Effect of Surfactant-Induced Modifications on CuCoMn Catalysts for Higher Alcohol Synthesis
2014-07-19ZhaoxiaZhangPeiyanBiPeiwenJiangQuanxinLi
Zhao-xia Zhang,Pei-yan Bi,Pei-wen Jiang,Quan-xin Li
Department of Chemical Physics,Anhui Province Key Laboratory of Biomass Clean Energy,University of Science and Technology of China,Hefei 230026,China
Effect of Surfactant-Induced Modifications on CuCoMn Catalysts for Higher Alcohol Synthesis
Zhao-xia Zhang,Pei-yan Bi,Pei-wen Jiang,Quan-xin Li∗
Department of Chemical Physics,Anhui Province Key Laboratory of Biomass Clean Energy,University of Science and Technology of China,Hefei 230026,China
A series of surfactant-modi fi ed CuCoMn-based catalysts were prepared for higher alcohol synthesis from biomass-based syngas.Three typical surfactants,cetyltrimethylammonium bromide(CTAB),sodium dodecyl sulfate(SDS)and pluronic P123 triblock copolymer(EO20PO70EO20),were employed.Compared to surfactant-free CuCoMn catalyst,CO conversion increased from 17.4%to 29.7%over SDS-modi fi ed CuCoMn catalyst,and the selectivity of higher alcohols increased from 22.0%to 41.2%over CTAB-modi fi ed catalyst. Besides,the proportions of higher alcohols in total alcohols increased over all surfactantmodi fi ed catalysts.The catalysts were characterized by N2adsorption/desorption,XRD, XPS and IR analysis.The results showed that several more favorable features rendered the CTAB-modi fi ed CuCoMn catalyst to be suitable for higher alcohol production,such as the larger pore size,better crystallinity of CuCoMnO4spinel,moderate surface atomic distribution and lower valence of metallic ions.In addition,it was veri fi ed that CTAB addition at the metal precipitation stage was bene ficial to higher alcohol synthesis.Surfactant-induced modi fication provides a promising alternative method for catalyst improvement in synthesis of higher alcohols.
Higher alcohol,Surfactant,Modi fication,CuCoMn catalyst
I.INTRODUCTION
Porousmaterialssynthesizedbyasurfactanttemplated process have attracted great interest in the past twenty years since the mesoporous M41S series were reported[1-4].The surfactant-templated materials showed high surface areas and uniform pore sizes in the mesoporous range(diameter 2-50 nm),and the pore diameters could be tuned by adjusting the diameter of templating micelles.The formation mechanism of the ordered mesoporous structure was postulated the micellar arrays of surfactants template inorganic precursor with the subsequent and/or simultaneous condensation for cooperative assembly.The ordered mesoporous materials provide various potential applications in molecular recognition,such as shape-selective catalysis,which show high activities and product selectivities due to the high surface areas with more exposure of active sites on the surface and enhanced dif f usion through the well-def i ned large mesopores[5-7].Zou et al.reported the synthesis of mesoporous MnOx-CeO2catalysts with high specific surface areas by using cetyltrimethylammonium bromide(CTAB)as templates,which was applied in the model reactions of CO and C3H8oxidation[6].Cao et al.also synthesized CuO-Fe2O3and CuO/Ce0.8Zr0.2O2catalysts by using CTAB template[8,9].These catalysts displayed obvious advantages over those prepared by other methods, including high surface areas and wormhole-like mesoporous structures.
Higher alcohols(HA,C2+OH),a mixture of C2-C6alcohols,have wide applications such as alternative fuels,fuel additives and chemical raw materials[10-12]. Therefore,the catalytic conversion of syngas(CO and H2)to HA has received renewed interest for both basic research and industrial application due to the abundant resources of syngas from coal,natural gas and biomass.Especially,biomass is an abundant and renewable resource which can contribute to a nonfossil-based feedstock for energy sources or chemicals[12].Several heterogeneous catalysts for HA synthesis from syngas have been explored and well reviewed[13],including the modified methanol synthesis catalysts[14,15],modified Fischer-Tropsch synthesis catalysts[16-21],noble metals-based synthesis catalysts[22],and Mo-based synthesis catalysts[23,24].Among them,CuCo-based modified Fischer-Tropsch catalysts developed by IFP (Institut Francais du Petrole)have been regarded as one of the most promising catalysts for HA synthesis owing to their relatively favorable reaction performance under mild operating conditions[25,26].These catalysts usually contained three or more metals from the groupCo,Cu,and Al,Mn,Zn,etc.and an alkali promoter (e.g.,La,K,Cs).They were generally prepared by coprecipitation method and the subsequent impregnation of alkali metal to obtain catalyst precursor with homogenous particles.The typical reaction conditions of HA synthesis for CuCo-based catalysts were 5-15 MPa, 490-620 K,4000-8000 h-1GHSV and a wide range of H2/CO ratio.The reaction products of HA synthesis were mainly primary alcohols with relatively high selectivity of especially ethanol,depending on the type of the employed metal compositions.We have recently reported Na-promoted CuCoMn catalysts for conversion of bio-syngas to HA[27].The results showed that the addition of sodium to the CuCoMn catalysts increased the selectivity of HA and promoted the dispersion of active elements(i.e.,Cu and Co).However,the rates of HA production(100-500 mg C2+OH/(gcath))were obviously lower than those achieved in methanol synthesis (1300-1500 mg MeOH/(gcath))[13].Thus,significant improvements in the HA synthesis must be further studied.
It is widely known that catalyst properties are greatly inf l uenced by the preparation method.In this work,we tried to improve reaction performance of HA synthesis by surfactant-induced modification on CuCoMn-based catalysts.Three typical surfactants,namely cationic surfactant CTAB,anionic surfactant SDS(sodium dodecyl sulfate),and nonionic surfactant Pluronic P123 triblock copolymer(EO20PO70EO20),were employed to investigate the different effects on the texture and surface chemical properties of CuCoMn oxides.The method of surfactant-induced modification provided a new approach for catalyst improvement in synthesis of higher alcohols.
II.EXPERIMENTS
A.Catalyst preparation
The chemicals used in this study were A.R.grade. The CuCoMn-based oxides were prepared with a settled molar composition:Cu/Co/Mn/Na=1/1/0.8/0.1. Three types of surfactants were employed:Pluronic P123(Sigma-Aldrich),SDS,and CTAB(both from SinopharmChemicalReagentCompanyLimited, Shanghai,China).The surfactants were added into the CuCoMn precursors by two different strategies, named as co-pricipitation and post-precipitation methods.Detailed preparation procedure was as follows. A specific surfactant(P123,SDS,or CTAB)was predissolved in the sodium carbonate solution(so-called co-pricipitation method).Accordingly,the f i nally obtained CuCoMn catalysts were designated as CCMP123,CCM-SDS,and CCM-CTAB respectively.Subsequently the mixture of metal nitrate solution(Cu,Co and Mn)was quickly added into the alkali solution at 343 K.The precipitate was kept in the mother liquor for 1 h,and then washed until the pH value of the f i nal suspension was near 7.0.After being dried at 393 K for 12 h,the solid was impregnated with appropriate amount of Na2CO3,aged at 343 K for 2 h,and then calcined in air at 723 K for 4 h.The obtained CuCoMn oxide was further crushed and pelletized to tablets,and then sieved into 40-60 mesh particles for kinetic experiments.In addition,for comparison of the inf l uence of different preparation method on CuCoMn catalyst,CTAB was introduced into inorganic precursors by physically mixing after metal precipitation and before calcination(so-called post-precipitation method,CCMCTAB-PP).The catalyst without adding any surfactant was marked as CCM-NS.
B.Catalyst characterization
PowderX-raydif f raction(XRD)patternswere recordedonanX’pertProPhilipsdif f ractrometer(Philips,Netherlands),using a Cu Kα radiation(λ=0.15418 nm).The N2adsorption/desorption isotherms were measured at 77 K using the Micromeritics ASAP 2020 V3.00 analyzer.Pore size distributions and pore volumes were calculated using the Barrett-Joyner-Halenda(BJH)method on the desorption branch while BET(Brunauer-Emmett-Teller)surface areas were evaluated from the linear part of the BET plots.X-ray photoelectron spectroscopy(XPS) measurements were performed on an ESCALAB 250 (Thermo-VG Scientific,USA)spectrometer with Al Kα radiation.The C1s peak at a binding energy of 284.6 eV was generally f i xed as a calibration standard for subtracting the surface charging effect.Infrared spectra(IR)of the samples were collected by a Bruker EQUINOX55 FTIR spectrometer,in the range of 400-4000 cm-1at 0.5 cm-1resolution.The specimens for measurements were prepared by mixing 3 mg of sample powder with 100 mg of KBr and then pressed into pellets.
C.Experimental setup and product analysis
The performance of HA synthesis from biomass-based syngas was evaluated in a f i xed-bed continuous-f l ow reactor using an on-line gas chromatograph(GC)detection system[12,27].Generally,1.0 g of catalyst diluted with 2.0 mL Pyrex beads was uniformly f i lled in the reactor(stainless steel 316 L,inner diameter: 10 mm,length:400 mm).Before kinetic tests,the catalyst was reduced by a f l owing 5vol%H2/Ar at 593 K for 12 h.Then,the biomass-based syngas,including H2,CO,CO2,N2,CH4and others with the volume contents of 62.80%,30.89%,2.96%,1.75%,1.20%,and 0.40%respectively[12,27],was introduced into the reactor for HA synthesis under the designed conditions: T=553 K,P=5.0 MPa,and GHSV=4000 h-1with dif-ferent CuCoMn catalysts.The gaseous products were analyzed on-line by GC(SP6890)with two detectors. H2,CO,CH4,and CO2were detected by a TCD detector with TDX-01 column,and gaseous hydrocarbons were determined by a FID detector with Porapak Q column.The organic condensates(mainly containing higher alcohols)cooled in a liquid tank were analyzed of f-line by a FID detector.The performance of HA synthesis was evaluated by carbon conversion(CC)of CO, carbon selectivity(SC)of alcohols(ROH),CO2or hydrocarbon(CxHy),carbon yield(YC)of higher alcohols and alcohol distribution(D),according to the following equations:

TABLE I Performance of HA synthesis over the CuCoMn-based catalysts modified by various surfactants.T=553 K, P=5.0 MPa,GHSV=4000 h-1.

where ainCO,aoutCO,and aCare carbon moles of CO in,CO out,and carbon of all alcohols respectively,b1, b2,and b3are carbon moles in a certain product,higher alcohols,and CxOH alcohol,respectively.The content of coke formed on the catalysts was negligent.
III.RESULTS AND DISCUSSION
A.Performance of HA synthesis

FIG.1 ASF plots of alcohol products over CuCoMn-based catalysts modified by different surfactants.Wn,the mass fraction of an alcohol containing n carbon atoms.
The performance of HA synthesis over CuCoMnbased catalysts modified by various surfactants was measuredundertheconstantreactionconditions: 553 K,5.0 MPa,and 4000 h-1,the results are shown in Table I.Table I shows that all CCincreased when the surfactants(SDS,P123 and CTAB)were introduced into the CuCoMn catalysts.However,SCof ROH followed different trends.The selectivities of alcohols over CCM-SDS and CCM-CTAB catalysts were enhanced to 27.3%and 41.2%respectively,while that over CCMP123 catalyst decreased.Oppositely,the selectivities of CO2and hydrocarbon over the modified catalysts by surfactants decreased except P123,compared with the CCM-NS catalyst.In addition,YCof higher alcohols, increased from 2.8%on CCM-NS catalyst to 6.3%,3.0% and 9.2%corresponding to CCM-SDS,CCM-P123 and CCM-CTAB catalysts respectively.CCM-CTAB catalyst showed the optimal yield of higher alcohols.Furthermore,in the alcohol distribution,Table I shows that the content of methanol decreased while that of higher alcohols(C2+OH)presented an increasing trend by addition of surfactants into the CuCoMn-based catalysts. Especially,the amount of ethanol achieved more than 40%in the alcohol products.The results indicated that surfactant-induced modification on CuCoMn-based catalysts facilitates the formation of higher alcohols,and the catalyst added CTAB exhibits the highest HA selectivity,yield and distribution under the performed reaction conditions.
Figure 1 shows the ASF(Anderson-Schulz-Flory)plots for the alcohol distribution over surfactantmodified CuCoMn-based catalysts.Similar to the CuCoMn-based catalyst without adding surfactants [27],the alcohol products over the surfactant-modified CuCoMn-based catalysts followed excellent ASF ditributions except for a little deviation of ethanol from the ASF plots over CCM-SDS and CCM-CTAB catalysts. The chain growth probability(α)calculated from the slope of the ASF plot over the CCM-NS catalyst was 0.346,while those over surfactant-modified CuCoMnbased catalysts were between 0.369 and 0.402.The results indicated that adding surfactants into CuCoMnbased catalysts enhances the chain growth probability, which is consistent with alcohol distribution in Table I.

TABLE II Textural parameters of CuCoMn-based catalysts modified by different surfactants.SBET:Brunauer-Emmett-Teller surface area,Vp:pore volume,dXRD:average crystallite size calculated by Sherrer equation.
B.Characterization of catalysts
It is well known that the reaction performance of catalysts is closely related to their textural and surface chemical properties.The CuCoMn-based catalysts were characterized by N2adsorption/desorption,XRD, XPS and IR to investigate the enhancement of reaction performance by adding surfactants.
Table II lists the textural properties(BET surface areas SBETand pore volume Vp)of CuCoMn-based catalysts added surfactants by co-precipitation methods. Interestingly,the SBETof the surfactant-modified catalysts monotonously decreased in the following order: CCM-P123>CCM-SDS>CCM-CTAB.The order was just the opposite to those of alcohol selectivities(shown in Table I)and carbon yields.Also,the Vpfollowed similar trends with the minimum of 0.16 cm3/g appearing in the CCM-CTAB catalyst.Generally,high surface areas favor more exposure of active sites on the surface consequently with high catalytic activities[6].Even so, an exception[21]was reported that Co3Cu1catalyst doped by activated carbon with 830 m2/g of SBETdisplayed lower conversion of CO and space-time-yields of (C1-8alcohols+dimethyl ether),compared to the CNTs (h-type)-doped catalyst with 135 m2/g of SBET.The results indicated that surface area of catalyst is not the only determinant of the reaction performance.
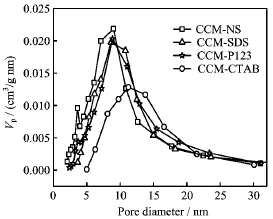
FIG.2 The BJH pore size distributions on the desorption branch for CuCoMn-based catalysts modified by different surfactants.

FIG.3 XRD patterns of CuCoMn-based catalysts modified by different surfactants.
The pore size distributions of the CuCoMn-based catalysts on the desorption branch calculated by BJH model are shown in Fig.2.The CCM-NS catalyst without adding surfactant had two pore sizes centred at 3.6 and 8.0 nm.The surfactant-modified CuCoMn-based catalysts(CCM-P123,CCM-SDS,and CCM-CTAB) possessed unique mesopores centered mainly at 8.8,9.7, and 11.2 nm,respectively.From the resluts,it is concluded that the pore diameters of CuCoMn-based catalysts are enlarged and adjustable by adding surfactants. Moreover,the order of mesopore size distributions was in accordance with those of alcohol selectivities and carbon yields,which was attributed to enhanced molecular dif f usions through the larger pores[5].
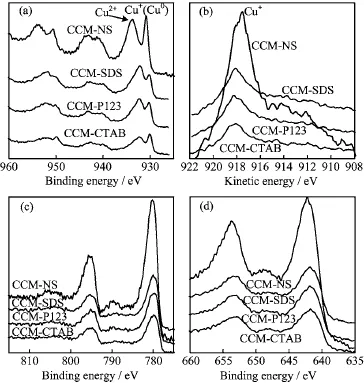
FIG.4 XPS spectra of catalysts modified by different surfactants.(a)Cu2p3/2,(b)Cu LMM,(c)Co2p3/2,and (d)Mn2p3/2.
The XRD patterns of the CuCoMn-based catalysts by adding different surfactants are presented in Fig.3.All of the dif f raction peaks in the f i gure can be attributed to spinel CuCoMnO4(JCPDS 47-0324).With addition of surfactants,the crystalline phases of CuCoMn-based catalysts were unchanged.However,the intensity of CuCoMnO4dif f raction peaks changed.The XRD line width for CCM-SDS catalyst was broader than that for CCM-NS catalyst.In contrast,the dif f raction peaks of CuCoMnO4for CCM-P123 and CCM-CTAB catalysts presented an increasing trend.The average crystallite sizes dXRDcalculated by XRD line widths of all peaks using Scherrer equation are listed in Table II.The crystallite size of CuCoMnO4in CCM-CTAB catalyst was the largest,which was consistent with its strongest intensity of dif f raction peaks.CuCoMnO4spinel has been reported to be beneficial to stabilizing the active species of Cu+[28].In addition,the increase of CuCoMnO4crystallite size in the CCM-CTAB was an indirect evidence for the remarkablely low specific surface area of 15 m2/g revealed by N2adsorption/desorption results.
XPS is a surface analysis technique that can provide chemical state and surface elemental composition of the samples.The binding energies of the detected elements (Cu,Co and Mn)were calibrated at 284.6 eV with the C1s peak.
The Cu2p3/2XPS spectra of CuCoMn-based catalysts are shown in Fig.4(a).The binding energies at 930.8 and 933.9 eV in the CCM-NS catalyst without adding surfactant were observed,while those at 930.1±0.1 and 932.2±0.1 eV in the surfactant-modified catalysts were discovered.On copper oxides,the 2p3/2peak of Cu+(Cu2O)is at 932.2±0.3 eV and that of Cu2+(CuO)is at 933.5±0.3 eV[28].The sharp Cu2p3/2peak at 930.8 eV in Mn-containing CuCoMn oxides(CCM-NS)could be attributed to either Cu+or Cu0.The Cu2p3/2peak at 933.9 eV in CCM-NS catalyst corresponding to Cu2+was just at the right position.The fact represented that Mn induces the shift of Cu+(Cu0)peak to lower binding energy.In addition, in the surfactant-modified catalysts(CCM-SDS,CCMP123,and CCM-CTAB),the binding energies corresponding to Cu2+(932.2±0.1 eV)in the Cu2p3/2spectra shifted as well as those corresponding to Cu+/Cu0(930.1±0.1 eV).This negative chemical shift implies the changes of microchemical environment of Cu2+due to the presence of surfactants.
Furthermore,the differentiation of Cu+and Cu0species could be achieved by combining with the Cu LMM Auger electron spectra(Fig.4(b))[29,30].The Cu LMM spectra exhibited kinetic energies at 917.6, 918.1,918.3,and 918.1 eV corresponding to the catalysts of CCM-NS,CCM-SDS,CCM-P123,and CCMCTAB respectively.Modified Auger parameter α′is proposed to distinguish Cu+and Cu0.This parameter,independent of the charging effect,is the sum of Cu+/Cu0binding energy of the photoelectron line and the kinetic energy of the Auger line.All values of α′calculated from Fig.4(a)and(b)were between 1848-1849 eV,which corresponds to Cu+rather than Cu0(1851-1852 eV)[29].Additional evidence is that Cu+has a broad LMM Auger peak with its FWHM (full width at half maximum)over 2 eV,while Cu0has a very sharp LMM Auger peak with its FWHM about 1 eV[28].In Fig.4(b),the Cu LMM Auger peaks of all catalysts were broad with their FWHM over 2 eV,thus indicating the presence of Cu+but not Cu0.Therefore, the Cu2p3/2spectra of all fresh CuCoMn-based catalysts exhibited copper mainly as Cu2+and Cu+.It is worth noting that the presence of Cu+in the unreduced catalysts suggests the internal redox reaction of Cu2+by Mn3+(Cu2++Mn3+→Cu++Mn4+).
A summary of copper relative contents estimated by Gaussian f i tting of the Cu2p3/2prof i les in Fig.4(a)is shown in Table III.For CCM-CTAB catalyst,higher Cu+/Cu2+ratio(0.40)was observed compared to the other catalysts.The result agrees with the weakest shake-up satellite peak(941.4 eV)of Cu2+about 9 eV higher than the binding energy of Cu2+in Fig.4(a).According to Yan et al.[25],the amount of surface Cu+sites was responsible for methanol production rate as Cu+sites were essential for CO adsorption.Therefore, the high Cu+/Cu2+ratio in the CTAB-modified Cu-CoMn catalyst is more favorable for the CO conversion shown in Table I and consequently high yield of higher alcohols.
XPS spectra of Co are shown in Fig.4(c).The primary peaks(780.0±0.2 eV)are attributed to Co3+or Co2+without Co0species.The differentiation of Co3+and Co2+species could be obtained with the satellite peaks of Co2p3/2.In the CCM-NS catalyst,Co3+is obviously observed by a weak satellite peak(789.6 eV) at about 10 eV higher than its main peak.However, the intensities of satellite peaks assigned to Co3+decreased in the surfactant-modified catalysts,and those assigned to Co2+at 787.5-785.5 eV comparatively increased especially in the CCM-CTAB.The presence ofCo2+also suggests internal reduction of Co3+by Mn3+(Co3++Mn3+→Co2++Mn4+).

TABLE III Surface composition of catalysts from XPS test.
Figure 4(d)shows Mn2p3/2XPS spectra of the CuCoMn-based catalysts.In general,the binding energy of Mn4+in MnO2is at 642±0.2 eV and that of Mn3+in Mn2O3is at 641.3 eV[28].From Fig.4(d), the Mn2p3/2peak of surfactant-free CCM-NS catalyst was at 642.2 eV which was the right position of Mn4+. The Mn2p3/2peaks of CCM-SDS and CCM-P123 catalysts are both at 641.9 eV.This value was slightly lower than that of Mn4+,but noticeably higher than that of Mn3+.It suggests that the surface Mn is mainly as Mn4+in CCM-SDS and CCM-P123 catalysts.Unlikely,the Mn2p3/2peak of CCM-CTAB catalyst was at 641.7 eV.This value was between those of Mn4+and Mn3+,implying the coexistence of Mn4+and Mn3+. It can be concluded that Mn3+was more attracted to the surface on the CTAB-modified CuCoMn catalyst. Moreover,the enriched Mn4+on the surface is in agreement with the internal reductions of Cu2+and Co3+as discussed above.
The surface atomic percentages are listed in Table III. Interestingly,the metallic contents of Cu,Co and Mn ions in CTAB-modified catalyst were significantly lower than those in the other catalysts.From XPS results discussed above,the addition of CTAB into CuCoMnbased catalyst is more favorable for lower metallic contents on the surface and lower valence metallic ions. This correlation may be related to the repulsive interaction of positive charge CTAB carries to the metallic ions.
The CTAB-modified CuCoMn-based catalyst was spectroscopically measured by infrared spectra since its significantly lower SBETand Vpwere observed by N2adsorption/desorption analysis.It was suspected of blocking the holes in the catalyst by adding CTAB.From Fig.5,pure CTAB showed the C-H stretching bands at 2914 and 2850 cm-1which was assigned to the-CH2-groups[31].Surprisingly,the C-H stretching bands in the range of 2980-2850 cm-1disappeared nearly completely in the uncalcinated CuCoMn precursor modified by CTAB.It seems that the CTAB-modified CuCoMn precursor contains little or no CTAB after washing.It is assumed that the CTAB-induced modification on Cu-CoMn catalyst mainly occurred during the metal precipitation stage.Further verification was conducted by comparison of catalytic activity to the CTAB-modified CuCoMn catalyst by post-precipitation method.

FIG.5 IR spectra of(a)CCM-CTAB precursor before calcination and(b)pure CTAB.
C.Comparison between co-precipitation and post-precipitation on CTAB addition
A comparison was made with regard to the CTAB addition methods by CCM-CTAB and CCM-CTAB-PP, as shown in Table I.From Table I,the CO conversions of these two catalysts were in close proximity(28%and 30%respectively).The distinct differences in selectivities of alcohols and hydrocarbons were caused.The selectivity of alcohols over CCM-CTAB catalyst achieved 41.2%while that over CCM-CTAB-PP catalyst was just 5.6%.On the other hand,the selectivity of hydrocarbons over CCM-CTAB catalyst was 28.2%while that over CCM-CTAB-PP catalyst reached 76.8%.In addition,the hydrocarbon products(CxHy)obtained from CCM-CTAB catalyst were mainly composed of C1-4gaseous hydrocarbons,while the content of C5+liquid hydrocarbons in the total hydrocarbon products from CCM-CTAB-PP catalyst achieved 44.8C-mol%.These results indicated that different reaction pathways for CO hydrogenation probably occur on different active sites over the catalysts CCM-CTAB and CCM-CTABPP.Meanwhile,it can be taken as an important evidence that CTAB addition during metal precipitation stage is more favorable for higher alcohol synthesis over the modified CuCoMn catalyst.
IV.CONCLUSION
It was demonstrated that higher alcohols can be efficiently synthesized from biomass-based syngas using surfactant-modified CuCoMn-based catalysts.It was found that CO conversion increased from 17.4% on surfactant-free CuCoMn catalyst to 29.7%on SDS-modified CuCoMn catalyst prepared by co-precipitation method.Selectivity toward higher alcohols(C2+OH)increased on the catalysts modi fi ed by SDS and CTAB. With respect to the alcohol products,the contents of higher alcohols in the total alcohols increased on surfactant-modi fi ed catalysts compared with that on surfactant-free catalyst.CTAB-modi fi ed CuCoMn catalyst showed the optimal catalytic performance.According to N2adsorption/desorption analysis,the pore diameters of surfactant-modi fi ed catalysts were enlarged and the pore diameter in CTAB-modi fi ed catalyst exhibited the largest in favor of molecular di ff usions.XRD results suggested that CuCoMnO4spinel structure was the dominant phase for all fresh catalysts, which was favorable for stabilizing Cu+species.XPS analysis showed Cu,Co,and Mn mainly presented as Cu+/Cu2+,Co2+/Co3+,and Mn3+/Mn4+in the fresh catalysts,indicating the internal reductions of Cu2+and Co3+by Mn3+.The CTAB-modi fi ed catalyst revealed lower metallic contents on the surface and lower valence metallic ions.Moreover,CTAB-induced modification during metal precipitation stage was con fi rmed to favor higher alcohol production.Present work reveals that surfactant modi fication is a promising method for catalyst improvement in synthesis of biomass-based higher alcohols.
V.ACKNOWLEDGMENTS
This work was supported by the National Key Basic Program of China(No.2013CB228105),and the National Natural Science Foundation of China (No.51161140331).The assistance of Song-bai Qiu and Tong-qi Ye from University of Science and Technology of China is gratefully acknowledged.
[1]L.Han and S.Che,Chem.Soc.Rev.42,3740(2013).
[2]M.Kruk,Accounts Chem.Res.45,1678(2012).
[3]C.T.Kresge,M.E.Leonowicz,W.J.Roth,J.C.Vartuli,and J.S.Beck,Nature 359,710(1992).
[4]S.Cabrera,J.El Haskouri,J.Alamo,A.Beltrn,D. Beltrn,S.Mendioroz,M.D.Marcos,and P.Amors, Adv.Mater.11,379(1999).
[5]J.Kim,M.Choi,and R.Ryoo,J.Catal.269,219 (2010).
[6]Z.Q.Zou,M.Meng,and Y.Q.Zha,J.Phys.Chem.C 114,468(2010).
[7]J.Zhong,J.Li,F.Feng,S.Huang,and J.Zeng,Mater. Lett.100,195(2013).
[8]J.L.Cao,Y.Wang,T.Y.Zhang,S.H.Wu,and Z.Y. Yuan,Appl.Catal.B 78,120(2008).
[9]J.L.Cao,Y.Wang,X.L.Yu,S.R.Wang,S.H.Wu, and Z.Y.Yuan,Appl.Catal.B 79,26(2008).
[10]M.Ding,M.Qiu,J.Liu,Y.Li,T.Wang,L.Ma,and C.Wu,Fuel 109,21(2013).
[11]J.J.Spivey and A.Egbebi,Chem.Soc.Rev.36,1514 (2007).
[12]S.Qiu,W.Huang,Y.Xu,L.Liu,and Q.Li,Chin.J. Chem.Phys.24,77(2011).
[13]V.Subramani and S.K.Gangwal,Energ.Fuel 22,814 (2008).
[14]E.Heracleousa,E.T.Liakakoua,A.A.Lappas,and A. A.Lemonidou,Appl.Catal.A 455,145(2013).
[15]R.G.Herman,Catal.Today 55,233(2000).
[16]V.Mahdavi and M.H.Peyrovi,Catal.Commun.7,542 (2006).
[17]N.Tien-Thao,M.H.Zahedi-Niaki,H.Alamdari,and S.Kaliaguine,J.Catal.245,348(2007).
[18]R.Xu,W.Wei,W.H.Li,T.D.Hu,and Y.H.Sun,J. Mol.Catal.A 234,75(2005).
[19]R.Xu,C.Yang,W.Wei,W.H.Li,Y.H.Sun,and T. D.Hu,J.Mol.Catal.A 221,51(2004).
[20]H.B.Zhang,X.L.Liang,X.Dong,H.Y.Li,and G. D.Lin,Catal.Surv.Asia 13,41(2009).
[21]X.Dong,X.L.Liang,H.Y.Li,G.D.Lin,P.Zhang, and H.B.Zhang,Catal.Today 147,158(2009).
[22]M.Ojeda,M.L.Granados,S.Rojas,P.Terreros,F.J. Garcia-Garcia,and J.L.G.Fierro,Appl.Catal.A 261, 47(2004).
[23]J.Iranmahboob,H.Toghiani,D.O.Hill,and F.Nadim, Fuel Process.Technol.79,71(2002).
[24]Z.Li,Y.Fu,J.Bao,M.Jiang,T.Hu,T.Liu,and Y. N.Xie,Appl.Catal.A 220,21(2001).
[25]Q.Yan,P.T.Doan,T.Hossein,C.G.Amit,and G. W.Mark,J.Phys.Chem.C 112,11847(2008).
[26]P.Mohanty,K.K.Pant,J.Parikh,and D.K.Sharma, Fuel Process.Technol.92,600(2011).
[27]T.Q.Ye,Z.X.Zhang,Y.Xu,S.Z.Yan,J.F.Zhu, Y.Liu,and Q.X.Li,Acta Phys.Chim.Sin.27,1493 (2011).
[28]B.L.Yang,S.F.Chan,W.S.Chang,and Y.Z.Chen, J.Catal.130,52(991).
[29]G.Deroubaix and P.Marcus,Surf.Interface Anal.18, 39(1992).
[30]S.Velu,K.Suzuki,and C.S.Gopinath,J.Phys.Chem. B 106,12737(2002).
[31]Y.Mitsunori,H.Hiroaki,O.Kaoru,M.Masato,and K.Tsuyoshi,Inorg.Chem.36,5565(1997).
ceived on April 28,2014;Accepted on May 29,2014)
∗Author to whom correspondence should be addressed.E-mail:liqx@ustc.edu.cn,FAX:+86-551-63606689
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Hydrothermal Synthesis and Efficient Visible Light Photocatalytic Properties of InVO4Hierarchical Microspheres and InVO4Nanowires
- Uniform B-C-N Ternary Monolayer from Non-Metal Filled g-C3N4Sheet
- Insights into Elastic and Thermodynamics Properties of Binary Intermetallics in Ni-Al Alloys under Extreme Condition:Full-Electronic Quasi-Harmonic Study
- Negative Differential Resistance of Au-MgB2-Au Nanoscale Junctions
- Molecular Dynamic Simulation of Melting Points of Trans-1,4,5,8-tetranitro-1,4,5,8-tetraazadacalin(TNAD)with Some Propellants
- Composite Cathode based on Mn-doped Perovskite Niobate-Titanate for Efficient Steam Electrolysis
