Study on Tribological Properties of CVT Fluid Containing Inert and Active Functional Elements
2014-07-25LiMaoshengDuQungui
Li Maosheng; Du Qungui
(1. School of Mechanical & Automotive Engineering, South China University of Technology, Guangzhou 510640;2. Guangzhou Mechanical Engineering Research Institute, Guangzhou 510700)
Study on Tribological Properties of CVT Fluid Containing Inert and Active Functional Elements
Li Maosheng1,2; Du Qungui1
(1. School of Mechanical & Automotive Engineering, South China University of Technology, Guangzhou 510640;2. Guangzhou Mechanical Engineering Research Institute, Guangzhou 510700)
The lubricating characteristics of CVTF (continuously variable transmission fluid) mixed with a multi-functional complex additive were studied. The said complex additive contained an organic borate ester and a new type of friction improver comprising phosphorus element and poly-methylmethacrylate (PMMA), and a viscosity index improver. The viscosity-pressure characteristics were evaluated by a high-pressure quartz viscometer, and the anti-wear property was investigated by a four-ball friction tester. The mechanism of lubrication by the CVTF was studied using X-ray photoelectron spectroscopy (XPS). The results showed that CVTF T10, which contained a multi-functional complex additive, exhibited excellent properties, featuring greater solidification pressure and pressure-viscosity coefficient, improved oil film strength, and low wear value. These attributes meet the special CVTF requirements for “high friction and low wear” that make it possible to provide both traction and lubrication. The lubricating mechanism was varied using different functional elements, such as inert and active elements. Sulfur and phosphorus are active extreme pressure elements that can react on the metal friction surface and produce an extreme pressure lubrication film. Boron is an inert functional element and does not react upon the metal surface; boron is only adsorbed onto the metal surface to act as a lubricant for adsorption film and fillers.
CVT fluid; pressure viscosity characteristics; high friction; low wear; lubrication
1 Introduction
Belt-drive continuously variable transmission (CVT) technology has developed rapidly in recent years thanks to its low fuel consumption, reasonable cost, simple mechanical structure and driving comfortableness[1-3]. It is extremely important to use a special functional fluid (continuously variable transmission fluid, CVTF) to provide lubrication and friction transmission during CVT operation. The commercial application of CVT technology was delayed for decades due to the lack of an adequate CVTF. The first-generation CVT used automatic transmission (AT) fluid instead of CVT fluid. However, the friction pair material and torque transmission of AT and CVT fluids are significantly different, and therefore, many problems occurred when AT fluid was used, such as an insufficient friction transmission efficiency, poor anti-jitter performance, and prevalent slipping. Since the late 1990s, the number of studies on the application and mechanism of CVTFs has been gradually increasing[4-7]. K. Narita (Idemitsu Kosan Co., Ltd.) and M. Priest (University of Leeds) studied the transmission efficiency and metal friction properties of CVTs[8]. In 2012, K. Narita studied the friction property of CVT lubricants and noted that additives with good performance could significantly improve the transmission efficiency and maximum torque and provide good boundary film and anti-shudder performance[9]. Song[10-11], Zhang[12]and Zhou[13-14]studied the transmission characteristics and technology of control over a metal V-belt-type CVT system, and Qin[15]introduced the parameters required by CVTF for use in a CVT system. Research on the elasto-hydrodynamic lubrication characteristics and application of a lubricating additive, such as ZDDP, has been ongoing for more than 30 years both in China and in other countries, although studies have only concentrated on the use of internal combustion engine oil[16-18]. There have only been a few studies on the viscosity-temperature pressure characteristics and tribo-logical characteristics of CVTFs.
It should be noted that the CVTF is a key factor in the transmission efficiency of a CVT, though there are other influential factors, such as the structure of mechanical system and control system. The CVTF performance significantly influences the transmission efficiency of a CVT. In addition to normal oil properties, such as viscosity, viscosity index, low-temperature performance, and shear resistance, there are several other properties that have significant effects, such as the drag force, the elastohydrodynamic lubrication oil film thickness, the pressureviscosity coefficient, and the lubricating characteristics. One study[19]reported the performance of several commercial CVTFs from Japan; the viscosity of the CVTFs ranged from 29.8 to 40.3 mm2/s at 40 ℃, and the viscosity index was between 155 and 227. In another study[20], the author tested and discussed the primary performance of several CVTFs.
In this paper, a new type of CVTF was developed; this new CVTF consists of base oil (synthetic oil) and special functional additives and is applied in selected original equipment manufacturer (OEM) automobiles. This paper describes the developed CVTF T10 and its performance with a particular focus on the pressure-viscosity and lubricating characteristics.
2 Experimental
To satisfy the requirements for reducing wear and maintaining an adequate traction force from a CVT, the CVTF should exhibit excellent lubricating properties and a good comprehensive performance, which requires a proper viscosity and viscosity index, an appropriate external friction and internal friction, and a good lubricating ability.
2.1 Base oil
To obtain good physical and chemical properties, anti-oxidation ability and low-temperature performance in particular, the selection of the base oil is extremely important. Table 1 presents the classification and physicochemical index of the base oil. The traditional base oil, which contains aromatic hydrocarbons, unsaturated hydrocarbons and other impurities, is unsuitable to be used as the base oil for CVTFs due to its poor anti-oxidation stability and poor low-temperature fluidity. It is recommended that the primary components of the base oil should include hydrogenated oil and polyalphaolefin (PAO). Table 2 presents the physicochemical properties of several base oils.
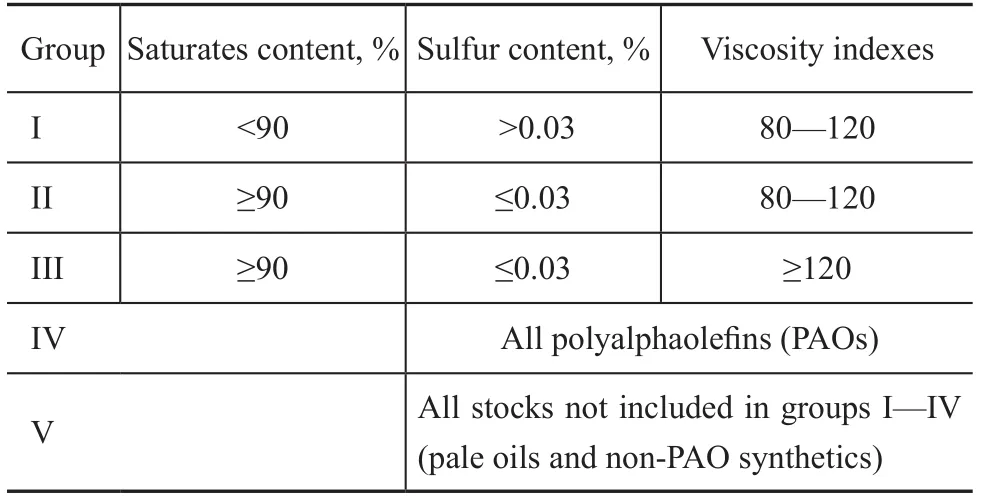
Table 1 API base oil categories (API publication 1509)

Table 2 Physicochemical properties of several base oils
2.2 Functional additives
2.2.1 Viscosity index improver
A viscosity index improver is a type of oil-soluble polymer that improves the viscosity temperature properties and viscosity index of the base oil. When the viscosity index improver is dissolved in a solvent (oil), different molecular structures are formed, such as reticulate, pectinate and stellate structures, which can greatly improve performance.
Poly(methyl acrylate) (PMA) is an acrylic polymer that is commonly used as an improver. By changing the degree of polymerization (molecular weight) and the molecular structure (the length and arrangement of the main chains and branched chains), the rheological property of PMA can be significantly changed to satisfy the special performance requirements for viscosity, viscosity index, lowtemperature properties and dispersion properties.
2.2.2 Lubricant additives
Lubricant additives are widely used in transmission fluids. Oiliness, anti-wear, and extreme pressure agents are generally included. Lubricant additives can improve the lubrication performance and reduce wear. Because CVTFs provide friction transmission, the selection of a friction improver is extremely important because it results in a suitable friction coefficient for CVTs. Synthetic esters exhibit good viscosity-temperature characteristics, excellent low-temperature fluidity, good anti-oxidation ability and good pressure-viscosity characteristics, which are typical requirements of CVTFs. In addition, borate ester, overbased calcium petroleum sulfonate and sulfurphosphorous type additives are used in the formula design of CVTF T10.
CVTF T10 is a special formula design, in which a mixture of hydrogenated oil and polyalphaolefin is used as the base oil, organic borate ester is used as a load-carrying additive containing sulfur and phosphorus, and polymethylmethacrylate is used as a viscosity index improver. The batch formula and physicochemical index of CVTF T10 are shown in Table 3. The physicochemical analysis results are presented in Table 4.
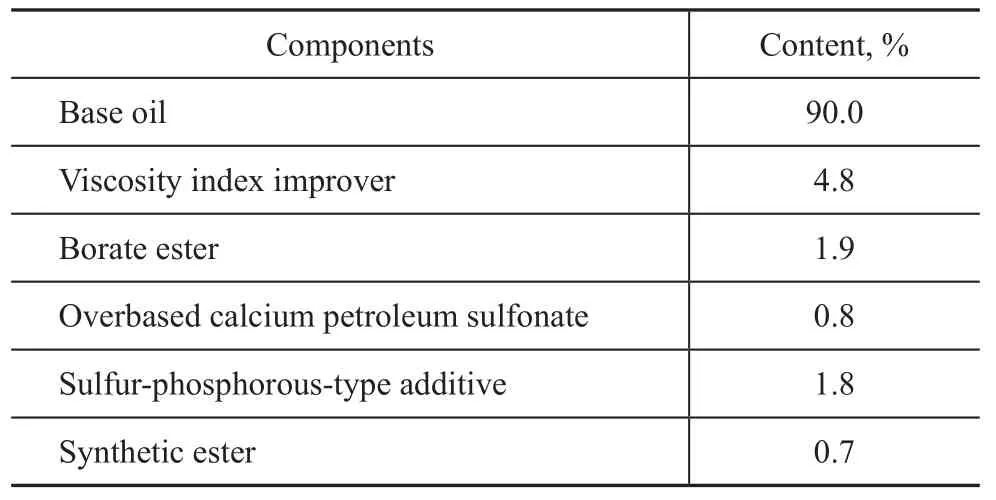
Table 3 Batch formula of CVTF T10
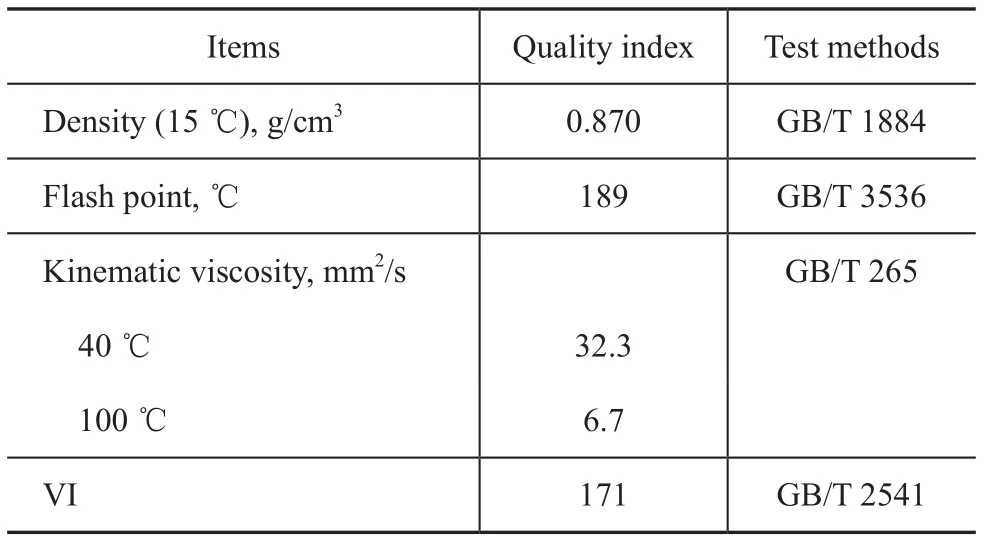
Table 4 Physicochemical analysis results of CVTF T10
The tests used to determine the viscosity-pressure and viscosity-temperature performance were performed using a high-pressure quartz viscosity measurement instrument manufactured by Bolenz and Schafer in Germany. The oil sample was compressed in the high-pressure cavity within the instrument at a constant temperature, and the viscosity was measured via the vibrations of the quartz vibrator in high-pressure oil at temperatures of 20 ℃, 40 ℃, and 60 ℃, respectively. The testing pressures ranged from atmospheric pressure to the oil-curing pressure. The samples were measured three times at each temperature, and the average value was reported.
The friction lubricating performance was investigated by a MS-10A four-ball tester manufactured by the Xiamen Tankey Automation Co., Ltd. The steel balls, which had a diameter of 12.7 mm, were made from the GCr15 steel. The oil film strength and sintering load were measured according to the national standard GB/T 3142. However, the wear scar diameter and average friction coefficient were also measured based on the national standard. The elemental analysis of the worn steel ball surfaces was conducted using an X-ray photoelectron spectrometer.
3 Results and Discussion
3.1 Pressure-viscosity characteristics
The pressure-viscosity (p-v) relationship of the test fluids at 20 ℃, 40 ℃, and 60 ℃ are shown in Figure 1 (a), (b), and (c), respectively. The pressure-viscosity characteristics of the oil can influence the elastohydrodynamic film thickness, thereby affecting the transfer capacity and oil film bearing capacity[21]. The results indicate that CVTF T10 has a higher solidification pressure and more stablep-vcoefficient than that of the base oil as the temperature changes, which increases the friction transmission capacity and oil film bearing capacity.
3.2 Lubrication characteristics
Comparison of the four-ball wear test results between the base oil and CVTF T10 are shown in Table 5. Figure 2 shows the wear scar diameters of the base oil and CVTF T10.

Figure 1 Pressure-viscosity relationship of the test fluids at different temperatures

Table 5 Comparison of the four-ball wear test results

Figure 2 Wear scar diameters of the base oil and CVTF T10

Figure 3 Trends of the friction coefficient
4 Mechanism of High Friction and Low Wear
To investigate the mechanism of high friction and low wear in the developed CVTF, the elemental types and chemical states of the worn surfaces on the steel ball were examined by X-ray photoelectron spectroscopy. The test was conducted according to the national standard method GB/T 19500. The pressure in the analysis room was 2.0×10-7Pa; the X-ray source was equipped with a monochromator Al-Kα (1 486.6 eV) at a power of 150 W. The results are shown in Figure 4 and Figure 5.

Figure 4 XPS spectra of the steel surface and worn steel surface

Figure 5 Typical element XPS spectra of the worn steel surface
Judging from the binding energy of the P element at 132 eV and the binding energy of 713 eV in the Fe2Pspectra, it can be concluded that FePO4is present, which indicatesthat the P element has reacted on the metal surface. In the case of the S element, its peak corresponding to Fex(SO4)yprimarily appeared at 169 eV, which was in good agreement with the characteristic peak in the Fe2Pspectra. The binding energy of 713 eV in the Fe2Pspectra can be treated as an overlapping peak from FePO4or Fex(SO4)y. The absence of a B peak in the spectra suggests that the B element was still in an organic state and did not react upon the metal surface. This absence also suggests the presence of a strong adsorption film of borate esters during the friction process[22]. In the literature[23], details were analyzed, and the conclusion was consistent with the results in this paper. These products in the worn steel surface formed a boundary lubricating film, which effectively improved the tribological properties of the oil.
5 Conclusions
CVTF T10 has a greater solidification pressure and more stable pressure-viscosity coefficient with the changing temperature than that of the base oil; thus, CVTF T10 shows an improved friction transmission capacity and oil film bearing capacity.
CVTF T10 exhibits good oil film strength and low wear along with a high friction coefficient. CVTF T10 provides a good lubricating performance and appropriate friction during CVT operation.
The elements S and P, which are the active extreme pressure agents, reacted upon the metal friction surface during the friction process and formed an extreme pressure lubricating film on the surface of the friction pair, whereas the element B, an inert functional agent, did not react on the metal surface but did provide lubrication in the form of an adsorption film and fillers.
Acknowledgements:This work was financially supported by the China National Machinery Industry Corporation Science & Technology Development Fund (SINOMACH12 No.180).
[1] Feng Ying, Luo Yongge. CVT—Review of the development of CVT[J]. Journal of Hubei Automotive Industrial Institute, 1999, 13(4): 15-18 (in Chinese)
[2] Cheng Naishi. Automotive Metal Belt CVT: CVT Principle And Design[M]. Beijing: Mechanical Industry Press, 2008 (in Chinese)
习总书记精准扶贫内涵可以高度概括为“六个精准”和“五个一批”。六个精准,即“对象要精准、项目安排要精准、资金使用要精准、措施到位要精准、因村派人要精准、脱贫成效要精准”。五个一批,即“通过扶持生产和就业发展一批,通过易地搬迁安置一批,通过生态保护脱贫一批,通过教育扶贫脱贫一批,通过低保政策兜底一批”,广泛动员全社会力量共同参与扶贫[5],做到“扶真贫、真扶贫”。
[3] Sun Dezhi, Tan Zhenjiang. Analysis of metal belt CVT transmission efficiency[J]. Journal of Northeastern University: Natural Science, 2002, 23(1): 52-56 (in Chinese)
[4] Ishikawa T, Murakami Y, Yauchibara R, et al. The effect of belt-drive CVT fluid on the friction coefficient between metal components[J]. Evaluation, 1997, 2013: 5-13
[5] Nakazawa K, Mitsui H, Kakegawa K, et al. Performance of a CVT fluid for high torque transmitting belt-CVTs[R]. SAE Technical Paper 982675, 1998
[6] Narita K, Abe A, Deshimaru J, et al. Improvement of torque capacity of metal V-belt type CVT fluids[J]. SAE Technical-Paper 2003-01-15.
[7] Pennings B, Drogen M, Brandsma A, et al. Van doorne CVT fluid test—A test method on belt-pulley level to select fluids for push belt CVT application[J]. SAE Paper 2003-01-3253, 2003
[8] Narita K, Priest M. Metal-metal friction characteristics and the transmission efficiency of a metal V-belt-type continuously variable transmission[J]. Proceedings of the Institution of Mechanical Engineers, Part J: Journal of Engineering Tribology, 2007, 221(1): 11-26
[9] Narita K. Tribological properties of metal V-Belt type CVT lubricant[J]. SAE Peper 476028, 2012
[10] An Ying. Research on transmission characteristics and comprehensive control technology of metal V-belt type CVT[D]. Changchun: Jilin University, 2012 (in Chinese)
[11] Zhang Shupei. Study on controlling method of metal belt continuously variable transmission (CVT) in clamping force aiming slip rate[D]. Changchun: Jilin University, 2007 (in Chinese)
[12] Wang Cheng. Research on the strategy of ratio control of metal belt CVT[D]. Changchun: Jilin University, 2007 (in Chinese)
[13] Gao Shuai. Development of the electric-hydraulic system for continuously variable transmission and research on the key technology[D]. Changchun: Jilin University, 2012 (in Chinese)
[14] Liu Jingang. Study on key technology of electro-hydraulic control system for metal-belt continuously variable transmission[D]. Changchun: Jilin University, 2008 (in Chinese).
[15] Yang Yalian. Study on some key problems of metalbelt continuously variable transmission[D]. Chongqing:Chongqing University, 2002 (in Chinese)
[16] Martin J M, Grossiord C, Le Mogne T, et al. The twolayer structure of ZnDTP tribofilms: Part I: AES, XPS and XANES analyses[J]. Tribology International, 2001, 34(8): 523-530
[17] Yin Z, Kasrai M, Bancroft G M, et al. Application of soft X-ray absorption spectroscopy in chemical characterization of antiwear films generated by ZDDP. Part II: The effect of detergents and dispersants[J]. Wear, 1997, 202(2): 192-201
[18] Fujita H, Spikes H A. The formation of zinc dithiophosphate antiwear films[J]. Journal of Engineering Tribology, 2004, 218(4): 265-278
[19] Sato T. Trends of metal pushing V-belt CVT fluid[C]. CVT-HYBRID 2007 Yokohama, 20074558(2007): 95-98
[20] Li Maosheng, Du Qungui, Jia Jiixin. Current technology and development trends of automatic transmission fluids[J]. Lubrication Engineering, 2014(1): 101-106 (in Chinese)
[21] Yang Wenzhi, Yang Siquan. Determination of mechanical continuously variable transmission pressure viscosity coefficient[J]. Lubrication Engineering, 1996(3): 32-35 (in Chinese)
[22] Wang Yonggang, Li Jiusheng, Ren Tianhui. Tribological study of a novel borate ester containing dialkylthiophosphate group as multifunctional additive[J]. Industrial Lubrication and Tribology, 2009, 61(1): 33-39
[23] Li Maosheng, Du Qungui. Study of friction lubrication characteristics research for CVT fluids in field test[J]. Advanced Materials Research, 2014, 915-916: 183-188
Received date: 2014-05-28; Accepted date: 2014-08-14.
Prof. Li Maosheng, Telephone: +86-20-32385265; E-mail: 827341372@qq.com.
猜你喜欢
杂志排行
中国炼油与石油化工的其它文章
- Preparation of Tungsten Film and Its Tribological Properties under Boundary Lubrication Conditions
- Preparation of Spherical MgCl2/SiO2/THF-Supported Late-Transition Metal Catalysts for Ethylene Polymerization
- Spray Characteristics Study of Combined Trapezoid Spray Tray
- Viscoelastic Characteristics of Asphalt Binders at Softening Point Temperature
- Solvothermal Synthesis of V2O3Catalysts for Oxidative Desulfurization of Dibenzothiophene
- Synergetic Effect of Y Zeolite and ZSM-5 Zeolite Ratios on Cracking, Oligomerization and Hydrogen Transfer Reactions
