption capacity thanks to its loose structure and porous surface. In this paper, the attapulgite clay was treated effectively with acid under microwave thermal activation and ultrasonic vibration, respectively, and characterized by XRD, N2adsorption, FT-IR and SEM. The desulfurization performance of the modified attapulgite clay was then evaluated by using simulated gasoline as the feed. The test results showed that the thiophene removal rate increased with an increasing dosage of hydrochloric acid during microwave modification of attapulgite clay. When the concentration of hydrochloric acid reached 15%, the increase of desulfurization rate became slower, and the desulfurization rate was about 69%.
attapulgite clay; modification; adsorption; desulfurization
1 Introduction
In recent years, as various environmental laws and regulations have been taking effect, low sulfur content or even sulfur-free demand has become an inevitable trend for the development of gasoline and diesel quality[1-3]. Currently, some American and European countries have conducted strict control over the sulfur content in gasoline and diesel fuels. The mass fraction of sulfur in gasoline is limited to less than 10 μg/g[4-6]. In China, 80% of the gasoline pool comes from fluid catalytic cracking (FCC) naphtha. The sulfur in FCC gasoline accounts for 90%—98% of the whole sulfur in gasoline pool. The sulfur mainly exists in the form of active thiols and inactive sulfides, thioethers, thiophene, benzothiophene and other derivatives. As the thiophene compounds are difficult to remove, they become the main study objects in the research of desulfurization processes. Currently, there are various desulfurization technologies, but they have their own advantages and disadvantages. Hydrodesulfurization (HDS) is an effective technique for removing sulfur from various petroleum distillates as it can greatly reduce the sulfur content[7-8]. However, as it requires special catalyst and high temperature and high pressure for achieving desulfurization, the equipment investment and operating cost are high. Although the researches on biological desulfurization have been conducted for more than 60 years[3,9], the biocatalytic desulfurization entered into the period of rapid development until Kilbane,et al.[10]in the late 1980s found the microbes capable of selectively breaking the C—S bonds. On the whole, this technology is still under development. Li Wangling,et al.[11]prepared the π complexation adsorbent Cu (I)-Y zeolite. In addition, they have studied the adsorption properties of Cu (I)-Y zeolite using DBT as the model compound. In 2003, Yang,et al.[12]applied the adsorbent which took the π complexation as the major interaction force to the in-depth desulfurization of oil. This technology has been attracting more attention finally. In general, the researches on adsorptive desulfurization mainly focused on the specific application, such as the screening of adsorbents, and optimization of adsorption conditions[13-20]. However, few researchers used the clay as the carrier and adopted this route to prepare the adsorptive desulfurization agent.
Attapulgite clay is a kind of silicate clay mineral and thiskind of crystal has a rod-shaped and fibrous structure. The loose structure and porous surface can provide excellent adsorption performance[2,17,21-23]. In this paper, the attapulgite clay was treated with acid under microwave thermal activation and ultrasonic vibration, respectively, the active component TiO2was then added as the adsorbent and the desulfurization performance was evaluated.
2 Experimental
2.1 Preparation of modi fied attapulgite clay
2.1.1 Microwave treatment
30 g of attapulgite clay was mixed with dilute hydrochloric acid with a concentration of 3%, 6%, 10%, 15% and 20%, respectively, and stirred for 2 hours. Then the mixture was placed in a chemical reactor with a microwave for activation under the programmed temperature. After that, deionized water was added for washing the clay until the discharged water showed a pH value of 6—7. The treated attapulgite clay was then filtered, and the filter cake was placed in a drying oven to be preheated at the temperature of 120 ℃ for drying.
2.1.2 Ultrasonic treatment
In this experiment, the acidified attapulgite clay was treated with ultrasonic wave instead of microwave as described in 2.1.1 under the same conditions.
2.2 Preparation of modi fied attapulgite clay adsorbent
The active component TiO2was added to the modi fied attapulgite clay at a certain proportion, and then the granulating machine was used to press them into particles. Afterwards these particles were placed in a muf fle furnace for heat activation.
2.3 Adsorbent characterization
All samples were characterized by XRD on a DANalytical X’Pert PRO MPD spectrometric diffractometer using Cu–Kα radiation, N2adsorption on a Micromeritics TriStar 3 000 instrument, FT-IR on a Micromeritics Nexu STM spectrometer by the KBr-disk method, and SEM on a Hitachi S-4800 instrument.
2.4 Performance evaluation of modified attapulgite clay adsorbent
The simulated FCC gasoline was made by dissolving thiophene into a mixture of octane and n-hexane. Firstly, 40 mL of the simulated FCC gasoline was placed into a three-necked flask. And then 5.0 g of adsorbent was added into the flask. Afterwards the flask with the mixture was placed in a water-bath equipped with a thermostat and stirred for 30 minutes for the separation of gasoline from the adsorbent. Finally, the sulfur content (determined by microcoulometry) in the simulated FCC gasoline after the absorption by the attapulgite clay adsorbent was measured to calculate the desulfurization rate.
3 Results and Discussion
3.1 Effect of microwave treatment on adsorptive desulfurization
Table 1 shows the pore structures data of the attapulgite clay samples treated with different concentrations of hydrochloric acid. It can be seen from Table 1 that in contrast with the original clay, the specific surface area, pore volume and average pore diameter of the treated attapulgite clay all increased obviously. This phenomenon occurred because some octahedrons in the attapulgite clay crystal were dissolved in the hydrochloric acid solution. The pore size and pore volume thus increased accordingly[2].
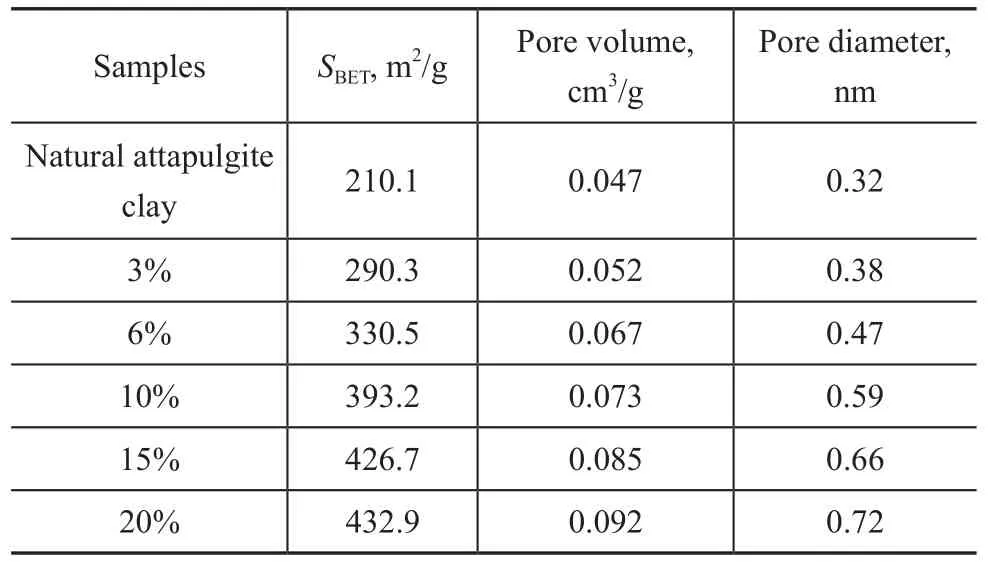
Table 1 Contrast of structural parameters of attapulgite clay samples modified by different concentrations of HCl under microwave heat activation
It can be seen from the XRD patterns presented in Figure 1 that the crystalline phase of the attapulgite clay changed little before and after the microwave activation and acid modification, and the XRD structural analysis showed that the peak of attapulgite clay was identified at about 8° and 20°, which meant that the structure of the attapulg-ite clay was not damaged by acid modification under microwave activation. After the acid modification under microwave activation and repeated washing, the impurity peaks of the attapulgite clay decreased substantially, which indicated that a large number of impurities in the attapulgite clay were removed. It could be seen clearly from the XRD patterns that the characteristic peak area of attapulgite increased after the treatment, which showed that the purity of attapulgite clay had improved[21,23].

Figure 1 XRD patterns of the microwave and acid modified attapulgite clay
Figure 2 shows the thiophene removal rate of the simulated FCC gasoline by the modified attapulgite clay. Experimental results showed that with the increase of the hydrochloric acid concentration, the desulfurization rate achieved by modified attapulgite clay increased. For example, when the concentration of hydrochloric acid was 15%, the desulfurization rate could reach 69%. However, the desulfurization rate changed little when the hydrochloric acid concentration continued to rise. The reason was that when the cations in the attapulgite clay were replaced by H+, the adsorption capacity of alkaline compound (such as thiophene) increased.

Figure 2 Change of thiophene removal rate with change in concentration of hydrochloric acid
Figure 3 is the XRD pattern of the acid modified attapulgite clay under microwave activation after the adsorptive desulfurization. It can be seen from Figure 3 that the sharpness of characteristic peak was improved. In addition, when 2θwas 40°, the characteristic peak of sulfides appeared more obviously.

Figure 3 XRD patterns of the microwave acid modified attapulgite clay after the adsorptive desulfurization of simulated gasoline
3.2 Influence of ultrasonic treatment on the adsorption effect
Figure 4 shows the FT-IR characteristic spectrum of the acid modified attapulgite clay under ultrasonic treatment. Obviously, the characteristic peak of adsorbed water was seen at 3 500 cm-1, and the characteristic peak of the attapulgite clay was identified at around 1 000 cm-1. It can beseen from Figure 4 that there was no significant difference in IR spectra of the original clay and the modified clay.
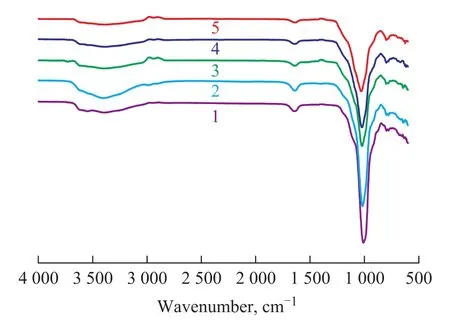
Figure 4 FT-IR spectra of the acid modified attapulgite clay samples under ultrasonic treatment
The XRD characteristic spectra of the ultrasonic treated acid modified attapulgite clay samples are shown in Figure 5. Obviously, the ultrasonic treated acid modification approach did not change the crystal structure of attapulgite clay. The characteristic peaks (2θ) still appeared at about 8° and 20°. After the modification treatment, the number of impurity peaks decreased significantly. The peak value of attapulgite clay increased, and most of the impurities were removed. The modified attapulgite clay had a relatively high purity.
It can be seen from SEM patterns of Figure 6 that, after the solid phase reaction between raw materials, many new crystals were formed. Most of these samples appeared in the form of particles with different sizes, and few of them clustered together.
Figure 7 shows the change of acid modified attapulgite clay after ultrasonic treatment in terms of the adsorptive desulfurization rate. It is not difficult to see that the desulfurization rate achieved by the modified attapulgite clay after ultrasonic treatment was higher at the time when the thiophene adsorption reached the equilibrium. When the hydrochloric acid concentration was 15%, the modification effect was the best under the ultrasonic treatment conditions. At this time, the desulfurization rate reached 69%.

Figure 5 XRD patterns of the acid modified attapulgite clay after ultrasonic treatment

Figure 6 SEM patterns of the acid modified attapulgite clay after ultrasonic treatment

Figure 7 Change in adsorptive desulfurization rate achieved by acid modified attapulgite clay after ultrasonic treatment

Figure 8 XRD patterns of acid modified and ultrasound treated attapulgite clay samples after adsorptive desulfurization
Figure 8 shows the XRD patterns of acid modified attapulgite clay under ultrasonic treatment after the adsorptive desulfurization. It could be seen from Figure 8that the characteristic peak of its structure increased in intensity after the acid modi fication. The results showed that the internal impurities had been removed. In addition, when 2θwas about 40o, the characteristic peak of sul fide appeared more obviously. The reason could be that the acid modi fied attapulgite clay under ultrasonic treatment may react with thiophene.
3.3 Adsorption mechanism analysis
The adsorption mechanism is one of main gut issues in the study on adsorptive desulfurization process. In order to better develop the adsorbents with high adsorption capacity and high selectivity, it is necessary to study the mechanism of adsorptive desulfurization by the modi fied attapulgite clay.
The adsorptive desulfurization mechanism was studied by analyzing the structural features of adsorbate and adsorbent. The modified attapulgite clay had larger surface area and better pore structure which was provided with richer surface groups. Therefore this kind of special surface structure and surface functional groups (e.g., π complexation, Lewis acid-base effect, and size selection effect) was favorable for the removal of sul fides from the fuel. The metal oxide components contained in the modified attapulgite clay could provide more capability for the modi fied attapulgite clay to remove the sul fides, such as the enhancement of polarization and formation of S-M coordination.
Upon studying the adsorptive desulfurization of model compounds, it had been found out that, with respect to the same kind of sulfides, the adsorption of thiophenic sulfur compounds on the modi fied attapulgite clay mainly depended on the π-π complexation. Then the molecular component of thiophene was distributed horizontally and could form a large delocalized conjugated π bond. Because of such structure, thiophene was easily adsorbed on the surface of the modi fied attapulgite clay with similar structure. In addition, the weak acting force (S-M coordination) existed between the metal oxides and sulfur atoms, which improved the desulfurization capacity of loaded modi fied attapulgite clay.
Therefore, to improve the adsorptive desulfurization capacity of modi fied attapulgite clay during desulfurization of gasoline, the desulfurization selectivity should be improved on the basis of the following two aspects.
1) Adsorbent: The polarity of adsorbent should be enhanced, and the bonding effect of S should be improved and the selectivity of S should be increased to avoid excessive reliance on the π-π effect.
2) Adsorbate: For the adsorptive desulfurization process, the test oil with low aromatic content and low sulfur content should be selected as the raw material to be used for adsorption by modified attapulgite clay, or the unsaturated hydrocarbons contained in the test oil must be reduced before its contact with modified attapulgite clay to avoid the impact of unsaturated hydrocarbons and sulfur compounds.
4 Conclusions
In this paper, the method of microwave thermal activation with acid treatment and the method of ultrasonic vibration with acid treatment were used for effective modification treatment of the attapulgite clay, and the following experimental results were observed:
(1) Under treatment by hydrochloric acid, the specific surface area, pore volume and pore size of the attapulgite clay increased;
(2) With the increase in hydrochloric acid concentration used for treating the clay, the thiophene removal rate of modified attapulgite clay increased. The increase in desulfurization rate became slower when the HCl concentration reached 15% or above;
(3) Both the microwave reaction and ultrasonic modification could effectively promote the adsorptive desulfurization efficiency of attapulgite clay used as the adsorbent. Meanwhile, the attapulgite clay could still retain its original good properties.
Acknowledgments:The project was supported financially by the National Natural Science Foundation of China (21306162), the National 973 Project of China (2010CB226903), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (14KJB430023), and the Union Innovation Funds-Prospective Joint Research Project of Jiangsu Province (BY2012152).
[1] Song C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel[J].Catalysis Today, 2003, 86(1): 211-263
[2] Boki K K, Tomioka H. Individual adsorption characteristics of beta-carotene and triolein on attapulgite and sepiolite[J]. Japanse Journal of Toxicology and Environmental Health, 1995, 41(6): 426-432
[3] Jia Y, Li G, Ning G. Efficient oxidative desulfurization (ODS) of model fuel with H2O2catalyzed by MoO3/gamma-Al2O3under mild and solvent free conditions[J]. Fuel Processing Technology, 2011, 92(1): 106-111
[4] Shahadat A, Tatarchuk B J. Adsorptive desulfurization of jet and diesel fuels using Ag/TiOx–Al2O3and Ag/TiOx–SiO2adsorbents[J]. Fuel, 2013,107: 465-473
[5] Pawelec B, Navarro R M, Campos-Martin J M, et al. Retracted article: Towards near zero-sulfur liquid fuels: A perspective review[J]. Catalysis Science & Technology, 2011, 1(1): 23-42.
[6] Stanislaus A, MarafiA, Rana M S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production[J]. Catalysis Today, 2010, 153(1): 1-68
[7] Zhu Y, Hua Z, Zhou X, et al. CTAB-templated mesoporous TS-1 zeolites as active catalysts in desulfurization process: the decreased hydrophobicity is more favourable in thiophene oxidation[J]. RSC Advances, 2013, 3: 4193-4198
[8] Skov E, England D. The ULSD oxidative desulfurisation option[J]. Hydrocarbon Engineering, 2007, 12(5): 33
[9] Campos-Martin J M, Capel-Sanchez M C, Perez-Presas P, et al. Oxidative processes of desulfurization of liquid fuels[J]. Journal of Chemical Technology and Biotechnology, 2010, 85(7): 879-890
[10] Kilbane J J. Desulfurization of coal: The microbial solution[J]. Trends in Biotechnology, 1989, 7(4): 97-101
[11] Li W, Xing J, Xiong X, et al. The biological regeneration of π complex desulfurization adsorbents[J]. Sci China Ser B Chem, 2005, 35(3): 258-264
[12] Yang R T, Hernandez-Maldonado A J, Yang F H. Desulfurization of transportation fuels with zeolites under ambient conditions[J]. Science, 2003, 301(5629): 79-81
[13] Araujo Melo D M, Ruiz J A C, Melo M A F, et al. Preparation and characterization of lanthanum palygorskite clays as acid catalysts[J]. Journal of Alloys and Compounds, 2002, 344(1): 352-355
[14] Xue M, Chitrakar R, Sakane K, et al. Preparation of cerium-loaded Y-zeolites for removal of organic sulfur compounds from hydrodesulfurizated gasoline and diesel oil[J]. Journal of Colloid and Interface Science, 2006, 298(2): 535-542
[15] Chen L, Guo S, Zhao D. Oxidative desulfurization of simulated gasoline over metal oxide-loaded molecular sieve [J]. Chinese Journal of Chemical Engineering, 2007, 15(4): 520-523 (in Chinese)
[16] Zhao D, Wang J, Zhou E. Oxidative desulfurization of diesel fuel using a Brønsted acid room temperature ionic liquid in the presence of H2O2[J]. Green Chemistry, 2007, 9(11): 1219-1222
[17] Zhang J, Xie S, Ho Y S. Removal of fluoride ions from aqueous solution using modified attapulgite as adsorbent[J]. Journal of Hazardous Materials, 2009, 165(1): 218-222
[18] Li B, Ma W, Liu J, et al. Synthesis of the well-ordered hexagonal mesoporous silicate incorporated with phosphotungstic acid through a novel method and its catalytic performance on the oxidative desulfurization reaction[J]. Catalysis Communications, 2011, 13(1): 101-105
[19] Zhang K, Liu Y, Tian Sh, et al. Preparation of bifunctional NiPb/ZnO-diatomite-ZSM-5 catalyst and its reactive adsorption desulfurization coupling aromatization performance in FCC gasoline upgrading process[J]. Fuel, 2013, 104: 201-207
[20] Dasgupta S, Gupta P, Nanoti A, et al. Adsorptive desulfurization of diesel by regenerable nickel based adsorbents[J]. Fuel, 2013, 108: 184-189
[21] Cao E, Bryant R, Williams D J. Electrochemical properties of Na–attapulgite[J]. Journal of Colloid and Interface Science, 1996, 179(1): 143-150
[22] Frost R L, Cash G A, Kloprogge J T. Rocky Mountain leather, sepiolite and attapulgite: An infrared emission spectroscopic study[J]. Vibrational Spectroscopy, 1998, 16(2): 173-184
[23] Huang J, Liu Y, Jin Q, et al. Adsorption studies of a water soluble dye, reactive red MF-3B, using sonication-surfactant-modified attapulgite clay[J]. Journal of Hazardous Materials, 2007, 143(1): 541-548
Received date: 2014-07-09;Accepted date: 2014-08-07.
Dr. Jiang Ruiyu, Telephone: +86-515-88298923; E-mail: jiangry@yeit.cn.
杂志排行
中国炼油与石油化工的其它文章
- Solvothermal Synthesis of V2O3Catalysts for Oxidative Desulfurization of Dibenzothiophene
- Identification and Characterization of Sulfur Compounds in Straight-Run Diesel Using Comprehensive Two-Dimensional GC Coupled with TOF MS
- Effects of Promoters on the Ignition Process over NiO/Al2O3Catalyst for Autothermal Reforming of Methane to Hydrogen
- Oligomerization of 1-Decene: Catalyzation by Immobilized AlCl3/γ-Al2O3Catalyst in Fixed-bed Reactor
- Study on Tribological Properties of CVT Fluid Containing Inert and Active Functional Elements
- Study on the Influence of Ni Modifying on Phase Transformation and Photocatalytic Activity of TiO2
