以聚苯胺表面包覆钛酸钡作为填料的环氧复合材料的微观结构与介电性能
2014-07-24陈秋婷梁先文于淑会孙蓉谢盛辉汪正平
陈秋婷梁先文于淑会孙 蓉谢盛辉汪正平
1(中科院深圳先进技术研究院 深圳 518055)2(深圳大学材料学院 深圳 518060)3(香港中文大学工程学院 香港 999077)
以聚苯胺表面包覆钛酸钡作为填料的环氧复合材料的微观结构与介电性能
陈秋婷1,2梁先文1于淑会1孙 蓉1谢盛辉2汪正平3
1(中科院深圳先进技术研究院 深圳 518055)2(深圳大学材料学院 深圳 518060)3(香港中文大学工程学院 香港 999077)
通过原位聚合的方法合成了表面包覆钛酸钡的聚苯胺复合纳米颗粒(BT@PANI),并将该复合纳米颗粒作为填料制备了具有特殊结构的 BT@PANI/EP 三相复合材料。实验发现由于导电聚苯胺增强了界面极化,因此随着 BT@PANI 中 PANI 质量的增加(即 BT 在复合材料中的质量分数减少),该复合材料的介电常数也随之增加。当PANI 的质量分数从 0% 增加至 26% 时,其介电常数也从 17 提高到了 53,并且当 BT@PANI 中 PANI 的质量分数达到 26% 时,该复合材料并没有出现明显的渗流效应,且其导电率保持在 1.64×10—6S/m 这一较低值。此外,当测量温度范围在 60℃ 到 100℃ 之间时,该复合材料的介电常数发生了明显的上升,这一现象可以说明随着温度的上升,导电聚苯胺、环氧分子链在 Tg 温度(90℃)下运动增强及钛酸钡在居里温度(120℃)下的相变共同产生了强烈的界面极化。
聚合物;钛酸钡;聚合物复合材料;介电性能
1 Introduction
Flexible polymer-matrix composites with high dielectric constant have drawn much attention due to their potential applications in many fields[1,2]such as embedded capacitors[3,4], actuators[5,6]gate dielectrics[7]andso on. Efforts have been madeto prepare ceramics/polymer composites composedof ferroelectric ceramic fillers including BaTiO[8-10],3BST[11], PZT[12]and PMN-PT[13]and polymer matrix. However, these composites possess low dielectric constant due to the low dielectric constant of polymer matrix. Dang et al.[9]reported that the dielectric constant of BaTiO3/polyimide composites containing 40 vol% BaTiO3was only 18. Dielectric constant of BaTiO3/epoxy composites with a high loading of 60 vol% BaTiO3was about 27[10].In order to obtain a dielectric constant larger than 20, the ceramic concentration has to be increasedto 50 vol% or higher, which however, will deteriorate the flexibility of ceramic/polymer composites and limit their applications[14]. On the other hand, some attempts have been focused on the conductor/ polymer composites with high dielectric constant and excellent flexibility. The concentration dependence of dielectric constant of the conductor/polymer composites is given by the following power law on the basis of percolation theory[15]∶
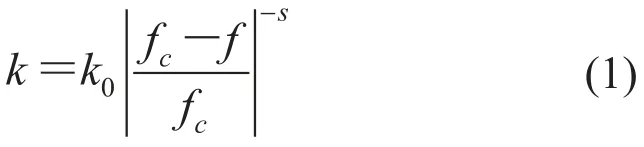
where k is the dielectric constant of the composite, k
0
is the dielectric constant of polymer matrix, f
c
is the percolation threshold, s is a critical exponent (0<s<1), and f is the volume concentration of conducting fillers. As is mentioned above, when the volume concentration of conducting fillers is close to a critical value, the dielectric constant of the composites increases a few orders of magnitude due to the formation of conductive networks induced by the conducting fillers. Rao and Wong et al.
[16]
reported that a high dielectric constant of 2000 was obtained in the Ag flake/epoxy composites. And a dielectric constant of about 110 was observed in the Al/epoxy composites
[17]
. Unfortunately, some disadvantages have been found in such percolative composites including (1) the large loss is caused by leakage current near percolation threshold; (2) the dielectric constant of the composites will drop remarkably with the increase of frequency; (3) the dielectric constant remains a low value unless the concentration of conducting fillers reaches percolation threshold; and (4) the dielectric properties of the composites are sensitive to the content of conducting fillers when the filler concentration is close to percolationthreshold
[15-18]
. In order to improve the dielectric constant of materials, some groups have introduced conducting fillers and ferroelectric ceramics simultaneously into polymer matrix to prepare the three-phase dielectric composites. For instance, Dang et al.
[14]
reported the three-phase Ni-BaTiO
3
/ PVDF composites. George et al.
[15]
reported the three-phase Silver-Ca[(Li
1/3
Nb
2/3
)
0.8
Ti
0.2
]O
3-δ
(CLNT)/ epoxy composite. However, these composites also show a typical percolation effect.
In this paper, polyaniline(PANI) was deposited on the BT surface to obtain hybrid nanoparticles (BT@ PANI) through in-situ oxidative polymerization. BT@PANI particles were incorporated into the epoxy matrix to prepare the special three-phase BT@PANI/epoxy composites. Effects of PANI deposition amount, on the dielectric properties of the composites were investigated. Results showed that the dielectric properties of the composite can be well-tailored through controlling the PANI content in the BT@PANI hybrid. The dependence of dielectric behavior on frequency and temperature was analyzed systematically.
2 Experimental
2.1 Materials
Barium titanate nanoparticles with an average diameter of 100 nm(BT) were obtained from Guoci Co., Ltd. Aniline (C6H7N, 99.5%), Ammonium persulfate (APS, (NH4)2S2O8, reagent grade, 98%), Sodium dodecyl benzene sulfonate (SDBS) (all from Shanghai Lingfeng Chemical Reagent Co., Ltd) and Hydrochloric acid (HCl) (Dongguan Dongjiang Chemical Reagent Co., Ltd) were used as monomer, initiator, emulsifier and dopant, respectively. The epoxy resin(trademark E51) (Sunstar Technology Co., Ltd) was employed as polymer matrix. Dicyandiamide and 2-Ethyl-4-methylimidazole (2E4MI) (both from Shanghai Lingfeng Chemical Co., Ltd) were used as curing agent and catalyst, respectively. Butanone, Ethanol (both from Tianjin Kaitong Chemical Co., Ltd) and Dimethyl formamide (DMF) (Shanghai Lingfeng Chemical Reagent Co., Ltd) were selected as solvent. Copper foil was purchased from Changchun Chemical Co., Ltd, Taiwan, China. All the chemicals were used as received without any treatment.
2.2 Preparation of BT@PANI Nanoparticles
The polyaniline deposited BT nanoparticles were prepared through in-situ oxidative polymerization. Firstly, 20.00 g BT particles with a mean size of 100 nm and 0.60 g SDBS were dispersed in deionized water in a 500 mL beaker with ultrasound treatment for 2 h to get a fine aqueous dispersion. Then mixture was transferred into a 500 mL threeneck flask, followed by the addition of aniline in 2 mol/L HCl solution. The monomer-BT-water dispersion was mixed by mechanical stirring at room temperature for 1 h to produce a homogenous mixture, and then was cooled down to 0℃ in an ice bath. After that, an aqueous solution of APS was dropwise added into the above solution (15-20 min intervals) to initiate a polymerization. The molar ratio of aniline ∶ HCl ∶ APS was adjusted to 1 ∶ 1 ∶ 2. The polymerization was carried out at 0℃ for 1 h, accompanied with mechanical stirring. A dark green colour of precipitate was formed at the end of reaction. The precipitate was collected by filtration and washed with distilled water and ethanol until SDBS was removed absolutely. At last, the obtained products were dried in a vacuum oven at 80℃ for 24h. According to above method, the PANI deposited BT (BT@PANI) nanoparticles with different weight fractions of PANI in the BT@PANI (5 wt%, 10 wt%, 17 wt%, 20 wt% and 26 wt% PANI) were synthesized, as were given in Table 1.
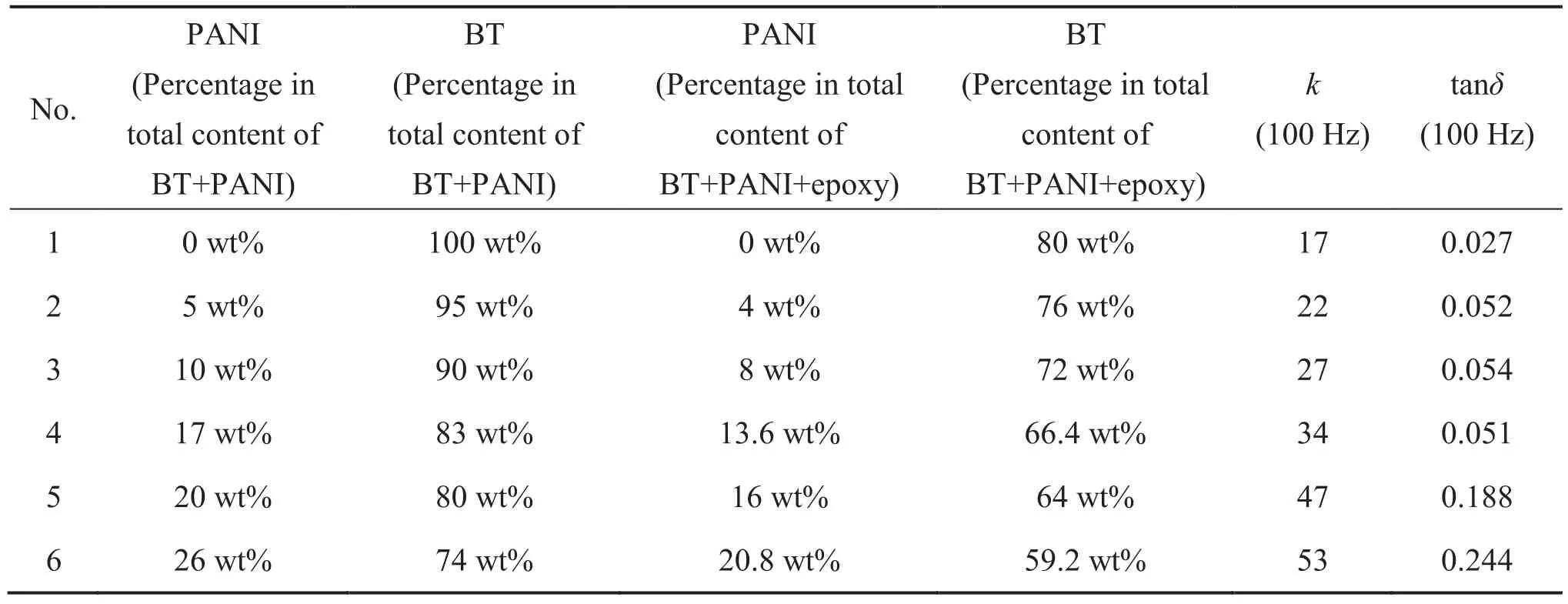
Table 1 The parameters of BT@PANI nanoparticles and BT@PANI/epoxy composites
2.3 Fabrication of the BT@PANI/Epoxy Composites
For preparation of the BT@PANI/epoxy composites, firstly, 2.50 g epoxy and 0.25 g dicyandiamide were dissolved in butanone and DMF, respectively. And 10.00 g BT@PANI nanoparticles were dispersed into butanone with ultrasound treatment. Then the above three solutions were mixed with stirring. Finally, uniform BT@PANI/epoxy composite slurry was obtained through adding 2E4MI into the above mixture, and was deposited on copper foil via a bar coating process. The composite films were heated at 120℃ for 1 h to evaporate the residual solvent and then cured at 150℃ for 2 hrs.
2.4 Characterization
The morphology of BT@PANI nanoparticles was examined via scanning electron microscopy (SEM) of Nova Nona 450. Thermogravimetry analyses (TGA) was performed by TA DSC Q20 in nitrogen with a heating rate of 10℃/min from 30℃ to 1000℃. The Fourier transform infrared (FT-IR) spectra were recorded from 4000 cm—1to 400 cm—1through Bruker Vertex 70. The conducting silver paste was painted on the surface of specimens as electrode for dielectric measurement with impedance analyzer Agilent 4294A at the frequencies of 102—107Hz. The effect of temperature on dielectric properties of BT@PANI/epoxy composites was characterized with impedance analyzer Agilent 4294A equipped with a THMS 600 temperature controlled stage (Linkam scientific instruments) and a T95-LinkPad temperature programmer (Linkam scientific instruments) in a temperature range from—50℃ to 100℃.
3 Results and Discussion
3.1 The Morphology of BT@PANI NanoparticlesFig. 1 presents SEM images of pure BT and BT@ PANI nanoparticles with different weight fractions of PANI. As is shown in Fig. 1 (a), the surface of pure BT nanoparticles is smooth. In contrast, the surface of BT@PANI hybrid nanoparticles is rough withsome PANI particles distributed on it, as is shown in Fig. 1(b)—(d). It is noted that the PANI particles and layer coexist on the surface of BT when PANI content is increased to 26 wt%, as is shown in Fig. 1(d).

Fig. 1. SEM images of (a) pure BT and BT@PANI nanoparticles with (b) 5 wt%, (c) 17 wt% and (d) 26 wt% PANI, respectively
3.2 FTIR and TGA Analysis of BT@PANI Nanoparticles
Fig. 2 shows FT-IR spectra of pure BT, PANI and BT@PANI nanoparticles with different amounts of PANI. As is shown in Fig. 2, the absorption peaks of pure BT nanoparticles at 578 cm—1and 450 cm—1correspond to Ti-O vibration and characteristic of BaTiO3, respectively. And the absorption peaks of PANI at 1584, 1504, 1303, 1240, 1154 and 820 cm—1are attributed to the main absorption bands of PANI. Among these peaks, the absorption bands at 1584, 1504 and 1303 cm—1are due to the stretching mode of N=quinine (Q)=N ring, N-benzene (B)-N ring and C-N (C aromatic-N) deformation, respectively[19-21]. The strong characteristic peak at 1154 cm—1originates from “electron-like band”and is considered to be a measure of electron delocalization, leading to the conductive feature of PANI, as is reported by Macdiarmid et al.[22-23]. The absorption band at 1240 cm—1(C-N+stretching vibration) indicates the doped form of PANI. The FT-IR curves of BT@PANI nanoparticles contain the peaks of both BT nanoparticles and PANI, and the characteristic peaks from PANI become much stronger with the increase of PANI content. However, the absorption peak at 578 cm—1from BT nanoparticles is suppressed as a result of the coverage of PANI on BT surface, as is illustrated in Fig. 2 (a) to (e), further confirming the successful synthesis of the BT@PANI nanoparticles.
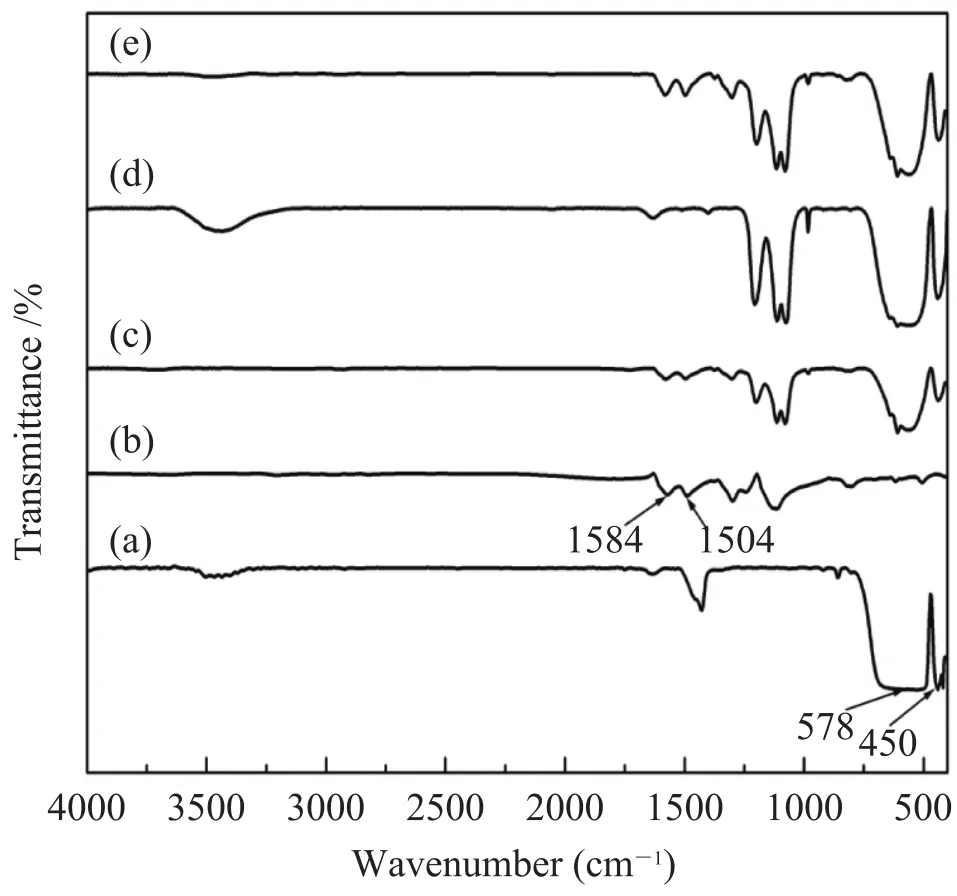
Fig. 2. FT-IR spectra of (a) pure BT, (b) PANI and BT@PANI nanoparticles with (c) 5 wt%, (d) 17 wt% and (e) 26 wt% PANI, respectively
Fig. 3 presents the thermogravimetric analysis of pure BT and BT@PANI nanoparticles with different PANI contents. The weight of pure BT nanoparticles almost maintains a constant value in the whole measured temperature range, as is shown in Fig. 3 (a). The weight loss of BT@PANI nanoparticles within100—250℃ may result from the loss of bound water molecules serving as secondary dopants in PANI, because the synthesis was carried out in an aqueous medium. The major weight loss starts at 700℃ for the BT@PANI nanoparticles[21]. According to the abovecurves, the final weight losses of BT@PANI nanoparticles are about 5 wt%, 17 wt% and 26 wt% through the whole curves, respectively.
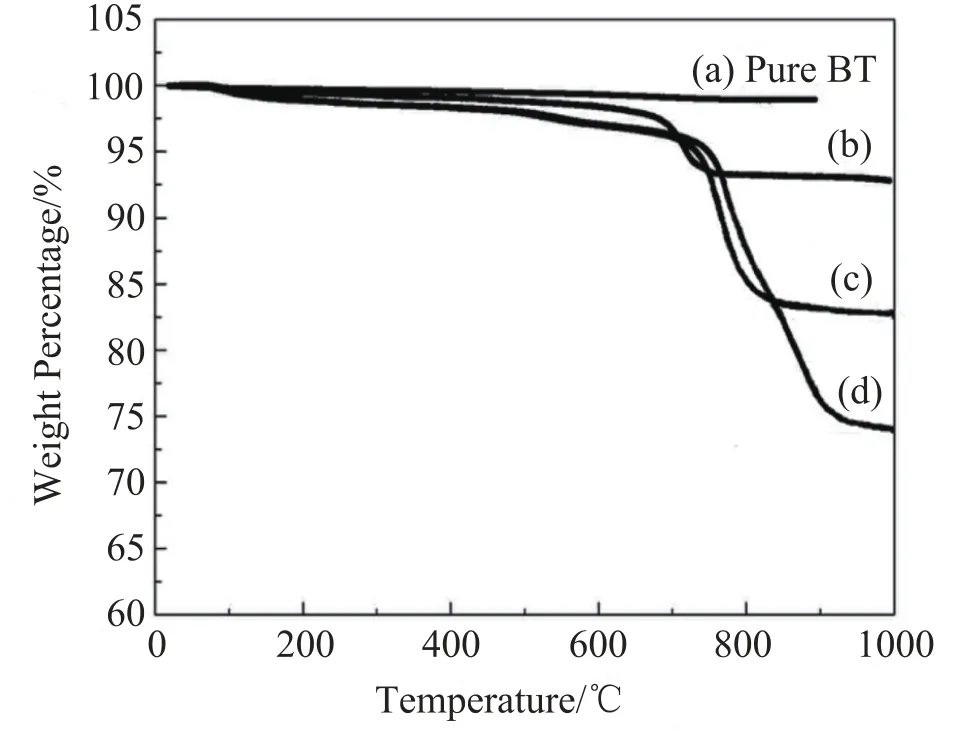
Fig. 3. The thermogravimetric analysis of (a) pure BT and BT@ PANI nanoparticles with (b)5 wt%, (c)17 wt% and (d)26 wt% PANI, respectively
3.3 The Dielectric Properties of the BT@PANI/ Epoxy Composites
Fig. 4 displays dependence of dielectric constant k and loss tanδ of the BT@PANI/epoxy composites on PANI weight fraction, based on the BT@PANI hybrid nanoparticles. As is shown in Fig. 4, the dielectric constant of the composite increases with the increase of PANI. When the content of PANI increases from 0 wt% to 26 wt%, the k rises from 17 to 53 at 100 Hz. The enhanced dielectric constant in the composite should be demonstrated by the interfacial polarization. According to the Maxwell-Wagner-Sillar (MWS) effect[24-27], the interfacial polarization takes place in heterogeneous system and the charge carriers are blocked at the internal surfaces or interfaces between matrix and fillers[28]. The BT@PANI/epoxy composites consist of PANI with conducting feature, and both of BT and epoxy with insulating nature. The protons (hydrogen ions) provided by the doping acid in the PANI on the surface of BT move along the PANI chains, but these charge movements will be blocked by the BT and epoxy matrix, leading to large amount of space charges accumulating at the interfaces of PANI-BT and PANI-epoxy, which results in a large interfacial polarization[29,30]. For the loss of the BT@PANI/ epoxy composites, when the PANI content ranges from 5 wt% to 17 wt%, the tanδ almost keeps a constant low value of 0.05, but it exceeds 0.1 as the content of PANI approaches 20 wt% or above, as is present in Fig. 4. The reason is that when the PANI content was low, a few discrete PANI particles were deposited on the surface of BT. The charge carriers are limited in the area of a single PANI particle, and they only need to move a short distance and reach the interfaces between PANI and other phasesto establish interfacial polarization under external field, which leads to a low energy consumption. In contrast, when the concentration of PANI is increased to higher values, a continuous PANI layeris formed on the surface of BT, as is shown in Fig. 1 (d). The external field has to drive these charge carriers to move a longer distance than that of a single PANI particle to accomplish interfacial polarization, which consumes more energy and results in increased loss. Meanwhile, they introduce a large amount of charge carriers (hydrogen ions) and interfaces, which results in the larger interfacial relaxation process.
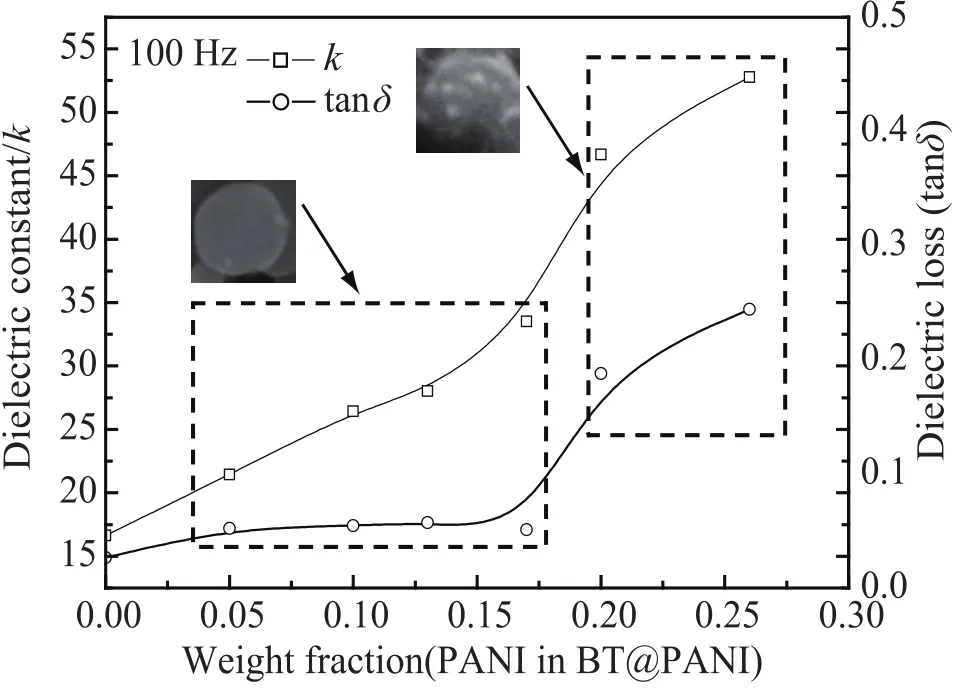
Fig. 4. Dependence of dielectric constant k and loss tanδ of the BT@PANI/epoxy composites on the weight fraction of PANI(based on the BT@PANI hybridnanoparticles) at 100 Hz
Fig. 5 presents variation of the dielectric constant k and loss tanδ of the BT@PANI/epoxy composites with the weight fraction of BT in the total content of (BT+PANI+epoxy) at 100 Hz and 100 kHz. Herein, we set the total weight fraction of BT@PANI hybrid nanoparticles in the BT@PANI/epoxy composites as a constant value of 80 wt% for all samples, and the calculated results are depicted in Table 1. Actually, when the content of PANI in the BT@PANI increases from 0 wt% to 26 wt%, the responding fractionof BT in the total content of (BT+PANI+epoxy) decreases from 80 wt% to 59.2 wt%. It is worth noting that the values of k and tanδ increase with the decrease of BT, as is shown in Fig. 5, which is assigned to the strong interfacial polarization caused by the conducting PANI and corresponds to the result given in Fig. 4. This phenomenon is in favor of the enhancement of dielectric constant and flexibility of materials. When the BTcontent is 66.4 wt% in the BT@PANI/ epoxy composite (17 wt% PANI in the BT@PANI), the k and tanδ of the composite are 34 and 0.051 at 100 Hz, respectively, which is superior to that of the BaTiO3/polyimide composites with 40 vol% BaTiO3(k~18)and the BaTiO3/epoxy composites with a high loading of 60 vol% (k~27)[9,10]. Additionally, the dielectric constant and loss of the BT@PANI/epoxy composites at 100 kHz are less than that of 100 Hz, originating from the weakening of interfacial polarization with the measured frequency increasing.
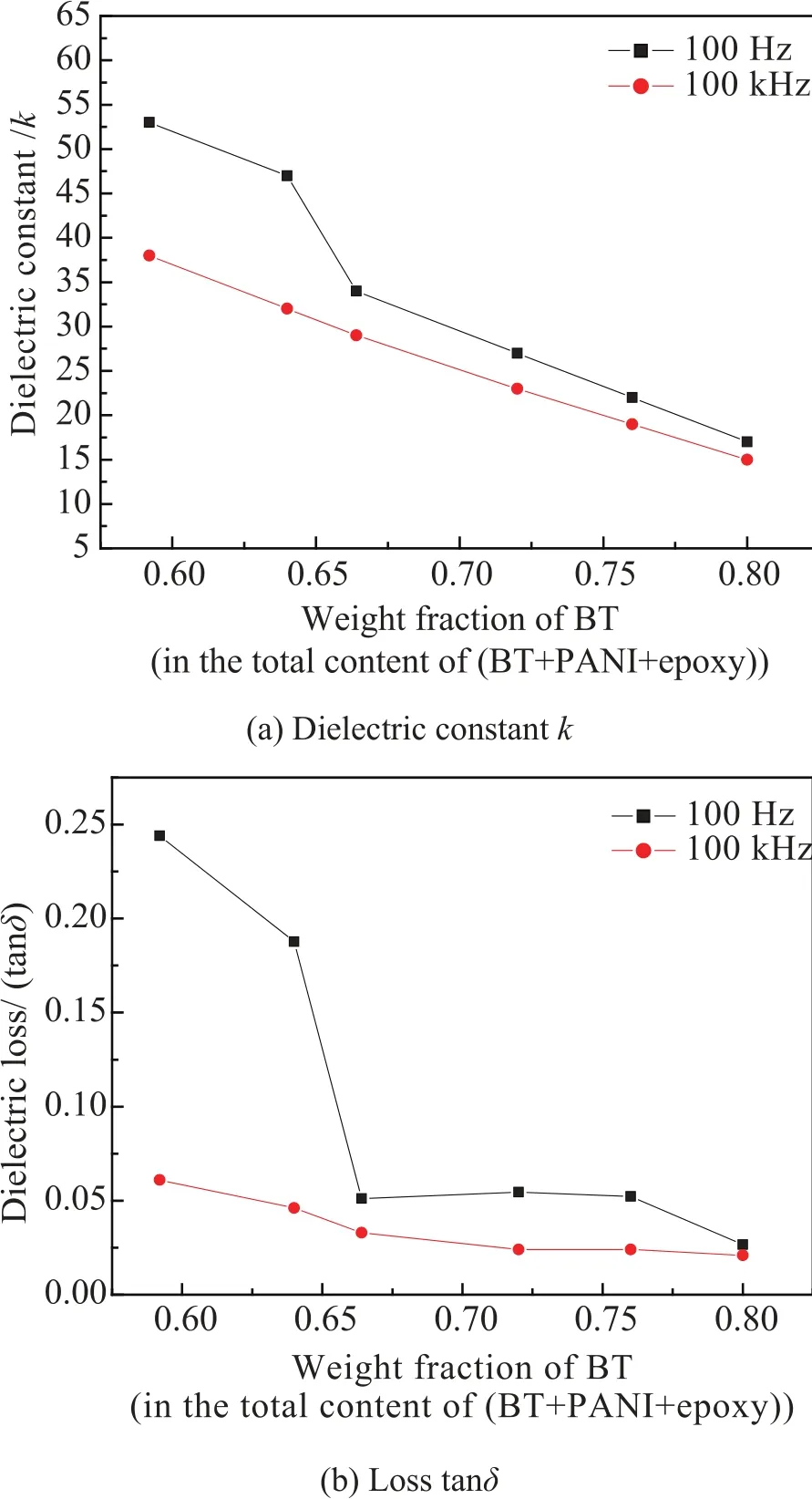
Fig. 5. Dielectric constant k and loss tanδ of the BT@PANI/ epoxy composites versus the weight fraction of BT in the total content of (BT+PANI+epoxy) at 100 Hz and 100 kHz
Fig. 6 presents dependence of dielectric constant k and loss tanδ of the BT@PANI/epoxy composites on the measured frequency. As is shown in Fig. 6 (a), the dielectric constant of the composite with BT@ PANI (17 wt%) or less shows weak dependenceon the frequency in the whole measured frequency range. However, when the contents of PANI exceed 17 wt%, the k of the composites shows obvious decrement with the frequency at low frequencies of 100 Hz to 10 kHz. For example, the k value of the composite with BT@PANI (26 wt%) decreases from 53 (100 Hz) to 40 (10 kHz). This phenomenon is demonstrated through the interfacial polarization caused by accumulated charges, which is sensitive to the frequency[31]. Interestingly, it is noticed that the k of the composites with higher PANI contents still maintains a higher value in the high frequency range of 10 kHz to 1 MHz due to the diffused electrical double layer in the internal interfaces between different phases. For instance, the k of the composite with BT@PANI(26 wt%) remains a relatively high value of 38, more than that of other composites at 100 kHz, as is depicted in Fig. 6(a). It can be explained that the PANI becomes charged positively owing to the protons (hydrogen ions) provided by the doping acid, as is mentioned above, and the epoxy matrix will respond by establishing an excess counter charge atmosphere screening the charge on the surface of PANI, which induces the polarization of epoxy matrix involving both electronic polarizability and the orientation of permanent dipoles, leading to the enhanced dielectric constant in the composites with higher PANI contents at high frequencies[32]. The loss of the BT@PANI/epoxy composites shows similar trend with that of the dielectric constant, as is displayed in Fig. 6 (b).
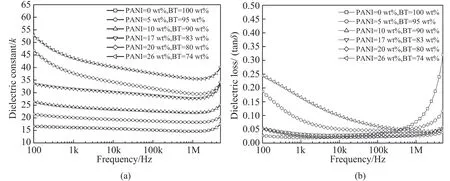
Fig. 6. Dielectric constant k and loss tanδ of the BT@PANI/epoxy composites as a function of frequency
Fig. 7 presents variation of dielectric constant and loss of the BT@PANI/epoxy composites with temperature. The k of all the composites shows a slight increase below 60℃, but increases fast in the temperature range of 60℃ to 100℃, as is shown in Fig. 7(a). The increasing temperature is in favor of charge movements along PANI chains and also promotes the motion of polymer PANI molecule chains, which enhances the interfacial polarization and improves dielectric constant. Additionally, the increasing dielectric constant is associated with the motion of epoxy molecule chains around the transition temperature (Tg) of pure epoxy. In this case, the Tg of pure epoxy as given by Fig. 7 is about 90℃. It is wellknown that the dipoles with enough mobility can enhance the dielectric constant. The polymer in the amorphous statedemonstrates a relaxation field associated with the glass transition, and the relaxation process is usually named as α and used to present the primary or glass-rubber relaxation. From the view of a dipolar point, the enhanced dielectric constant is induced by large-range motions “dipole-elastic process”corresponding to micro-Brownian segmental motion of chain near epoxy Tg[33]. On the other hand, the dielectric constant could also be influenced by the temperature near the curie point (around 120℃) of BT, which undergoes a phase transition, resulting in the increase of dielectric constant. Correspondingly, the loss of the BT@PANI/epoxy composites shows the similar variation, as is presented in Fig. 7 (b). The tanδ increases with the temperature, which possibly results from thermally activated conduction of BT with a pseudo-cubic structure.
Fig. 8 shows DSC curve of pure epoxy. We can see that the transition temperature (Tg) of pure epoxy is 90℃, and the cure reaction peak is 152℃.
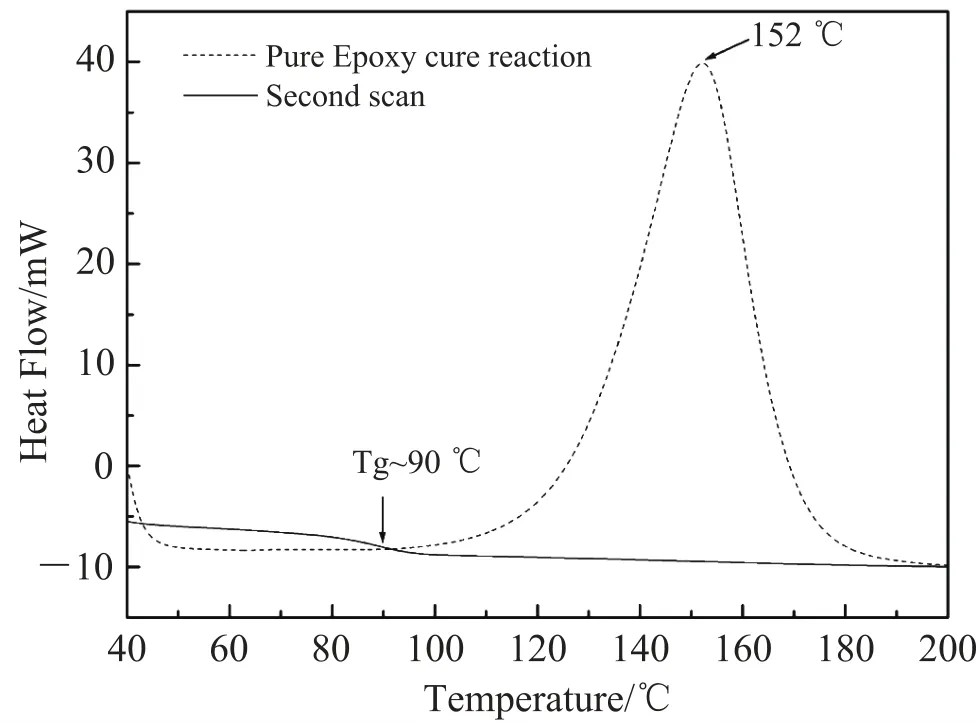
Fig. 8. Differential calorimeter analysis (DSC) of pure epoxy
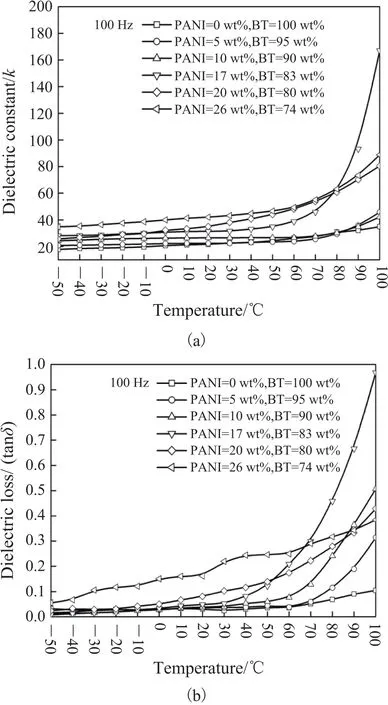
Fig. 7. Dependence of (a) dielectric constant k and (b) loss tanδ
3.4 The Conductivity of the BT@PANI/Epoxy Composites
Fig. 9 shows the effects of frequency and temperature on the conductivity of the BT@PANI/epoxy composites. It is noticed that the conductivity of the composites increases with the frequency. At 100 Hz, the conductivity of all specimens keeps low values of less than 10—5S/m, and it is 1.64×10—6S/m for 26 wt% of PANI in the BT@PANI, further indicating insulating nature of the composites, as is depicted in Fig. 9 (a). Besides, the decreasing conductivity of the composites with the temperature is observed in Fig. 9 (b). It is demonstrated that the volume of epoxy matrix will expand when the temperature is increased, especially near the Tg point, resulting in the increasing gap between BT@PANI nanoparticles.Therefore, the conducting path developed by PANI is destroyed, which leads to the reduced conductivity. In contrast, the conductivity of pure BT filled epoxy composite almost keeps a constant value at the whole temperature range, which indicates the evidence of this phenomenon.
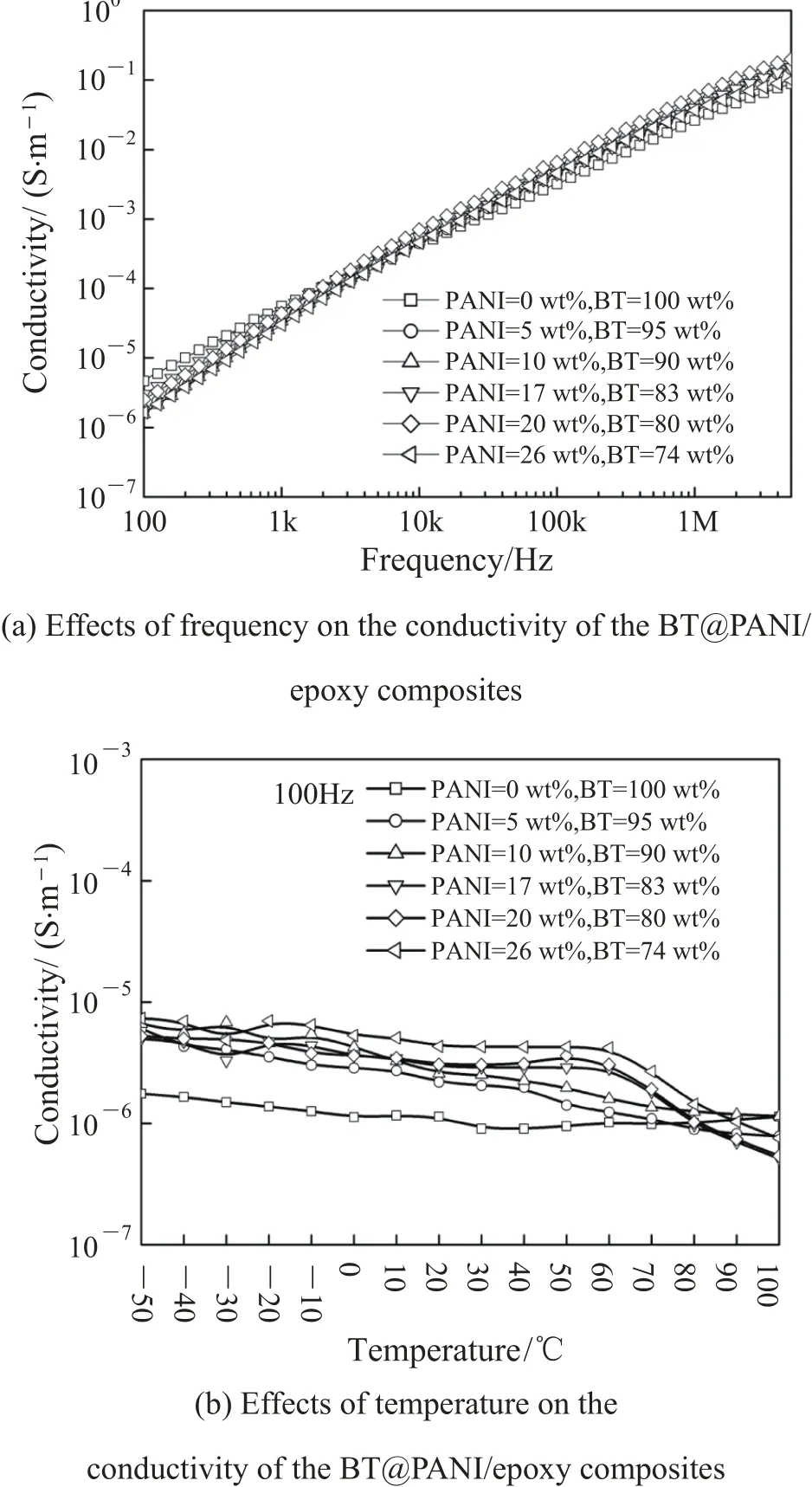
Fig. 9. Effects of frequency and temperature on the conductivity of the BT@PANI/epoxy composites at 100 Hz
4 Conclusion
PANI deposited BaTiO3hybrid nanoparticles (BT@PANI) were synthetized through in-situ polymerization successfully. As-prepared BT@PANI nanoparticles were introduced into epoxy matrix to fabricate the special three-phase BT@PANI/epoxy composites. The enhancement of dielectric constant of the composites with the increase of PANI content was due to the interfacial polarization caused by the conducting PANI. There was no percolation effect appearing in the composite system. Additionally, when the measured temperature ranged from 60℃to 100℃, the dielectric constant of the composites increased remarkably. This phenomenon was explained by the enhanced interfacial polarization induced by the increasing temperature, the strengthened motion of epoxy molecule chains near Tg (90℃) and the phase transition of BT near the curie point 120℃. The composite with a tunable dielectric property was potential for practical applications.
[1] Zhang QM, Li H, Poh M, et al. An all-organic composite actuator material with a high dielectric constant [J]. Nature, 2002, 419∶ 284-287.
[2] Li JY. Exchange coupling in P(VDF-TrFE) copolymer based all-organic composites with giant electrostriction [J]. Physical Review Letters, 2003, 90(2)∶ 217601.
[3] Panda M, Srinivas V, Thakur AK. Role of polymer matrix in large enhancement of dielectric constant in polymer-metal composites [J]. Applied Physics Letters, 2011, 99(4)∶ 042905. doi∶10.1063/1.3600345.
[4] Li M, Deng Y, Wang Y, et al. High dielectric properties in a three-phase polymer composite induced by a parallel structure [J]. Materials Chemistry and Physics, 2013,139 (2-3)∶ 865-870.
[5] MolbergM, Crespy D, Rupper P, et al. High breakdown field dielectric elastomer actuators using encapsulated polyaniline as high dielectric constant filler [J]. Advanced Functional Materials, 2010, 20(19)∶ 3280-3291.
[6] Huang C, Zhang Q. Fully functionalized highdielectric-constant nanophasepolymers with highelectromechanical response advanced functional materials [J]. Advanced Materials, 2005, 17(9)∶1153-1158.
[7] Maliakal A, Katz H, Cotts PM, et al. Inorganic oxide core, polymer shell nanocomposite as a high kgate dielectric for flexible electronics applications [J]. Journal of the American Chemical Society, 2005, 127(42)∶ 14655-14662.
[8] Zeng X, Yu S, Sun R, et al. Microstructure, thermal and dielectric properties of homogeneous bismaleimide-triazine/barium titanate nanocomposite films [J]. Materials Chemistry and Physics, 2011, 131(1-2)∶ 387-392.
[9] Dang Z, Yu Y, Xu H, et al. Study on microstructure and dielectric property of the BaTiO3/epoxy resin composites [J]. Composite Science and Technology, 2008, 68(1)∶ 171-177.
[10] Guo Z, Lee SE, Kim H, et al. Fabrication, characterization and microwave properties of polyurethane nanocomposites reinforced with iron oxide and barium titanate nanoparticles [J]. Acta Materialia, 2009, 57∶ 267-277.
[11] Hu T, Juuti J, Jantunen H, et al. Dielectric properties of BST/polymer composite [J]. Journal of the European Ceramic Society, 2007, 27(13-15)∶ 3997-4001.
[12] Ploss B, Ng W, Chan HL, et al. Poling study of PZT/P(VDF-TrFE) composites [J]. Composite Science and Technology, 2001, 61(7)∶ 957-962.
[13] Lam KH, Chan HLW. Piezoelectric and pyroelectric properties of 65PMN-35PT/P(VDF-TrFE) 0-3 composites [J]. Composites Science and Technology, 2005, 65(7-8)∶ 1107-1111.
[14] Dang Z, Shen Y, Nan C. Dielectric behavior of three-phase percolative Ni-BaTiO3/polyvinylidene fluoride composites [J]. Applied Physics Letters, 2002, 81(25)∶ 4814-4816.
[15] George S, Sebastian MT. Three-phase polymerceramic-metal composite for embedded capacitor applications [J]. Composites Science and Technology, 2009, 69(7-8)∶ 1298-1302.
[16] Rao Y, Wong CP. Ultra high dielectric constant epoxy silver composite for embedded capacitor application [C] // Proceedings of 52nd Electronic Components and Technology Conference, 2002∶ 920-923.
[17] Xu JW, Wong CP. Low-loss percolative dielectric composite [J]. Applied Physics Letters, 2005, 87(8)∶082907. doi∶ 10.1063/1.2032597.
[18] Baibarac M, Baltog I, Lefrant S, et al. Polyaniline and carbon nanotubes based composites containing whole units and fragments of nanotubes [J]. Chemistry Materials, 2003, 15(21)∶ 4149-4156.
[19] Markovic MG, Matisons JG, Cervini R, et al. Synthesis of new polyaniline/nanotube composites using ultrasonically initiated emulsion polymerization [J]. Chemistry Materials, 2006, 18(26)∶ 6258-6265.
[20] Zhu Y, Fu Y, Natsuki T, et al. Fabrication and microwave absorption properties of BaTiO3nanotube/polyaniline hybrid nanomaterials [J]. Polymer Composites, 2013, 34(2)∶ 265-273.
[21] Zengin H, Zhou W, Jin J, et al. Carbon nanotube doped polyaniline [J]. Advance Materials, 2002, 14(20)∶ 1480-1483.
[22] Boyer MI, Quillard S, Rebourt E, et al. Vibrational analysis of polyaniline∶ a model compound approach [J]. The Journal of Physical Chemistry B, 1998, 102(38)∶ 7382-7392.
[23] Ramajo L, Castro MS, Reboredo MM. Dielectric response of Ag/BaTiO3/epoxy nanocomposites [J]. Journal of Materials Science, 2010, 45∶ 106-111.
[24] Dang Z, Wang L, Yin Y, et al. Giant dielectric permittivities in functionalized carbon-nanotube/ electroactive-polymer nanocomposites [J]. Advance Materials, 2007, 19(6)∶ 852-857.
[25] Huang C, Zhang QM, Su J. High-dielectric-constant all-polymer percolative composites [J]. Applied Physics Letters, 2003, 82(20)∶ 3502-3504.
[26] Liang X, Yu S, Sun R, et al. Microstructure and dielectric behavior of the three-phase Ag@SiO2/ BaTiO3/PVDF composites with high permittivity [J]. Journal of Materials Research, 2012, 27(7)∶991-998.
[27] Schönhals A, Goering H, Costa FR, et al. Dielectric properties of nanocomposites based on polyethylene and layered double hydroxide [J]. Macromolecules, 2009, 42(12)∶ 4165-4174.
[28] Lu J, Moon K, Kim B, et al. High dielectric constant polyaniline/epoxy composites via in situ polymerization for embedded capacitor applications [J]. Polymer, 2007, 48(6)∶ 1510-1516.
[29] Fang F, Kim JH, Choi HJ, et al. Organic/inorganic hybrid of polyaniline/BaTiO3composites and their electrorheological and dielectric characteristics [J]. Journal of Applied Polymer Science, 2007, 105(4)∶1853-1860.
[30] Yang W, Yu S, Sun R, et al. Nano- and micro-size effect of CCTO fillers on the dielectric behavior of CCTO/PVDF composites [J]. Acta Materialia, 2011, 59 (14)∶ 5593-5602.
[31] Lewis TJ. Interfaces are the dominant feature of dielectrics at the nanometric level [J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2004, 11(5)∶ 739-753.
[32] Ramajo L, Reboredo M, Castro M. Dielectric response and relaxation phenomena in composites of epoxy resin with BaTiO3particles [J]. Composites Part A, 2005, 36(9)∶ 1267-1274.
[33] Xu HP, Dang ZM, Bing NC, et al. Temperature dependence of electric and dielectric behaviors of Ni/polyvinylidene fluoride composites [J]. Journal of Applied Physics, 2010, 107(3)∶ 034105. doi∶10.1063/1.3289731.
Microstructure and Dielectric Properties of Epoxy Composites with Polyaniline-Deposited BaTiO3as Fillers
CHEN Qiuting1,2LIANG Xianwen1YU Shuhui1SUN Rong1XIE Shenghui2WONG Chingping31
( Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China )2( Department of Materials, Shenzhen University, Shenzhen 518060, China )3( Faculty of Engineering, The Chinese University of Hong Kong, Hong Kong 999077, China )
Polyaniline deposited BaTiO3hybrid nanoparticles (BT@PANI) were synthesized via in-situ polymerization process and then used as fillers to fabricate the BT@PANI/epoxy composites. The dielectric constant of the composites increased with the increment of PANI in BT@PANI(while the fraction of BT decreased in the composite), which wasattributed to the enhanced interfacial polarization induced by the conducting PANI. The value of k increased from 17 for 0 wt% PANI to 53 for 26 wt% PANI. No typical percolation effect was observed in the composite and when the content of PANI in the BT@PANIreached 26 wt%, the conductivity maintained a low value of 1.64×10—6S/m. Besides, the dielectric constant of the composites jumped remarkably inthe measured temperature range from 60℃ to 100℃ as anevidenceof the stronger interfacial polarization generated by conducting PANI with temperature increasing, theenhanced motion of epoxy molecule chains around Tg (90℃)and the phase transition of BT near the curie point 120℃.
polymers; BaTiO3; polymeric composites; dielectricproperties
2014-09-22
TM 21
A
Foundation:National Natural Science Foundation of China(51377157);Guangdong Innovative Research Team Program(2011D052);Shenzhen Peacock Pragram(KYPT20121228160843692);ShenzhenElectronic Packaging Materials (深发改【2012】372 号)
Author:Chen Qiuting, Master’s degree candidate. Her research interest are preperation and characterization of nano composite dielectric materials; Liang Xianwen, Research Assistant. His research interests are manufacturing and application of nano materials; Yu Shuhui (corresponding author), Ph.D, Associate Research Professor. Her research interests are nano functional materials and embedded components, E-mail:sh.yu@siat.ac.cn; Sun Rong (corresponding author), Ph. D, Research Professor. Her research interests are electronic packaging materials, E-mail:rong.sun@siat.ac.cn; Xie Shenghui, Associate Professor. His research interest is composite material; Wong Chingping, Ph. D, Professor.His research interests are polymer nanocomposites and high-density electric packaging materials.
