Kinetics of Forward Extraction of Boric Acid from Salt Lake Brine by 2-Ethyl-1,3-hexanediol in Toluene Using Single Drop Technique*
2014-07-18Jianhua吕建华LIUJidong刘继东SUNYujie孙玉洁andLIChunli李春利SchoolofChemicalEngineeringHebeiUniversityofTechnologyTianjin300130China
LÜ Jianhua (吕建华), LIU Jidong (刘继东), SUN Yujie (孙玉洁) and LI Chunli (李春利)**School of Chemical Engineering, Hebei University of Technology, Tianjin 300130, China
Kinetics of Forward Extraction of Boric Acid from Salt Lake Brine by 2-Ethyl-1,3-hexanediol in Toluene Using Single Drop Technique*
LÜ Jianhua (吕建华), LIU Jidong (刘继东), SUN Yujie (孙玉洁) and LI Chunli (李春利)**
School of Chemical Engineering, Hebei University of Technology, Tianjin 300130, China
The kinetics of forward extraction of boric acid from salt lake brine by 2-ethyl-1,3-hexanediol in toluene was investigated using the single drop technique. The factors affecting the extraction rate include interfacial area between aqueous phase and organic phase, initial concentration of boric acid in aqueous phase, initial concentration of 2-ethyl-1,3-hexanediol in organic phase, and extraction temperature. The experimental results show that the extraction rate increases with the increase of the initial concentration of boric acid and 2-ethyl-1,3-hexanediol, interfacial area of two phases, and temperature. With the temperature-dependence study, it is showed that the extraction is a diffusion-controlled process. The kinetic equation is presented for pH 1.0 in the aqueous phase and temperature of 318 K.
kinetics, boric acid, extraction, mass transfer, single drop technique
1 INTRODUCTION
Boric acid is widely used in industry due to its excellent characteristics, such as glass, ceramic, detergent, plastic, agricultural and textile industries [1]. Although the boric acid production is increasing, the supply does not meet the demand. The raw materials used to produce boric acid are boron ore and salt lake brine. After decades of mining, boron ore resources decline sharply. Brine contains quite a lot of boric acid, but its utilization is still at the primary stage because of low concentration of boric acid [2]. Solvent extraction has many advantages for extracting low-concentration boric acid. Some alcohols present good properties for extracting boric acid from salt lake brine, such as monohydric alcohol [3-5], dibasic alcohol [6-8] and mixed alcohols [9, 10].
Lü et al. studied the kinetics of boric acid extraction from salt lake brine with monohydric alcohol [11]. Although the extraction effect of dibasic alcohol is better than monohydric alcohol, the kinetics has not been reported.
The single drop technique is an appropriate technique for studying kinetics of extraction [12-14]. In this study, the kinetics of forward extraction of boric acid from salt lake brine by 2-ethyl-1,3-hexanediol in toluene is investigated. The effects of interfacial area between aqueous and organic phases, initial concentration of boric acid in aqueous phase, initial concentration of 2-ethyl-1,3-hexanediol in organic phase, and extraction temperature on the extraction rate are examined. The results provide reliable basic data for the selection and design of industrial device.
2 EXPERIMENTAL
2.1 Chemicals and reagents
The pH value is one of the most important factors in the extraction. H3BO3polymerizes and gradually transforms to polymeric borate anions in the range of pH 3.0 to 5.0. In this pH range, H3BO3does not react with alcohol. When the pH value is less than 3.0, almost all boric acid is in the molecular form of H3BO3[15], and the change of [H+] has little influence on the coefficient of distribution [11]. The salt lake brine used in this study was a crystal mother liquid of boric acid, from Jinaier salt lake, Qinghai province, China, the pH value of which is approximately equal to 1.0, so the experiment on extraction rate were carried out at pH=1.0.
The component and physical properties of the salt lake brine are listed in Table 1. 2-ethyl-1,3-hexanediol was supplied from Jinlong Industry, Tianjin, China. Toluene was supplied from Jiangtian Chemical Technology, Tianjin, China. All chemicals were of analytical reagent grade and used without further purification.

Table 1 Component and physical properties of the salt lake brine (293.15 K)
2.2 Experimental setup and operation
The experimental setup of single drop technique is shown in Fig. 1. The drop size was changed by usingcapillaries with different sizes (1.0, 1.5, 2.0, 2.5, 3.0, and 3.5 mm). The velocity of organic phase was controlled by the needle valve at 100±5 drops per minute. The contact-time of the two phases was controlled by the height of the aqueous phase (150, 300, 450, or 600 mm). The average volume of drops was calculated from the volume of 400 drops collected in a graduated flask. For a particular height of aqueous phase, the contact-time of organic drop with aqueous phase was calculated by averaging the rising time of 10 drops. The concentrations of boric acid in aqueous and organic phases were estimated by double indicator titration method [16].

Figure 1 Schematic diagram of experimental set-up of single drop technique1—dropping funnel; 2—column of organic phase; 3—jacketed pipe of constant temperature bath; 4—needle valve; 5—piston; 6—pipe of flowing back; 7—capillary; 8—extraction column; 9—overflow vent; 10—feed hopper of aqueous phase
2.3 Data processing
The r is defined as the amount of boric acid through unit area per unit time. At a constant temperature, r can be calculated by

where r is the rate of mass transfer, A and V are the surface area and volume of a drop, [H3BO3] is the concentration of boric acid, t is the rising time of droplet, and subscript o refers to organic phase.
For the single drop technique, a great shortcoming is the end effect, which is the mass transfer of boric acid in the period for droplets to form at the bottom of extraction column and coalesce at the top of the column, but is not considered in Eq. (1). Therefore, the mass transfer per unit interfacial area is measured by changing the height of columns, then the slopes of lines represent33Od[H BO]/dt in Eq. (1), so that the experimental results are not affected by the end effect (the intercept of line shows the influence of the end effect). In the process of drop rising in the extraction column, it is assumed that the drop behaves as a rigid sphere and the organic phase is relatively stable. The volume V is the average volume of drops and the surface area A is calculated with the average volume.
The experiments of single drop technique were carried out under the condition far from extraction equilibrium. Horner [17] has pointed out that reverse mass transfer rate is far less than forward mass transfer rate. Therefore, the reverse mass transfer rate of H3BO3from organic phase to aqueous phase is neglected in this study. The measurement results are forward extraction rate of H3BO3from aqueous phase to organic phase. In addition, the volume of aqueous phase is much larger than that of organic phase, so changes of H3BO3concentration in aqueous phase are neglected. The end effect of drops at the top and bottom of extraction column is the same. At fixed [H+] and temperature, the extraction rate of boric acid is expressed as

where k is extraction rate constant, a and b are the reaction order with respect to [H3BO3] and [R(OH)2], respectively.
Equation (2) can be written as
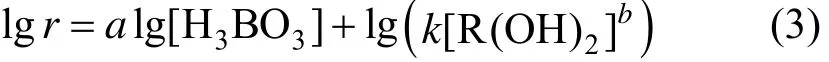
For fixed [R(OH)2], the relationship between lgr and lg[H3BO3] is linear with slope a; for fixed [H3BO3], the relationship between lgr and lg[R(OH)2] is linear with slope b. From Eq. (2), k value can be calculated.
The effect of temperature on the extraction rate can be expressed by the Arrhenius equation. The value of apparent activation energy (Ea) can be calculated by [18]

where R is the universal gas constant (8.314 J·mol−1·K−1), T is the reaction temperature, and B is the constant in the Arrhenius equation. The plot of lnk vs. 1/T yields a straight line, whose slope multiplying (R−) is the activation energy (Ea).
According to classic transition state theory [19, 20], we have

where h is Planck’s constant (6.626068×10−34J⋅s), k∗is the Boltzmann constant (1.3806503×10−23J·K−1), H± Δ is the enthalpy of activation, and S±Δ is the entropy of activation.
Equation (5) shows that the plot of ln(/)rh k T∗vs. 1/T is a straight line with slope (/HR±−Δ) and intercept332+). Thus theln([H BO][R(OH)]) a b value of G±Δ at a particular temperature may be evaluated as

where G±Δ is the Gibbs free energy of activation.
3 RESULTS AND DISCUSSION
3.1 Effect of specific surface area on extraction rate Figure 2 presents the effect of specific surface area on extraction rate at five volume ratios of 2-ethyl-1,3-hexanediol to toluene (1︰1, 2︰3, 3︰8, 1︰5, 1︰9) with fixed H3BO3concentration. Different specific surface areas are from capillaries with different sizes. The chemical reaction between boric acid and 2-ethyl-1,3-hexanediol in the extraction process may occur in the bulk aqueous phase or organic phase or at the interface. Fig. 2 shows that the extraction rate of boric acid is directly proportional to the specific surface area. Because the increase in H3BO3concentration is directly proportional to the number of H3BO3molecules crossing the interface, the chemical reaction occurs at the interface. The result is similar to the extraction results of 2-ethyl hexanol and boric acid [11]. The extraction rate by 2-ethyl-1,3-hexanediol is higher than the transfer rate of boric acid.

Figure 2 Effect of specific surface area on extraction rate at different concentrations of 2-ethyl-1,3-hexanediol ([H3BO3]=
3.2 Effect of initial boric acid concentration in aqueous phase on extraction rate
Table 2 shows the effect of initial boric acid concentration on extraction rate at fixed 2-ethyl-1,3-hexanediol concentration. The extraction rate of boric acid increases with the increase of boric acid concentration in aqueous phase, since more boric acid molecules are brought to the interface and participate in the esterification reaction.
3.3 Effect of initial concentrations of 2-ethyl-1,3-hexanediol in organic phase on extraction rate
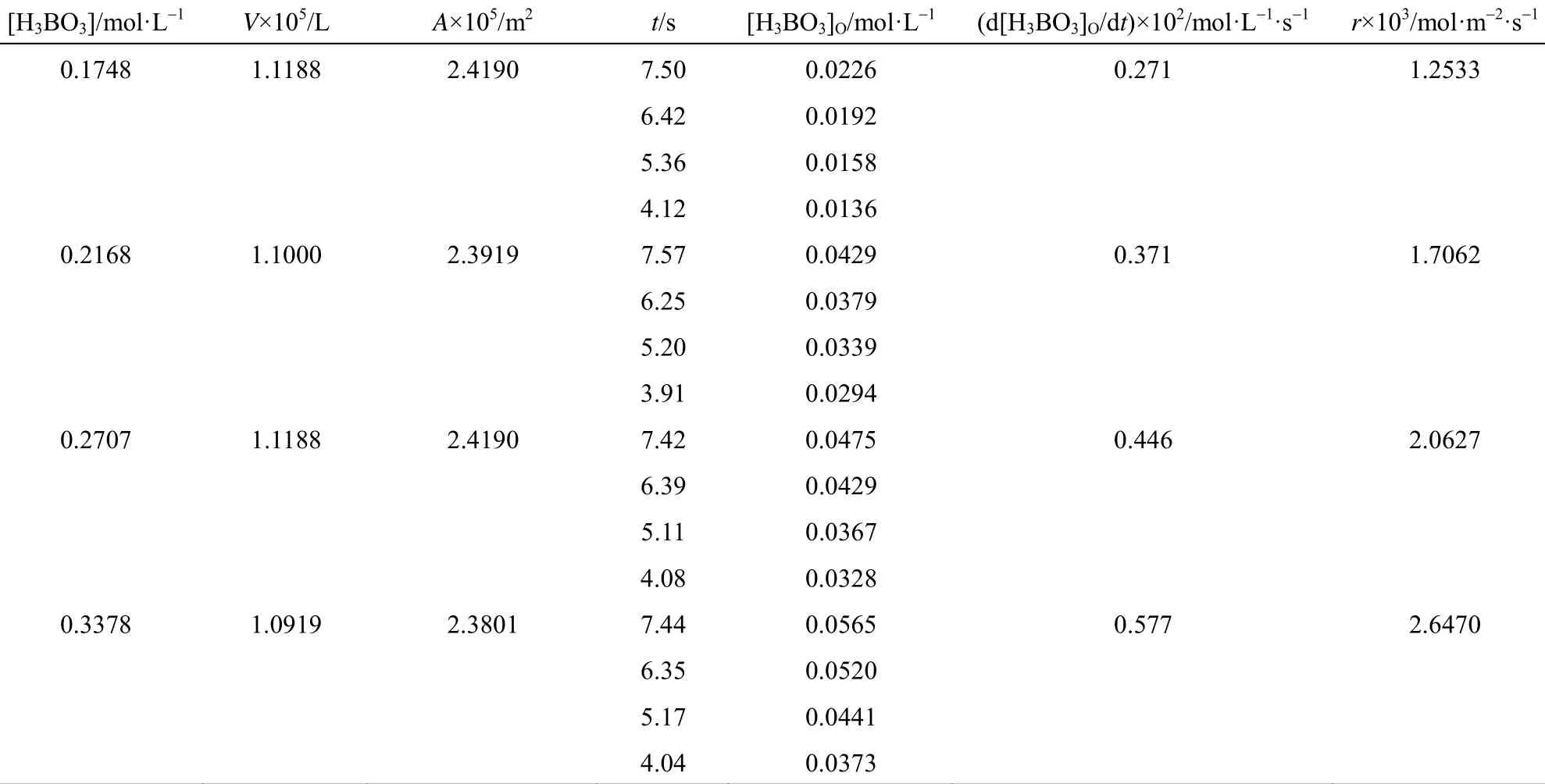
Table 2 Effect of boric acid initial concentration in aqueous phase on extraction rate([R(OH)2]=0.9205 mol·L−1, pH=1.0, T=318 K)
The effect of initial concentrations of 2-ethyl-1,3-hexanediol on extraction rate at fixed H3BO3concentration is shown in Table 3. The extraction rate increases with the increase of initial concentrations of 2-ethyl-1,3-hexanediol. The variation of 2-ethyl-1,3-hexanediol concentration changes the drop diameter from the same capillary, resulting in different specific surface area. In order to evaluate the effects of initial concentrations of 2-ethyl-1,3-hexanediol on extraction rate in the same specific surface area, we define the corrected extraction rate (r′) as the one at certain specific surface area and volume of drops. The corrected extraction rates are calculated by the linear relation between extraction rate (r) and specific surface
area in Fig. 2 and shown in Table 3, with the drop volume of 1.1188×10−5L and its specific surface area of 2162 m−1.
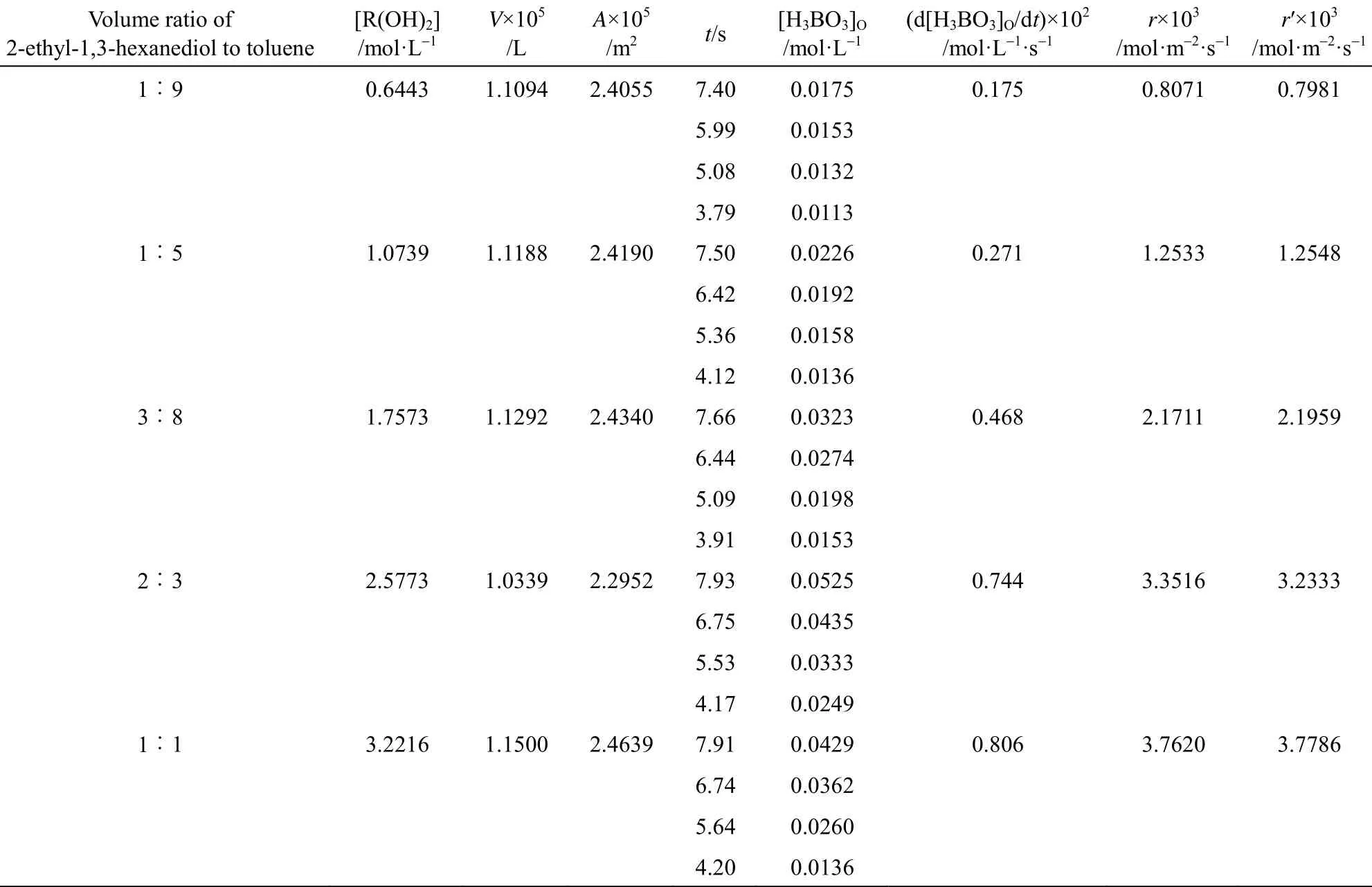
Table 3 Effect of initial 2-ethyl-1,3-hexanediol concentration in organic phase on extraction rate ([H3BO3]=0.1748 mol·L−1, pH=1.0, T=318 K )

Table 4 Effect of temperature on extraction rate ([H3BO3]=0.1748 mol·L−1, [R(OH)2]=1.0739 mol·L−1, pH=1.0)
Equation (2) is used for regression of the data in Tables 2 and 3 with the linear least square method. The equation of extraction kinetics at 318 K can be expressed as
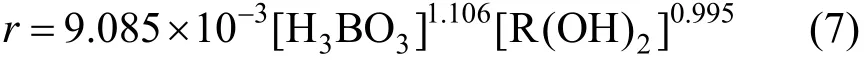
The extraction rate constant is k=9.085×10−3L2.101·mol−1.101·m−2·s−1, with relative standard deviation of 5.92%. Thus the extraction reaction is approximately first-order with respect to 2-ethyl-1,3-hexanediol and H3BO3.
3.4 Effect of temperature on extraction rate
The effect of temperature on extraction rate at fixed concentrations of H3BO3and 2-ethyl-1,3-hexanediol is shown in Table 4. The extraction rate increases with the increase of extraction temperature. The relationship between extraction rate constant and temperature is shown in Fig. 3.

Figure 3 Effect of temperature on reaction rate constant ([H3BO3]=0.1748 mol·L−1, [R(OH)2]=1.0739 mol·L−1, pH=1)
In general, the controlling step of chemical reaction can be judged by the value of activation energy. Wang et al. [21] have classified the controlling step of this system in this way. When the activation energy is larger than 42 kJ·mol−1, the controlling step is chemical reaction; when the activation energy is less than 13 kJ·mol−1, the reaction is diffusion-controlled; when the activation energy is 20-34 kJ·mol−1, the rates of mass transfer and reaction are in the same order of magnitude. In this study, the activation energy is Ea= 10 kJ·mol−1from Eq. (4), so the reaction of extracting boric acid is considered as a diffusion-controlled process.
calculated results are: ΔH± = 7 kJ·mol−1, ΔS± =−262 J·mol−1·K−1, and ΔG± = 91 kJ·mol−1.
3.5 Analysis on reaction mechanism
The reaction between boric acid and 2-ethyl-1,3-hexanediol is

In the experiment, the aqueous phase containing boric acid and organic phase were mixed in a separating funnel. After a period of time the concentration of boric acid in aqueous phase was not changed, and the boric acid concentrations in organic and aqueous phases were analyzed. At the ratio of O/A of 2︰1, 1︰1, 1︰2, 1︰3 and 1︰4, the distribution coefficients at different concentrations of 2-ethyl-1,3-hexanediol were measured by the slope method. B(OHare first formed by H3BO3ionization in acid solution, and one boric acid molecule and one 2-ethyl-1,3-hexanediol molecule react to form an intermediate

Figure 4 Distribution coefficient and 2-ethyl-1,3-hexanediol concentration
The straight lines of lgD against lg[R(OH)2] and their slops are shown in Fig. 4. These slopes are approximately equal to 2.0. It indicates that the ester form of boric acid is H3BO3·2R(OH)2. Eq. (7) shows that the extraction rate is nearly proportional to concentrations of boric acid and 2-ethyl-1,3-hexanediol, while Fig. 4 shows that the ultimate ester form of boric acid is H3BO3·2R(OH)2. These indicate that the extraction reaction between boric acid and 2-ethyl-1,3-hexanediol is a stepwise reaction. Traces of H+andmolecule, which reacts with one 2-ethyl-1,3-hexanediol molecule to the ultimate ester form H3BO3·2R(OH)2. The main reactions between boric acid and 2-ethyl-1,3-hexanediol in this extraction are as follows:

When the ionization equilibrium of H3BO3is broken by esterification of R(OH)2and4B(OH)−forms and participates in the reaction. Because the extraction reaction can be considered as diffusion-controlled, the reaction rates of reactions (12), (13) and (14) are very fast. However, since the diffusion rate of R(OH)2is not large enough to supply the reaction, reaction rate (14) is decreased. By increasing concentrations of boric acid in aqueous phase and 2-ethyl-1,3-hexanediol in organic phase, the diffusion rate is increased, so the overall extraction rate increases. In general, the chemical reaction mechanism can be judged by S±(OH)−, more 4 Δ. The value of S±Δ becomes negative when solvent molecules are tightly attached to H3BO3(SN2 mechanism), and S±Δ becomes positive when solvent molecules dissociate from H3BO3(SN1 mechanism). In this experiment, reaction (14) occurs via the SN2 mechanism (S±Δ=−261.3688 J·mol−1·K−1).
4 CONCLUSIONS
The reaction between boric acid and 2-ethyl-1,3-hexanediol occurs at the interface between the two phases and the extraction reaction is diffusion-controlled. The values of activation energy (Ea), enthalpy of activation (H±Δ), entropy of activation S±Δ, and G±Δ are obtained.
The kinetic equation of extraction is obtained, and the extraction reaction is approximately first-order with respect to concentrations of 2-ethyl-1,3-hexanediol and H3BO3. The main reaction steps with 2-ethyl-1,3-hexanediol and boric acid is described by the SN2 mechanism.
NOMENCLATURE


Superscripts

Subscripts

REFERENCES
1 Mergen, A., Demirhan, M.H., Bilen, M., “Processing of boric acid from borax by a wet chemical method”, Advanced Powder Technology, 14 (3), 279-293 (2003).
2 Li, J., Fan, Z.G., Liu, Y.L., Liu, S.L., Jiang, T., Xi, Z.P., “Preparation of boric acid from low-grade ascharite and recovery of magnesium sulfate”, Transactions of Nonferrous Metals Society of China, 20 (6), 1161-1165 (2010).
3 Vinogradov, E.E., “Extraction of boric acid with isoamyl alcohol from hydrochloric acid solutions”, Zh. Strukt. Khim., 7, 2813-2816 (1962).
4 Brown, C.G., Sanderson, B.R., “Solvent extraction of boron”, Chem. Ind., 1978a, 68-73 (1978).
5 Vinogradov, E.E., Shamiryan, P.S., Tarasova, G.N., Ivanov, A.A., Panasyuk, G.P., “The system, boric acid, lithium chloride,2-ethylhexanol”, Zh. Neorg. Khim., 46, 860-865 (2001).
6 Brown, C.G., Sanderson, B.R, “Solvent extraction of boron”, Chem. Ind., 1978b, 68-73 (1978).
7 Shvarts, E.M., Kalve, I., Telzhenskaya, P.N., “Extraction of boric acid in the boric acid, sulphuric acid, water system”, Latv. Kim. Z., 3/4, 88-93 (1995).
8 Svares, E., Putnina, A., Kalve, I., Sennikova, L.M., Kirchanov, A.,“Extraction of boric acid by normal 1,3-diols”, Zh. Neorg. Khim., 28, 2333-2337 (1983).
9 Lü, J.H., Sun, Y.J., Li, C.L., “Mass transfer coefficient of extracting boric acid by 2-ethyl-1,3-hexanediol”, CIESC Journal, 63 (S1), 145-153 (2012). (in Chinese)
10 Ayers, P., Dudeney, A.W.L., Kahraman, F., “Solvent extraction of boron with 2-ethyl-1,3-hexanediol and 2-chloro-4-(1,1,3,3-tetramethylbutyl)-6-methylol-phenol”, Journal of Inorganic and Nuclear Chemistry, 43, 2097-2100 (1981).
11 Lü, J.H., Li, C.L., Geng, H., “Kinetics for extraction of boric acid from salt lake brine by 2-ethyl hexanol-toluene”, CIESC Journal, 61 (12), 3124-3129 (2010).
12 Zhu, P., Hong, D., Wu, J.H., Qian, G.R., “Kinetics of forward extraction of Ti(IV) from H2SO4 medium by P507 in kerosene using the single drop technique”, Rare Metals, 30 (1), 1-7 (2011).
13 Biswas, R.K., Hayat, M.A., “Kinetics of extraction of Zr(IV) from chloride solution after 30-day ageing by D2EHPA using the single drop technique”, Hydrometallurgy, 75, 45-54 (2004).
14 Muhammad, I.S., Md, F.B., Md, S.J., Bahruddin, S., “Kinetics of lanthanum (III) extraction from nitrate-acetato medium by Cyanex 272 in toluene using the single drop technique”, Hydrometallurgy, 67, 45-52 (2002).
15 Ingri, N., Lagerstrom, G., Frydman, M., Sillen, L.G., “Equilibrium studies of polyanions. 2. Polyborates in NaClO4medium”, Acta Chemica Scandinavica, 11, 1034-1058 (1957).
16 Qinghai Institute of Salt Lakes., Analysis of Brine and Salt, Science Press, Beijing (1973). (in Chinese)
17 Horner, D.E., “Process for recovery of strontium values from fission product waste solutions”, U.S. Pat., 3122414(1964).
18 Pannetier, X., Souchay, P., Chemical Kinetics, Elsevier, Amsterdam (1967).
19 Biswas, R.K., Habib, M.A., Bari, M.F., “Kinetics of backward extraction of Mn(II) from Mn-D2EHP complex in kerosene to hydrochloric acid medium using single drop technique”, Hydrometallurgy, 46 (3), 493-362 (1997).
20 Maron, S.H., Frutton, F., Principles of Physical Chemistry, 4th edition, MacMillan, New York, 571-584 (1975).
21 Wang, K.Y., Cheng, B.C., Shu, W.Y., Solvent Extraction Chemistry, Central South University of Technology Press, Hunan, 74 (1991).
2012-09-21, accepted 2012-12-17.
* Supported by the Natural Science Foundation of the Education Department Hebei Province (2009426), and Educational Commission of Hebei Province (ZH2011221).
** To whom correspondence should be addressed. E-mail: ctstljh@hebut.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Soft Sensor Model Derived from Wiener Model Structure: Modeling and Identification*
- Influence of Solvent on Reaction Path to Synthesis of Methyl N-Phenyl Carbamate from Aniline, CO2and Methanol*
- Effect of Adsorbent Diameter on the Performance of Adsorption Refrigeration*
- High-Thermal Conductive Coating Used on Metal Heat Exchanger*
- A Facile Route for Synthesis of LiFePO4/C Cathode Material with Nano-sized Primary Particles*
- Filtering Surface Water with a Polyurethane-based Hollow Fiber Membrane: Effects of Operating Pressure on Membrane Fouling*
