室温超声键合中 Cu/Sn 固液界面间的超声声化学效应
2014-07-24李卓霖李明雨
李卓霖 李明雨 肖 勇
(哈尔滨工业大学深圳研究生院深圳市新材料技术重点实验室 深圳 518055)
室温超声键合中 Cu/Sn 固液界面间的超声声化学效应
李卓霖 李明雨 肖 勇
(哈尔滨工业大学深圳研究生院深圳市新材料技术重点实验室 深圳 518055)
文章通过对 Cu/Sn/Cu 互连结构短时间施加超声波实现了 Cu/Cu6Sn5/Cu 或Cu/Cu3Sn/Cu 高性能的焊接接头。由于超声空化效应的作用 Cu/Sn 固液界面产生了快速元素扩散从而加速了金属间化合物的形成。研究发现,空化气泡在固液界面附近坍塌会对固相铜界面造成严重的空化腐蚀,并且在液相锡中会形成铜过饱和区导致金属间化合物的快速形成。值得说明的是,这种室温超声键合所形成的金属间化合物接头具有良好的机械可靠性并且可实现超高温服役的优势。
超声化学;空化作用;扩散;超声键合;金属间化合物
1 Introduction
Homogeneous intermetallic phase joints have been widely used in semiconductor electronic industry because they have higher melting points than common solder joints and can elegantly improve the reliability of interconnections in the electronic assemblies and systems operated at elevated temperatures. The conventional joining method for fabricating this type of joint is using transientliquid-phase (TLP) bonding process[1-7]in which the intermetallic compounds (IMCs) or solid solution phase is formed through a lower melting point depressant (MPD) in an interlayer, or filler, diffusing into a surrounding bulk base metal under extended isothermal solidification. However, an inevitable drawback of TLP bonding is that it often necessitates a long processing time[1,4]up to several hours, which may lead extra thermal stresses to bond components and seriously affect the reliability of packaging system. In the present work, we applied ultrasonic bonding process to a similar Cu/Sn foil/Cu interconnection system as that employed in previous TLP bonding studies, forming homogeneous Cu6Sn5and Cu3Sn IMCs joints in a dramatically reduced short bonding time of several seconds, respectively. Our main focus was on the sonochemical effects of ultrasonic waves at the interface between liquid Sn and solid Cu, which were vital for exploring the ultrarapid formation mechanism and kinetics of these full intermetallic phase joints.
2 Experimental
The sandwich Cu/Sn foil/Cu interconnection system and ultrasonic bonding we used are schematically illustrated in Fig. 1(a) and (b). The interlayer is one piece of pure Sn foil with thickness of 25 μm, and the base metals are two pieces of pure Cu plate with thickness of 0.3 mm. The reason for choosing Cu/ Sn foil/Cu system as joining couple is its wide usage in electronic packaging interconnecting. The bonding is performed with the assembly initially at room temperature, using only a pressure of 0.6 MPa and a horizontal ultrasonic vibration of 20 kHz for a short period of 3 s. To clarify the formation of liquid solder interlayer, a thermal couple was placed underneath the interconnection system to detect its overall temperature profile during the bonding procedure. As is shown in Fig. 1(c), it indicates that the temperature of the entire assembly firstly underwent a steep rise to 297℃ and then sustained around 277℃ until the bonding was over. Considering that the melting point of Sn is 232℃, the formation of liquid Sn interlayer during bonding is doubtless. Thereason for the initial abrupt temperature increase can be attributed to the frictional heat generation at the rubbing interfaces between the solid solder and base metal. As to the thermal effect after liquid interlayer formation, it is because the propagation of ultrasonic waves in the liquid interlayer will induce acoustic cavitation phenomenon, where bubble collapse results in enormous concentration of energy from the conversion of the surface energy, kinetic energy of liquid motion into heat and chemical energy[8,9]hence sustaining the bonding temperature.
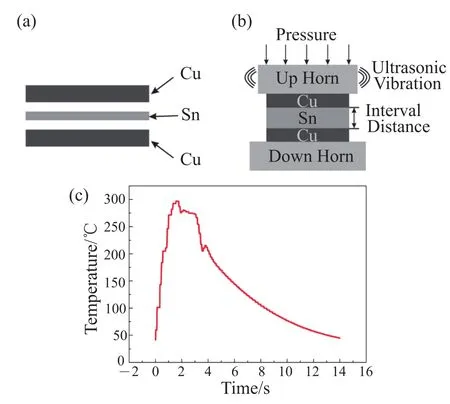
Fig. 1. (a), (b) Schematic illustration of the Cu/Sn foil/Cu interconnection system and the ultrasonic bonding; (c) Overall temperature profile of the interconnection system during the bonding procedure
3 Results and Discussion
The cross-sectional backscattered electron (BSE) image and X-ray diffraction (XRD) analysis of one produced joint are shown in Fig. 2(a) and (e), the connection layer of joint has a thickness of 20 μm, where the Sn interlayer has been completely consumed and remained homogeneous Cu6Sn5(η) intermetallic phase. At the boundary between connection layer and base metal, the Cu6Sn5phase advanced into the Cu base metal, forming potshape damage pits on the original flat Cu surface. Reviewing previous study of TLP soldering in similar Cu/Sn foil/Cu system[7], a long processing time of at least 90 minutes is necessary to form the same homogeneous Cu6Sn5joint as that we formed using present method with a processing time of 3 s. This ultrarapid development of homogeneous Cu6Sn5phase joint can be wholly attributed to the sonochemical effects induced by the propagation of ultrasonic waves in the liquid solder interlayer.

Fig. 2. Cross-sectional BSE images and XRD analysis of ultrarapidly formed joints using ultrasonic waves
Propagation of ultrasonic waves in the liquid interlayer can generate acoustic cavitation, which is the formation, growth, and rapid implosive collapse of a vapor filled microbubble. Sonochemical effects are primarily derived from this acoustic cavitation. Rapid bubble implosion can induce tiny hot spots with the localized extreme temperature and pressure estimated to be 5000 K and 0.1 GPa, respectively[10-13]. But the action region itself is so small and the heat dissipates in a short period of 2 μs[10], so only the bubbles imploding adjacent to the interface can generate effects on the solidsurface. Actually in our study, the cavitation was confined in a thin liquid solder interlayer, therefore most bubble collapse could be seen to occur in the vicinity of either base metal surface.
When the bubble is close to the base metal surface, the liquid motion in its vicinity is hindered, leading to micro-jet phenomenon[14]. In addition, the collapse of the bubble leads to the emission of a shock wave supposed to reach a pressure of several GPa with a starting shock velocity of 4000 ms—1[15]. The conjoint work of liquid-solder micro-jets, shock waves, and localized high temperature could result in microdamages (pits) on base metal surface, which is known as cavitation erosion[16], thus an excessive amount of Cu was detached from the base metal and released into the molten Sn during bonding. When the detached Cu particle entered into the action region of an imploding bubble, they would be dissolved into the molten Sn instantly under the effect of localized high temperature. Although single cavity collapse would only last for several microseconds, the bubbles confined in this thin layer of molten Sn were imploding consecutively. Therefore, on macro perspective, the liquid Sn layer was throughout kept in a dynamically unequilibrium state with high supersaturation of Cu. On subsequent cooling stage, Cu6Sn5nucleus would rapidly precipitate and grow as a result of reaction crystallization, driven by the high level Cu supersaturation in the liquid solder interlayer, and eventually forming homogeneous Cu6Sn5phase connection layer. Just due to these sonochemical effects, the ultrarapid formation of homogeneous Cu6Sn5joint is realized.
Furthermore, through adjusting the interval distance between the two base metal plates during bonding (solder interlayer will be pressed thin by the up horn and the fallen position of up horn is controllable), we can change the IMC phase consisting in the produced joint as shown in Fig. 2(a)—(e). It indicates that, with the thickness of connection layer hcin the joint decreasing from 20 μm to 12 μm (overlooking the slight volume reduction of liquid solder during solidification, the thickness of connection layer is roughly equal to the interval distance between base metal plates during bonding), the consisting intermetallic phase of joint transformed from single Cu6Sn5(η) phase, coexistences of Cu6Sn5(η) and Cu3Sn (ε) phases, to single Cu3Sn (ε) phase, and a correlated increaseof Cu-consisting ratio in the connection layers was characterized by electron probe micro-analyzer (EPMA) and energy dispersive X-ray spectroscopy (EDS) as are shown in Fig. 3. This evolution of intermetallic phases is mostly attributed to the amplification of sonochemical effects induced by the changes in localized cavitation condition.

Fig. 3. EPMA and EDS analysis of the formed joints with different connection layer thickness hc, where an increasing trend in Cu concentration consisting in the connection layer is characterized
Cavitation erosion of solid surfaces is generally attributed to two principle effects[16]∶ micro-jets and shock waves, the relative importance of each effect depends on the localized cavitation conditions, such as bubble radius and its distance from the solid surface[14-16]. The source of impact pressure at the solid surface can be classified into three types depending on L/Rmax[17], where L is the distance between solid surface and bubble center, and Rmaxis the radius of bubble at its maximum size. The type and the region of their existence are∶
(1) L/Rmax<0.3 and >1.5, shock wave is dominant;
(2) 0.6<L/Rmax<0.8, liquid jet is dominant;
(3) 0.3<L/Rmax<0.6 and 0.8<L/Rmax<1.5, shock wave and liquid jet coexist.
The actual radius of bubble at its maximum size (Rmax) is difficult to measure in liquid Sn interlayer during bonding process, but as reported in the previous studies on the approximate size of cavitation bubble, the bubble size is mainly determined by the ultrasonic frequency[18]and for the 20 kHz ultrasonic waves employed in present work, the generated bubble has a roughly estimated radius around 5 μm[19-20]. As to the distance L between solid surface and bubble center, we assume the condition that the bubble center is localized at the middle point of liquid solder layer with equal distance to each surface of the two base metals. When the interval distance between the two Cu plates are approximated to the eventual thickness of connection layers in the bonded joints varying from 20 μm to 12 μm, the calculated L changes in a range of 5 μm to 1 μm, and the L/Rmaxis confined in an region from 1 to 0.2 in our work. Referring to classification of the source of impact pressure at the solid surface, for the fabricated joints in present study, the reduction of the interval distance between the two Cu plates in ultrasonic bonding process, would lead the mechanism of cavitation erosion at the liquid Sn/ solid Cu gradually transformed from a dominant work of micro-jets to shock waves. Through the comparison of the amplitudes of micro-jets and shock waves from ultrasonic cavitation at 20 kHz in water, the hammer pressure exerted by the micro-jet at the impact zone was 0.225 GPa and the average velocity of the microjet hitting the solid surface was 150 m/s[15]. However, shock wave pressure estimated from single bubble sonoluminescence was in a range of 4—6 GPa and its impacting velocity was almost 4000 m/s[21]. Obviously, more amplified damage effects would be resulted in by shock waves than by micro-jets. This was also confirmed by the gradual expansion of cavitation erosion pits generated on the Cu plate surfaces with the decreasing of the interval distance between base metals as is shown in Fig. 2. In Cu/Sn reaction system, there are two kinds of intermetallic phase, namely Cu6Sn5and Cu3Sn, whose formation is determined by the consisting ratio of Cu to Sn. Therefore, with more amplified damage effects generated on the Cu plate surfaces induced by the changes in localized cavitation condition, a larger volume of Cu was released into the liquid melt interlayer and increased the Cu-consisting ratio in reaction system, forming Cu3Sn phase in Cu6Sn5phase matrix. When the Cu-consisting ratio exceeded a critical threshold, homogeneous Cu3Snjoint was produced.
Following shear test performed with a shearing speed of 200 μm/s shows that the average shear strengths of the homogeneous Cu6Sn5joints and Cu3Sn joints are 57.3 MPa and 64.5 MPa, respectively, which are both higher than common Sn solder joints (45.7 MPa) and meet the requirement of reliability for the interconnections in electronic packaging.
4 Conclusion
Ultrarapid melting diffusion bonding in Cu/Sn foil/Cu system using ultrasonic waves at room temperature has been demonstrated in present work, yielding homogeneous Cu6Sn5and Cu3Sn joints with high melting points, which are especially suitable for the electronic systems operated at elevated temperatures. Ultrasonic vibration can serve as heating source to rapidly melt the solder interlayer and subsequent sonochemical effects induced by the propagation of ultrasonic waves in the liquid interlayer dramatically accelerate the melting diffusion kinetics. Resulted joints are verified to have reliable strengths. The efficiency and reliability of this joining method afford it a promising application in electronic packaging.
[1] Hong SM, Bartlow CC, Reynolda TB, et al. Ultrarapid transient-liquid-phase bonding of Al2O3ceramics [J]. Advanced Matererials, 2008, 20(24)∶ 4799-4803.
[2] MacDonald WD, Eagar TW. Transient liquid phase bonding [J]. Annual Review of Materials Science, 1992, 22∶ 23-46.
[3] Zhou Y, Gale WF, North TH. Modelling of transient liquid phase bonding [J]. International Materials Reviews, 1995, 40∶ 181-196.
[4] Cook GO, Sorensen CD. Overview of transient liquid phase and partial transient liquid phase bonding [J]. Journal of Materials Science, 2011, 46(16)∶ 5305-5323.
[5] Bosco NS, Zok FW. Critical interlayer thickness for transient liquid phase bonding in the Cu-Sn system [J]. Acta Materialia, 2004, 52(10)∶ 2965-2972.
[6] Li JF, Agyakwa PA, Johnson CM. Kinetics of Ag3Sn growth in Ag-Sn-Ag system during transient liquid phase soldering process [J]. Acta Materialia, 2010, 58(9)∶ 3429-3443 .
[7] Li JF, Agyakwa PA, Johnson CM. Interfacial reaction in Cu/Sn/Cu system during the transient liquid phase soldering process [J]. Acta Materialia, 2011, 59(3)∶1198-1211 .
[8] Shchukin DG, Ekaterina S, Belova V, et al. Ultrasonic cavitation at solid surfaces [J]. Advanced Matererials, 2011, 23(17)∶ 1922-1934.
[9] Suslick KS, Flannigan DJ. Inside a collapsing bubble∶sonoluminescence and the conditions during cavitation [J]. Annual Review of Physical Chemistry, 2008, 59∶659-683.
[10] Suslick KS, Hammerton DA, Cline RE. The sonochemical hot spot [J]. Journal of the American Chemical Society, 1986, 108(18)∶ 5641-5642.
[11] Suslick KS, Didenko Y, Fang MM, et al. Acoustic cavitation and its chemical consequences [J]. Philosophical Transactions of the Royal Society A, 1999, 357(1751)∶ 335-353.
[12] Flint EB, Suslick KS. The temperature of cavitation [J]. Science, 1991, 253(5026)∶ 1397-1399.
[13] Suslick KS, Price GJ. Application of ultrasound to materials chemistry [J]. Annual Review of Materials Science, 1999, 29∶ 295-326.
[14] Chen XG, Yan JC, Gao F, et al. Interaction behaviors at the interface between liquid Al-Si and solid Ti-6Al-4V in ultrassonic-assisted brazing in air [J]. Ultrasonics Sonochemistry, 2013, 20(1)∶ 144-154.
[15] Virot M, Chave T, Nikitenko SI, et al. Acoustic cavitation at the water-glass interface [J]. Journal of Physical Chemistry C, 2010, 114(30)∶ 13083-13091.
[16] Karimi A, Martin JL. Cavitation erosion of materials [J]. International Metals Review, 1986, 31(1)∶ 1-26.
[17] Shima A, Takayama K, Tomita Y. An experimental study on effects of a solid wall on the motion of bubbles and shock waves in bubble collapse [J]. Acoustica, 1981, 48(5)∶ 293-301.
[18] Brotchie A, Grieser F, Ashokkumar M. Effect of power and frequency on bubble-size distributions in acoustic cavitation [J]. Physical Review Letters, 2009, 102∶084302 .
[19] Kanthale P, Ashokkumar M, Grieser F. Sonoluminescence, sonochemistry (H2O2Yield) and bubble dynamics∶ frequency and power effects [J]. Ultrasonics Sonochemistry, 2008, 15(2)∶ 143-150.
[20] Ashokkumar M. The characterization of acoustic cavitation bubbles - an overview [J]. Ultrasonics Sonochemistry, 2011, 18(4)∶ 864-872.
[21] Pecha R, Gompf B. Microimplosions∶ cavitation collapse and shock wave emission on a nanosecond time scale [J]. Physical Review Letters, 2000, 84(6)∶1328-1330.
Sonochemical Effects at the Interface Between Liquid Sn and Solid Cu in Ultrasonic Bonding at Room Temperature
LI Zhuolin LI Mingyu XIAO Yong
( Shenzhen Key Laboratory of Advanced Materials, Harbin Institute of Technology Shenzhen Graduate School, Shenzhen 518060, China )
Homogeneous Cu6Sn5and Cu3Sn joints were formed in Cu/Sn foil/Cu interconnection system respectively, using ultrasonic bonding process for a short period of 3 seconds at room temperature. This ultrarapid development of full intermetallic compound (IMC) joints required an accelerated interdiffusion kinetics at the interface between liquid Sn and solid Cu which could be wholly attributed to the sonochemical effects induced by acoustic cavitation phenomenon. When bubble collapsed near the liquid/solid interface, excessive cavitation erosion was generated on the solid Cu surface, resulting in supersaturation of Cu in the liquid Sn and hence facilitating the formation of intermetallic phases as chemical reaction products. The resulted intermetallic joints performed high mechanical reliability.
sonochemistry; cavitation; interdiffusion; ultrasonic bonding; intermetallic compound
2014-09-03
TG 456.9
A
Foundation:National Natural Science Foundation of China(51175116)
Author:Li Zhuolin, Ph.D., lecturer of Harbin Institute of Technology at Weihai. His research interests include electronic interconnection processes, special joining technology; Li Mingyu (corresponding author), Ph.D., Professor at the Shenzhen Graduate School of HIT. His research interests include electronic interconnection processes, special joining technology, and electronic interconnection materials, E-mai:myli@hit.edu.cn; Xiao Yong, Ph.D. candidate. His research interest is electronic interconnection materials.
