Effect of Adsorbent Diameter on the Performance of Adsorption Refrigeration*
2014-07-18HUANGHongyu黄宏宇HEZhaohong何兆红YUANHaoran袁浩然KOBAYASHINoriyuki小林敬幸ZHAODandan赵丹丹KUBOTAMitsuhiro窪田光宏andGUOHuafang郭华芳GuangzhouInstituteofEnergyConversionChineseAcademyofSciencesGuangzhou50640ChinaSchoolofEngineeringNagoyaUnive
HUANG Hongyu (黄宏宇), HE Zhaohong (何兆红)**, YUAN Haoran (袁浩然) KOBAYASHI Noriyuki (小林敬幸), ZHAO Dandan (赵丹丹) KUBOTA Mitsuhiro (窪田光宏)and GUO Huafang (郭华芳)Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 50640, ChinaSchool of Engineering, Nagoya University, Nagoya 464-8603, Japan
Effect of Adsorbent Diameter on the Performance of Adsorption Refrigeration*
HUANG Hongyu (黄宏宇)1,2, HE Zhaohong (何兆红)1,**, YUAN Haoran (袁浩然)1, KOBAYASHI Noriyuki (小林敬幸)1,2, ZHAO Dandan (赵丹丹)1, KUBOTA Mitsuhiro (窪田光宏)2and GUO Huafang (郭华芳)11Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, China2School of Engineering, Nagoya University, Nagoya 464-8603, Japan
Adsorbents are important components in adsorption refrigeration. The diameter of an adsorbent can affect the heat and mass transfer of an adsorber. The effect of particle diameter on effective thermal conductivity was investigated. The heat transfer coefficient of the refrigerant and the void rate of the adsorbent layer can also affect the effective thermal conductivity of adsorbents. The performance of mass transfer in the adsorber is better when pressure drop decreases. Pressure drop decreases with increasing permeability. The permeability of the adsorbent layer can be improved with increasing adsorbent diameter. The effect of adsorbent diameter on refrigeration output power was experimentally studied. Output power initially increases and then decreases with increasing diameter under different cycle time conditions. Output power increases with decreasing cycle time under similar diameters. Keywords adsorption refrigeration, heat and mass transfer, adsorbent diameter, thermal conduction, permeability
1 INTRODUCTION
With the increasing economic development and environment protection, adsorption refrigeration technology as the green refrigeration method has received much attention. Adsorption refrigeration can be driven by low-grade heat source, such as waste heat from the process industry and solar energy [1, 2]. Thus, the adsorption technology can enhance the energy utilization efficiency for recovering low-grade heat and reduce fossil resource consumption. Furthermore, environment friendly refrigerants are used in adsorption refrigeration, making the refrigeration system simple and adsorption chiller with low noise performance.
Common working pairs include silica gel-water [3-6], zeolite-water [7, 8], and active carbon-methanol [9, 10]. Silica gel is widely used because of its better adsorption performance for vapor and low regenerated temperature. Thus, most products of adsorption refrigeration can use the silica gel-water working pair. Adsorbents are considered as important components in adsorption refrigeration systems and are directly associated with refrigeration performance. And the heat and mass transfer performance of adsorbent are studied generally to examine adsorption refrigeration performance.
The effective diffusivity of the adsorption refrigeration working pair 13X-H2O was determined through thermogravimetry (TG) under the unsteady diffusion by Wang et al [11]. The surface diffusion of water was found to be the main control factor in pellet diffusion. Wang and Wang [12] compared the temperature field and adsorbed mass distribution in the adsorbent bed, and discussed some thermodynamic analyses with various surface diffusing velocity coefficients. Enhancing the rate of adsorption or desorption can improve the coefficient of performance (COP) in an adsorption refrigeration system. A model for the heat and mass transfer process of an active carbon-methanol adsorption bed was developed [13]. The relationship among adsorption temperature, adsorption rate, adsorbed quantity, COP, and cycle time were investigated using numerical methods.
Permeability is one of the important parameters in the adsorption of adsorbents. It represents the mass transfer performance of an adsorbate and indicates the pressure drop when an adsorbate flows through an adsorbent layer. The distributions of temperature and adsorbate concentration were considerably affected by bed permeability [14]. The permeability of two types of monolithic activated carbons was investigated to develop a design for a high-performance generator for sorption refrigeration systems. Permeability was found to be highly anisotropic [15]. The porosity of the adsorbent bed in a zeolite-13X/water adsorption cooling system was numerically studied. It was found that the porosity of the adsorbent bed had a strong effect on the performance of the adsorption cooling system [16]. The permeability of the consolidated composite activated carbon adsorbent for refrigeration was studied. The simple solidified adsorbent was found to have a higher permeability than that of the consolidated adsorbent [17]. Wang [18] found that the permeability of compressed expanded natural graphite (ENG) treated with acid decreased with increasing density, which was much lower than that of compressed ENG without treatment. Tian et al. [19] tested the permeability of compacted graphite using nitrogen as the gas source. It was found that the permeability had dramatic differences when the curing density changed from 100kg·m−3to 400 kg·m−3and the permeability ranged from 10−14m2to 10−12m2. The thermal conductivities and the permeabilities of the AC/ENG consolidated composite adsorbents were studied using the steadystate method. With a density of 600 kg·m−3, the permeability grew with the size of the grains, and the thermal conductivities of the active carbon (AC) grains were constant at 0.36 W·m−1·K−1.
Considering the importance of the heat and mass transfer of the adsorbent, analyses of effective thermal conduction coefficient and permeability with different silica gel particle diameters were proposed. The effect of adsorption chiller with different silica gel diameters on cooling power was evaluated and presented in this paper.
2 MATERIALS AND ANALYSIS
2.1 Material
Four types of silica gels adsorbent with different diameter were used. The parameters (particle diameter, mass, void ratio, and specific surface area) of the silica gel adsorbent were shown in Table 1.
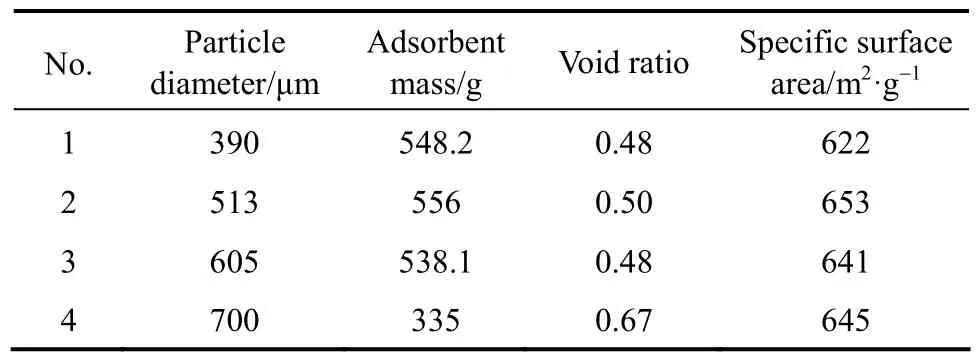
Table 1 Silica gel adsorbent parameters
2.2 Thermal conduction
The heat transfer process in adsorption packed beds consists of three parts. The first is the heat conduction in a porous particle adsorbent. The second is the heat transfer between the adsorbent and the metal surface of the heat exchanger. The last is the heat convection between the metal surface and the heating/cooling liquid, where the heat transfer coefficient is better than that of the others. Hence, the controlling thermal resistance of the adsorption packed bed is in the adsorbent side. The thermal conduction coefficient of the particle adsorbent is very poor because of the porosity of the adsorbent. The heat transfer performance of the adsorption packed bed is affected by the poor thermal conduction coefficient. The thermal conduction coefficients of silica gel, zeolite and active carbon are 0.1-0.2 W·m−1·K−1, 0.1 W·m−1·K−1and 0.3-0.5 W·m−1·K−1, respectively [20, 21].
These thermal conduction coefficients are tested under steady-state conditions. However, the adsorption quantity of the adsorbent of the adsorption packed bed in actual working conditions changes over time, and the thermal conduction coefficient of the adsorbent varies under unsteady state conditions. The latter is called effective thermal conductivity. Effective thermal conductivity dependent on the adsorption/desorption rate is higher than that under steady-state conditions and directly affects the performance of the adsorber. The equation of effective thermal conductivity (Krupiczka equation) can be expressed as follows:

where keis the effective thermal conductivity of the adsorbent, kvis the thermal conductivity of the refrigerant vapor, ksis the thermal conductivity of the adsorbent and n is given as

where ε is the void rate of the adsorbent layer, which is the ratio of space between particle adsorbents to total volume of adsorbent.
No temperature difference between adsorbent and heat transfer flow is considered as precondition using Krupiczka equation.
The relationship between the diameter and the effective thermal conductivity of silica gel is shown in Fig. 1. The effective thermal conductivity can remain stable without obvious changes at adsorbent diameters of 305, 390, 513 and 605 µm. However, the effective thermal conductivity at an adsorbent diameter of 700 µm obviously decreases and is far below those of the four sizes of adsorbent. The effective thermal conductivity decreases with decreasing n according to Eq. (1). Increasing void ratio can lead to a decrease in n according to Eq. (2). The effective thermal conductivity of 700 µm is much lower than that of other diameters because of higher void ratio.
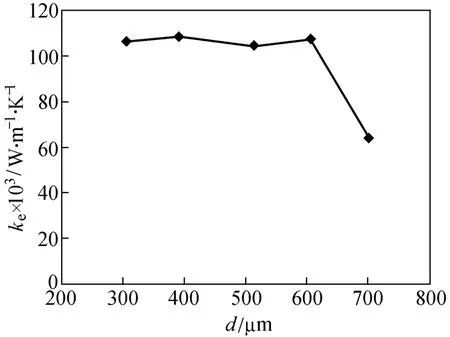
Figure 1 Effect of adsorbent diameter on effective thermal conductivity
2.3 Permeability
The mass transfer performance of the adsorber is an important characteristic of adsorption refrigeration. The mass transfer in the particle adsorbent of the packed bed in the adsorption chiller was studied. Therefrigerant flows through the adsorbent layer to reach the adsorbent surface by overcoming the mass transfer resistance in the adsorbent layer, and then goes deep into the micropore and mesopore to complete the adsorption process. Although the physical property of the adsorbent and the geometric property of the packed bed affect mass transfer, the mass transfer resistance in the adsorbent layer affected by the pressure drop in the adsorbent layer is an important parameter of mass transfer. The mass transfer in the adsorber improves with decreasing pressure drop in the adsorbent layer. The pressure drop in the adsorbent layer is given as

where Δp is the pressure drop in the adsorbent layer, μ is the viscosity coefficient of the refrigerant, u is the velocity though the adsorbent layer, l is the thickness of the adsorbent layer and kpis the permeability of the adsorbent. kp(Blake-Kozeny equation) can be expressed semi-empirically as
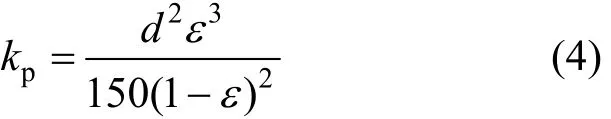
where d is the diameter of the adsorbent and ε is the void ratio.
Based on Eq. (3), Δp can be reduced to improve mass transfer when kpincreases. Based on Eqs. (3) and (4), mass transfer is enhanced when the diameter of the adsorbent d increases. In addition, the permeability kpis related to the square of the diameter d2, and it increases with increasing void ratio. The effect of adsorbent diameter on permeability is shown in Fig. 2. Permeability increases with increasing adsorbent diameter. The permeability at a diameter of 700 μm is obviously higher than that at other adsorbent diameters because of combined effect of higher void ratio and larger diameter.

Figure 2 Effect of diameter on permeability
3 EXPERIMENTAL
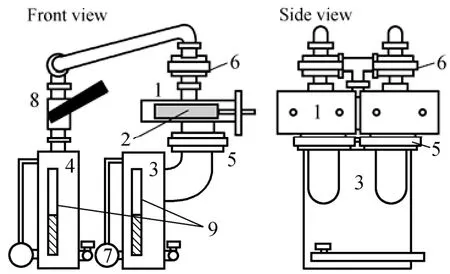
Figure 3 Experimental setup1—adsorber; 2—heat exchanger; 3—evaporator; 4—condenser; 5,6—air valve; 7—magnetic pumping; 8—valve; 9—level gage

Figure 4 Connection between TH and TM flows
To evaluate the effect on cooling performance, the adsorption-desorption experiments were carried out.
The experimental setup is shown in Fig. 3. The experimental installation consisted of adsorbers, evaporator, condenser, water pump, valves, level gage, and so on. Two adsorbers can provide continuous refrigeration. The liquid levels in the evaporator and condenser are observed through their level gages. The connection of heat transfer flow into the two adsorbers is presented in Fig. 4. Heat transfer flow (TH) can be used to heat the adsorber for desorption. TL flow can be for the evaporator, and TM flow can cool the adsorber to remove the sensible heat of the adsorbent. TM flow was also connected to the condenser to remove the latent heat of condensation. Pt temperature sensors were installed in the inlet/outlet of the TH, TL, and TM flows. And the processes of adsorber were described as follows:
(1) Adsorption process: One piece of adsorber was connected with TM flow. When the pressure in the evaporator reached the saturation pressure of the temperature TL flow in the evaporator, evaporation process of refrigerant began. And the refrigerant steam would flow from evaporator to the adsorber when the valve between the evaporator and adsorber was open. And the adsorption process in the adsorber would start.
(2) Desorption process: One piece of adsorber was connected with TH flow. The heat form TH flow provided the heat source of adsorber. The refrigerant steam from the adsorber would flow into the condenser with the valve between the adsorber and condenser opening.
Before the experiment, the packed bed with particle adsorbent must be dried.
The experimental conditions were listed as follows:
(1) The temperature of the TH, TM, and TL flows were at 353 K, 303 K, and 288 K, respectively.
(2) The flow rate in the different parts is shown in Table 2.

Table 2 Flow rates in the different parts
(3) The cycle times include 120, 150, 180, and 210 s.
The chilled water from the evaporator can provide the output power for refrigeration, which can be defined as follows:
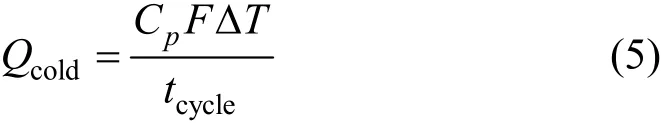
where Cpis the thermal capacity of flow in the evaporator, F is the flow rate in the evaporator, ΔT is the temperature difference between the inlet and outlet of the flow in the evaporator and tcycleis the cycle time of the experiment.
Figure 5 shows a set of output power curves under different diameters and cycle times. Output power initially increases and then decreases with increasing diameter under different cycle times. At a diameter of 605 μm, the output power with cycle times of 120 s and 180 s reach its maximum, respectively. This result can be attributed to the joined effect of heat and mass transfer in the adsorber on the output power of refrigeration. Considering the mass transfer, the permeability of the adsorbent layer can be improved with increasing diameter, leading to low pressure drop through the adsorbent layer. It can also help enhance mass transfer in the adsorbent.
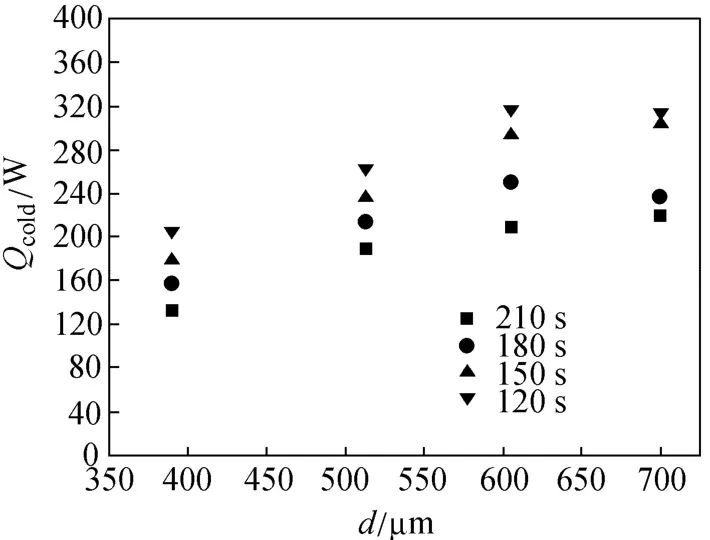
Figure 5 Effect of particle diameter on output power at different cycle times
In particular, the permeability of the adsorbent with a diameter of 700 μm is much higher compared with that of adsorbents with other diameters. Therefore, mass transfer performance would be the best at an adsorbent diameter of 700 μm. In terms of heat transfer, effective thermal conductivity can remain stable without obvious changes at adsorbent diameters of 305, 390, 513 and 605 µm. Effective thermal conductivity with adsorbent diameter of 700 µm obviously decreases and is much lower than that with other diameters. Considering the effects of mass and heat transfer, the output power of refrigeration reaches or be close to its maximum with an adsorbent diameter of 605 μm. In addition, output power with an adsorbent diameter of 700 μm is higher than that with adsorbent diameters of 390 and 513 μm. Hence, the effect of mass transfer on output power is greater than that of heat transfer. Otherwise, output power would increase when the cycle time is reduced under similar diameter conditions. The maximum output power is 320 W at a diameter of 605 μm and a cycle time of 120 s.
The effect of adsorbent diameter on heat and mass transfer is investigated. Effective thermal conductivity can remain stable without obvious changes at adsorbent diameters of 305, 390, 513 and 605 µm. Effective thermal conductivity obviously decreases at an adsorbent diameter of 700 µm. Permeability increases with increasing adsorbent diameter and void ratio. The permeability at an adsorbent diameter of 700 μm is obviously higher than that at other adsorbent diameters. Output power initially increases and then decreases with increasing adsorbent diameter under different cycle time conditions. Output power increases with decreasing cycle time under similar diameter conditions. The maximum output power is 320 W at an adsorbent diameter of 605 μm and a cycle time of 120 s. The results suggest that the effect of mass transfer on output power is greater than that of heat transfer under experimental conditions.
NOMENCLATURE

REFERENCES
1 Critoph, R.E., Zhong, Y., “Review of trends in solid sorption refrigeration and heat pumping technology”, J. Process Mech. Eng., 219, 285-300 (2005).
2 Wang, R.Z., Oliveira, R.G., “Adsorption refrigeration-An efficient way to make good use of waste heat and solar energy”, Prog. Energy Combust. Sci., 32, 424-458 (2006).
3 Chua, T., Ng, K.C., Chakraborty, A., Oo, N.M., Othman, M.A.,“Adsorption characteristics of silica gel-water systems”, J. Chem. Eng. Data, 47, 1177-1181 (2002).
4 Cho, S.H., Kim J.N., “Modeling of a silica gel / water adsorption cooling system”, Energy, 17 (9), 829-839 (1992).
5 Boelman, E.C., Saha, B.B., Kashiwagi, T., “Parametric study of asilica gel-water adsorption refrigeration cycle—the influence of thermal capacitance and heat exchanger UA-values on cooling capacity, power density and COP”, ASHRAE Transactions, 103, 139-148 (1997).
6 Saha, B.B., Kashiwagi, T., “Experimental investigation of an advanced adsorption refrigeration cycle”, ASHRAE Transactions, 103, 50-58 (1997).
7 Kakiuchi, H., Shimooka, S., Iwade, M., Oshima, K., Yamazaki, M., Terada, S., “Water vapor adsorbent FAM-Z02 and its applicability to adsorption heat pump”, J. Chem. Eng. Japan, 31, 273-277 (2005).
8 Kakiuchi, H., Shimooka, S., Iwade, M., Oshima, K., Yamazaki, M., Terada, S., “Novel water vapor adsorbent FAM-Z01 and its applicability to an adsorption heat pump”, J. Chem. Eng. Japan, 31, 361-364 (2005).
9 Critoph, R.E., Vogel, R., “Possible adsorption pairs for use in solar cooling”, Ambient Energy, 7, 183-190 (1986).
10 Meunier, F., Douss, N., “Performance of adsorption heat pumps. Active carbon-methanol and zeolite-water pairs”, ASHRAE Transactions, 2, 267-274 (1990).
11 Wang, Q., Chen, G.M., Han, B.Y., “Determination and analysis of the effective diffusivity of adsorption refrigeration working pair”, Acta Energiae Solaris Sinica, 22, 187-191 (2001).
12 Wang, W., Wang, R.Z., “Investigation of solid adsorption refrigeration cycle with non-equilibrium adsorption”, J. Eng. Thermophys., 22, 674-676 (2001).
13 Yang, P.Z., Chen, H.X., “Heat and mass transfer character on adsorption bed with consideration of non-equilibrium adsorption”, Cryogenics, (4), 15-20 (2008).
14 Demir, H., Mobedi, M., Ulku, S., “Effects of porosity on heat and mass transfer in a granular adsorbent bed”, Int. Commun. Heat Mass Transfer, 36, 372-377 (2009).
15 Tamainot-Telto, Z., Critoph, R.E., “Monolithic carbon for sorption refrigeration and heat pump applications”, Appl. Therm. Eng., 21, 37-52 (2001).
16 Leong, K.C., Liu, Y., “Numerical modeling of combined heat and mass transfer in the adsorbent bed of a zeolite/water cooling system”, Appl. Therm. Eng., 24, 2359-2374 (2004).
17 Wang, L.W., Tamainot-Telto, Z., Thorpe, R., Critoph, R.E., Metcalf, S.J., Wang, R.Z., “Study of thermal conductivity, permeability, and adsorption performance of consolidated composite activated carbon adsorbent for refrigeration”, Renewable Energy, 36, 2062-2066 (2011).
18 Wang, L.W., Metcalf, S.J., Critoph, R.E., Tamainot-Telto, Z., Thorpe, R., “Two types of natural graphite host matrix for composite activated carbon adsorbents”, Appl. Therm. Eng., 50 (2), 1652-1657 (2013).
19 Tian, B., Jin, Z.Q., Wei, D.S., Wang, L.W., Wang, R.Z., “Testing on permeability of compacted graphite”, CIESC J., 61, 35-38 (2010). (in Chinese)
20 Jin, Z.Q., Tian, B., Wang, L.W., Wang, R.Z., “Study on thermal conductivities and permeabilities of AC/ENG consolidated composite adsorbents”, Journal of Shanghai Jiaotong University, 45, 866-869 (2011).
21 Guilleminot, J.J., Chalfen, J.B, Choisier, A., “Heat and mass transfer characteristics of composites for adsorption heat pumps”, In: Proceedings of the International Absorption Heat Pump Conference, ASME, AES-Vol. 31, 401-405, New Orleans (1994).
2012-09-16, accepted 2012-11-19.
* Supported by the Chinese Academy of Science Visiting Professorship for Senior International Scientists project (2009Z2-1973).
** To whom correspondence should be addressed. E-mail: hezh@ms.giec.ac.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Soft Sensor Model Derived from Wiener Model Structure: Modeling and Identification*
- Kinetics of Forward Extraction of Boric Acid from Salt Lake Brine by 2-Ethyl-1,3-hexanediol in Toluene Using Single Drop Technique*
- Influence of Solvent on Reaction Path to Synthesis of Methyl N-Phenyl Carbamate from Aniline, CO2and Methanol*
- High-Thermal Conductive Coating Used on Metal Heat Exchanger*
- A Facile Route for Synthesis of LiFePO4/C Cathode Material with Nano-sized Primary Particles*
- Filtering Surface Water with a Polyurethane-based Hollow Fiber Membrane: Effects of Operating Pressure on Membrane Fouling*
