River Water Purification via a Coagulation-Porous Ceramic Membrane Hybrid Process*
2014-07-18ZHANGHuiqin张荟钦ZHONGZhaoxiang仲兆祥LIWeixing李卫星XINGWeihong邢卫红andJINWanqin金万勤
ZHANG Huiqin (张荟钦), ZHONG Zhaoxiang (仲兆祥), LI Weixing (李卫星), XING Weihong (邢卫红)** and JIN Wanqin (金万勤)
State Key Laboratory of Materials-Oriented Chemical Engineering, Membrane Science and Technology Research Center, Nanjing University of Technology, Nanjing 210009, China
River Water Purification via a Coagulation-Porous Ceramic Membrane Hybrid Process*
ZHANG Huiqin (张荟钦), ZHONG Zhaoxiang (仲兆祥), LI Weixing (李卫星), XING Weihong (邢卫红)** and JIN Wanqin (金万勤)
State Key Laboratory of Materials-Oriented Chemical Engineering, Membrane Science and Technology Research Center, Nanjing University of Technology, Nanjing 210009, China
Membrane filtration technology combined with coagulation is widely used to purify river water. In this study, microfiltration (MF) and ultrafiltration (UF) ceramic membranes were combined with coagulation to treat local river water located at Xinghua, Jiangsu province, China. The operation parameters, fouling mechanism and pilot-scale tests were investigated. The results show that the pore size of membrane has small effect on the pseudo-steady flux for dead-end filtration, and the increase of flux in MF process is more than that in UF process for cross-flow filtration with the same increase of cross-flow velocity. The membrane pore size has little influence on the water quality. The analysis on membrane fouling mechanism shows that the cake filtration has significant influence on the pseudo-steady flux and water quality for the membrane with pore size of 50, 200 and 500 nm. For the membrane with pore size of 200 nm and backwashing employed in our pilot study, a constant flux of 150 L·m−2·h−1was reached during stable operation, with the removal efficiency of turbidity, total organic carbon (TOC) and UV254 higher than 99%, 45% and 48%, respectively. The study demonstrates that coagulation-porous ceramic membrane hybrid process is a reliable method for river water purification.
ceramic membrane, surface water, membrane fouling mechanism, pilot study
1 INTRODUCTION
Lack of clean fresh water becomes a potential risk to public health [1]. The rapid increase of contaminants such as heavy metals, bacteria, virus, and disinfection by-products in the source water strongly affects the quality of drinking water [2-5]. Traditional processes (coagulation + settlement + sand filtration + chlorination) are often used to treat the contaminated water in many waterworks, but it is difficult for the treated water to meet the drinking water standards.
In recent years, membrane technologies such as ultrafiltration (UF) and microfiltration (MF) are used to purify river water in China. Polymeric UF or MF membrane has been widely used in water treatment [6]. For example, polyvinyl chloride membrane and polyvinylidene fluoride membrane were employed to purify the water from the Tai Lake (Wuxi, China) and Huangpu River (Shanghai, China), respectively [7, 8]. Ceramic membranes have also been used in pharmaceutical, electronic, petrochemical, metallurgical and environmental fields, because of their substantial thermal, chemical and mechanical stability compared to organic membranes. Recently, with the decrease in price, ceramic membranes have been adopted to treat surface water [9-12]. Hofs et al. investigated four ceramic membranes (TiO2, ZrO2, Al2O3, and SiC) and one polymeric membrane (polyethersulfone-polyvinylpyridine) and showed that the ceramic membranes had high flux and high removal rate for natural organic matter (NOM) and UV254 [11].
NOM plays an important role in membrane fouling [13]. Coagulation pretreatment is reported to be an effective method to alleviate the fouling by NOM [14, 15]. The performance of a coagulation-ceramic membrane system depends on characteristics of feed water. In-line coagulation prior to ceramic microfiltration was used for low turbidity (1 NTU) water treatment. The flux was maintained about 250 L·m−2·h−1[16]. The hybrid process of coagulation and ceramic membrane filtration was adopted to treat Ruhr River water, and stable operation at a constant flux of 80 L·m−2·h−1was obtained [17].
In our previous work, the effect of coagulation on this hybrid process was reported [18]. More work is needed to understand membrane fouling mechanism and stability of ceramic membranes processes. In this study, a coagulation-porous ceramic membrane hybrid process is used to purify the micro-polluted source water of Hengjing River in East China. Hemia model is employed to investigate the fouling mechanism for membranes with different pore sizes. River water is used as the feed water to investigate the effects of operation modes on ceramic membrane filtration. A pilot study is employed to study the feasibility of ceramic membranes for river water purification.
2 MATERIALS AND METHODS
2.1 Feed water and experimental system
Feed water was taken from Hengjing River,which is one of the drinking water sources for the city of Xinghua, China and a typical water source for small cities in East China. The experiment was carried out in winter with rich humic acid substance in the river. The schematic diagram of the membrane filtration process for river water purification is shown in Fig. 1. It was carried out in open mode, in which the permeate did not return to the feed tank.
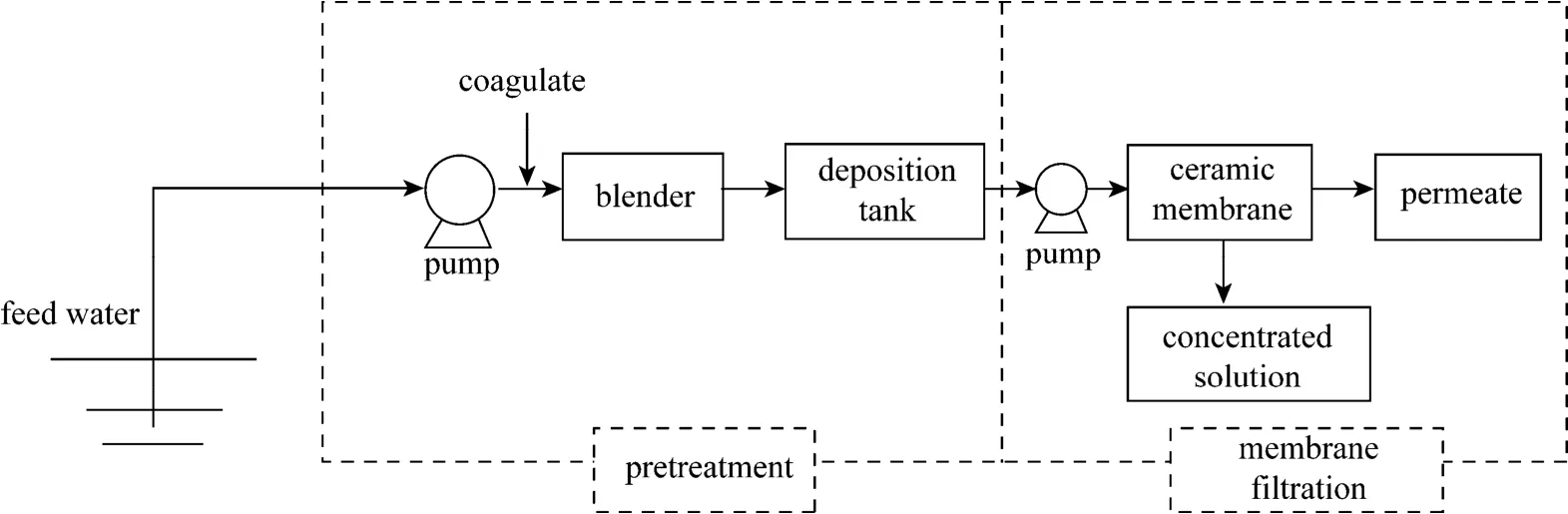
Figure 1 Schematic diagram of the experiment
2.2 Analytical methods
The concentrations of metal ions in the feed water and permeate were measured by inductively coupled plasma atomic emission spectroscopy (ICP, Optima 2000 DV, Perkin Elmer, USA). The concentrations of natural organic matters were determined by the TOC (TOC-VCPH, Shimadzu, Japan) and UV254(Gold S54, Shanghai Lengguang Technology Co. Ltd, China). The turbidity was measured with a turbidity meter (2100N, HACH, USA). Surface morphology and cross-section morphology of the membranes were observed by field emission scanning electron microscopy (FE-SEM, S-4800, Hitachi, Japan).
2.3 Membranes
The 19-channel ceramic membranes were used, supplied by Jiangsu Jiuwu Hitech Co., Ltd. (China), with external diameter of 31 mm, channel diameter of 4 mm, and membrane area of 0.1 m2. The mean pore size of membrane was 20, 50, 200 and 500 nm, determined by gas bubble pressure method. The pure water fluxes of membranes with pore size of 20, 50, 200 and 500 nm were 161.5, 568.9, 614.2 and 891.9 L·m−2·h−1(0.1 MPa, 20 °C), respectively. For membranes with pore size of 20 and 50 nm, the membrane material was ZrO2/α-Al2O3; for those with pore size of 200 and 500 nm, the material was α-Al2O3/α-Al2O3.
3 MODELING FOR MEMBRANE FOULING PROCESS
Constant pressure blocking filtration laws to Newtonian fluids was first reported by Hermia in 1982 [19]. Membrane fouling involves four mechanisms: standard blocking, intermediate blocking, complete blocking, and cake filtration. A general equation for these mechanisms is given
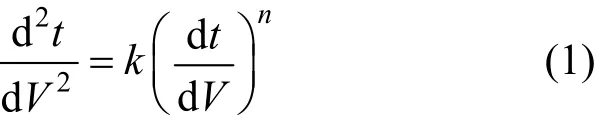
For n=0, the membrane fouling mechanism is cake filtration, with all particles deposited in the filtration cake at the membrane surface. For n=1, the fouling mechanism is intermediate blocking, with some particles entering and blocking some pores and others stay in the cake. For n=1.5, the fouling mechanism is general blocking, with all particles arriving at the membrane deposited on pore walls. For n=2, the membrane fouling is complete blocking, with particles blocking some pores without superposition of particles [20, 21].
Yuan et al. [22, 23] successfully applied this model to describe membrane fouling mechanism for MF process of humic acid aqueous solutions. The mathematical expressions of the four fouling mechanisms are described by Salahi et al. [24] and Beatriz et al [25].
For cake filtration (n=0), the flux vs. t is

For intermediate blocking (n=1), the flux vs. t is given by

For standard blocking (n=1.5), the relationship is

For complete blocking (n=2), the expression is

In this study, the fouling mechanism of the membrane with different pore sizes was evaluated by Eqs. (2)-(5). All the simulations were implemented with Origin 8 (OriginLab, USA).
4 RESULTS AND DISCUSSION
4.1 Effect of pore size on flux
Figure 2 shows the fluxes of membranes with different pore sizes for purification of river water. In the first hour of filtration, the flux decreased significantly, and then turned to be stable. The pseudo-steady flux was 52.6, 69.0, 66.8 and 93.1 L·m−2·h−1(75 kPa, 20 °C) for four membranes under dead-end conditions after filtration for 7 h. As the average pore size increases, the flux decreases more rapidly. The flux decline is similar for the membranes with pore size of 50, 200 and 500 nm, but it is different with the pore size of 20 nm. This indicates that the mechanism of membrane fouling for the membrane with pore size of 20 nm is different from that for other three membranes.

Figure 2 Effects of pore size on flux in dead-end filtration (75 kPa, 20 °C)
The analysis on membrane fouling resistance is employed to determine Rm/RTfor this filtration. Rmand RTare calculated by Darcy’s law,

The methods were described in details previously [26]. The results are shown in Table 1. As the pore size increases, Rm/RTdecreases. For the membranes with pore size 50, 200 and 500 nm, the difference in Rm/RTis regular, but the values are only one third of that with pore size of 20 nm.
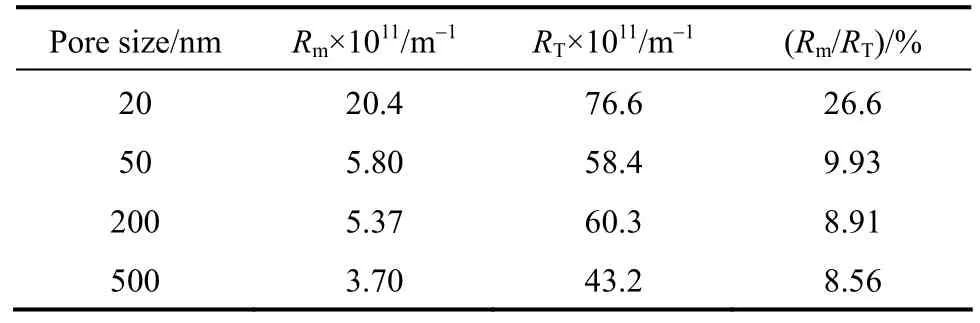
Table 1 Resistances of the membranes with different pore sizes
The quality of permeate with different membrane pore sizes is shown in Table 2. The pore size in the range of 20 nm to 500 nm has little influence on the water quality. Fig. 3 shows the field-emission scanning electron microscope (FE-SEM) images for the membrane surface with pore size of 50 nm withtrans-membrane pressure (TMP) of 0.1 MPa and cross-flow velocity (CFV) of 1 m·s−1. The fouling layer on the membrane surface is denser than a new membrane. During the filtration, the fouling layer that acts as a dynamic membrane has a more significant influence on the water quality than the ceramic membrane. Therefore, water quality is affected by pore size and porosity of the cake rather than the pore size of membrane. The results suggest that ceramic membrane with large pore sizes (200 nm or 500 nm) can be used for river water purification.

Table 2 Quality of feed water and permeate with different membranes
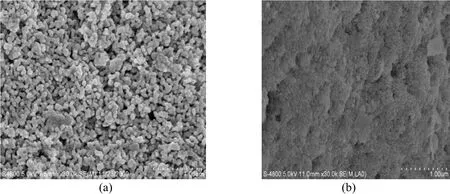
Figure 3 SEM images of new membrane (a) and fouled membrane (b) with pore size 50 nm

Figure 4 Analysis for membrane fouling process■ 20 nm; ● 50 nm; ▲ 200 nm; ▼ 500 nm
4.2 Effect of pore size on fouling mechanism
Equations (2)-(5) are used to fit the flux with filtration time t to determine the fouling mechanism, as shown in Fig. 4, with the correlation coefficients R2in Table 3. For the membrane with pore size of 20 nm, all of these models are not acceptable. For the membrane with pore size of 50 and 500 nm, the fouling is mainly caused by intermediate blocking (R2>0.97) and cake filtration (R2>0.98); for the membrane with pore size of 200 nm, it is mainly caused by standard blocking (R2>0.96), intermediate blocking (R2>0.98), and cake filtration (R2>0.98).
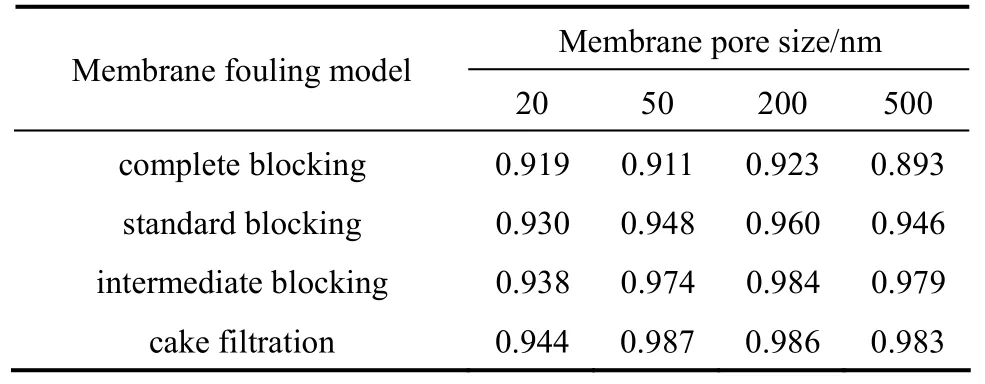
Table 3 Correlation coefficient R2by linear fitting with Hermia model for analysis on fouling mechanism
The fitting of cake filtration model is slightly better than that of intermediate and standard blocking. The intermediate blocking indicates the transition from pore blocking to cake filtration [20]. The pore blocking (intermediate blocking) occurs in the initial stage of filtration process. After filtration for about 1 h, the fouling mechanism changed to cake filtration because the flux decline became slowly (Fig. 3). Salahi et al. [24] investigated the membrane fouling for oil wastewater filtration, the fouling mechanism changed after 300 s filtration. The critical time may depend on the quality of feed water. At higher concentration of organic matter, the fouling mechanism changed earlier. According to the fouling mechanism analysis andorganic membrane fouling by river water reported by Qiao et al. [7], we infer that the membrane fouling process is divided into two steps. First, the membrane is fouled by the NOM and suspended solid with small particle size and pore blocking is the main fouling mechanism. Then the cake formed by the NOM and suspended solid with large particle size is the main reason for the slow decline of flux. Thus the cake filtration has significant influence on the process with ceramic membranes. In order to alleviate the resistance of the filter cake, backwashing was employed in pilot study.
4.3 Effect of operation mode
Two operation modes, dead-end filtration and micro-cross-flow filtration, were used to assess the membrane fouling in river water purification, as shown in Fig. 5. When the operation mode was changed from dead-end filtration to micro-cross-flow filtration (0.5 m·s−1), for UF membranes with pore sizes of 20 and 50 nm, the pseudo-steady flux was improved by 20.4% and 20.2%, respectively; for the MF membranes with pore sizes of 200 and 500 nm, it was increased by 69.1% and 78.3%, respectively. Compare with dead-end filtration, in micro-cross-flow mode, the contamination on the membrane surface was limited, increasing the flux [27]. The flux increase in MF processes was higher than that in UF processes with the same increase of CFV. The MF processes are significantly affected by concentration polarization. As the CFV increases, the thickness of the gel layer decreases, and the concentration polarization is alleviated. Without consideration for water recovery and energy consumption, microcross-flow filtration is more suitable for river water purification than dead-end filtration.
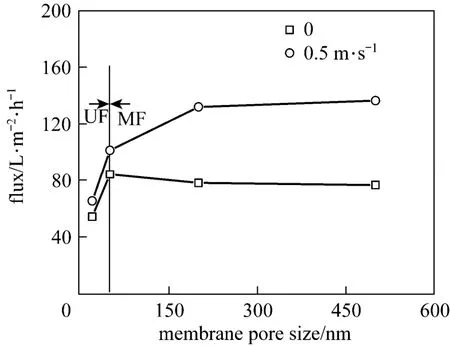
Figure 5 The relationship between pseudo-steady flux and CFV (TMP=0.1 MPa, 20 °C)
4.4 Pilot-scale study for river water purification
A pilot-scale study was conducted to determine the long-term performance of ceramic membranes for river water purification. The production capacity was set to 1.84 m3·h−1. 19-Channel ceramic membrane modules were employed throughout the experiments. The membrane area was approximately 12 m2and the pore size was 200 nm. For obtaining a high water recovery rate and low energy consumption, dead-end filtration was employed. The backwashing period was set to 1 h, and the membrane was backwashed with permeate for 30 s each time. The temperature of the feed water was (15±1) °C. The proposed process was operated steadily for more than a week at a constant flux of 150 L·m−2·h−1(1.84 m3·h−1). Water recovery rate was more than 92%, calculated by

Figure 6 shows the TMP as a function of time. In the pilot-scale study, the maximum TMP was 0.17 MPa and the minimum TMP was 0.05 MPa. The average growth rate of TMP was 3.71 kPa·h−1. The TMP after backwashing was about 0.5 times that before backwashing. Thus backwashing can effectively reduce membrane fouling. The calculated result by Hermia model is consistent with the experimental results. The chemical cleaning was carried out after every 70 h filtration. The aqueous solutions of sodium hypochlorite with volume concentration of 0.1% and sodium hydroxide with mass concentration of 0.1% were employed.
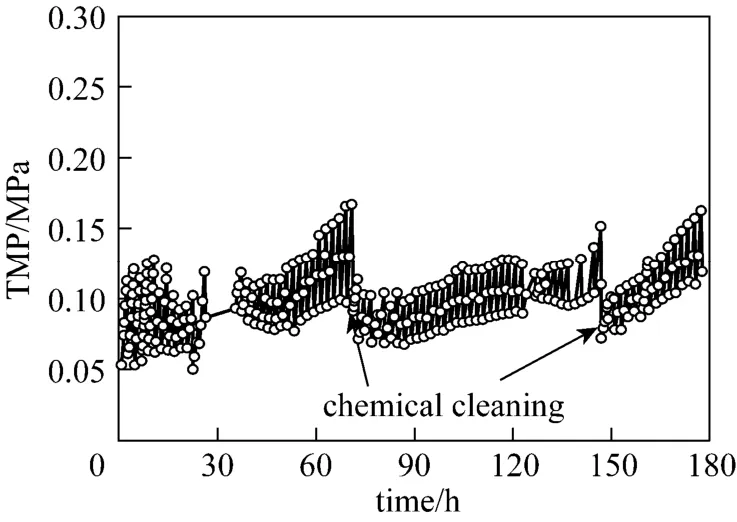
Figure 6 TMP of ceramic membrane for pilot-scale experiment (constant flux=150 L·m−2·h−1)
Water quality of Hengjing River and permeate is shown in Table 4. The removal efficiency of turbidity, UV254and TOC was higher than 99%, 45% and 48%, respectively. Al3+could be removed completely while the removal efficiency of Fe3+was 99.7%. A suitable dosage of coagulates can improve the pseudo-steady flux and retention rate of NOM [28]. Fe3+and Al3+make NOM molecules aggregate and are encapsulated by NOM. The Fe3+-NOM and Al3+-NOM are intercepted by the membranes, so the membrane has a high retention rate for Fe3+and Al3+. Similar results has been reported for ceramic membrane with pore size of 100 nm, with Fe added as coagulates [29]. The membrane filtration can not remove Ca2+and Mg2+from feed water. In this work, the concentration of Ca2+in the feed water was 64.56 mg·L−1, higher than that reported in literature, and Ca2+makes the gel layer denser [30].Turbidity/NTU UV254/cm−1TOC/mg·L−1Fe/mg·L−1Al/mg·L−1Total hardness (CaCO3)/mg·L−1
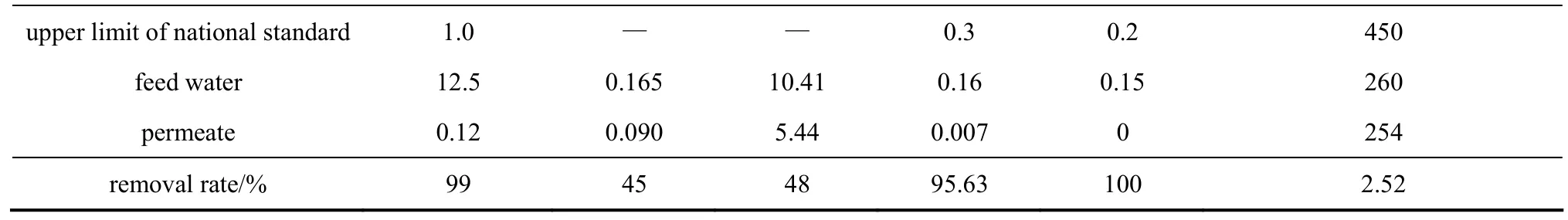
Table 4 Water quality analysis for the river water and permeate
The results for variation of TMP and water quality show that the ceramic membranes coupled with coagulation are steady for river water purification with long-term operation.
5 CONCLUSIONS
The coagulation-porous ceramic membrane hybrid process was used to purify Hengjing River water. The results show that this hybrid process is an effective way for purify micro-polluted source water. The MF process is more efficient than UF with the same increase of CFV. The membrane pore size in the range of 20 nm to 500 nm has a small effect on the pseudosteady flux for dead-end filtration and has little influence on the water quality of permeate. The analysis on membrane fouling shows that the cake filtration plays an important role in this process for the membrane with pore size of 50, 200 and 500 nm. The water quality is significantly influence by the cake on the membrane surface. In a pilot scale study, for the membrane with pore size of 200 nm with backwashing, stable operation at a constant flux of 150 L·m−2·h−1was attained, and the removal efficiency of turbidity, TOC and UV254 was higher than 99%, 45% and 48%, respectively. In summary, the proposed coagulationceramic membrane process is a potential process for the purification of surface water.
NOMENCLATURE
J flux, L·m−2·h−1
Jppseudo-steady flux, L·m−2·h−1
J0initial flux, L·m−2·h−1
k constant
ΔP trans-membrane pressure, Pa
R water recovery rate
Rffouling resistance of fouled membrane, m−1
Rmintrinsic resistance of new membrane, m−1
RTtotal resistance of fouled membrane, m−1
R2correlation coefficient
t filtration time, h
V filtrate volume, L
Vfeedvolume of feed water, L
Vpermeatevolume of permeate, L
μ viscosity of feed water, Pa⋅s
REFERENCES
1 Shannon, M.A., Bohn, W.P., Elimelech, M., Georgiadis G.J., Marinas, J.B., Mayes, M.A., “Science and technology for water purification in the coming decades”, Nature, 452, 301-310 (2008).
2 Xu, W.D., Chellam, S., “Initial stages of bacterial fouling during dead-end microfiltration”, Environ. Sci. Technol., 39 (17), 6470-6476 (2005).
3 Meyn, T., Leiknes, T.O., Koenig, A., “MS2 removal from high NOM content surface water by coagulation-ceramic microfiltration, for potable water production”, AIChE J., 58 (7), 2270-2281 (2012).
4 Sentana, I., Puche, R.D.S., Sentana, E., Prats, D., “Reduction of chlorination byproducts in surface water using ceramic nanofiltration membranes”, Desalination, 277, 147-155 (2011).
5 Hajdu, I., Bodnár, M., Csikós, Z., Wei, S., Daróczi, L., Kovács, B., Zoltán,G., Tamás, J., Borbély, J., “Combined nano-membrane technology for removal of lead ions”, J. Membr. Sci., 409-410, 44-53 (2012).
6 Hashino, M., Hirami, K., Katagiri, T., Kubota, N., Ohmukai, Y., Ishigami, T., Maruyama, T., Matsuyama, H., “Effects of three natural organic matter types on cellulose acetate butyrate microfiltration membrane fouling”, J. Membr. Sci., 379, 233-238 (2011).
7 Qiao, X.L., Zhang, Z.J., Wang, N.C., Wee, V., Low, M., Loh, C.S., Hing, N.T., “Coagulation pretreatment for a large-scale ultrafiltration process treating water from the Taihu River”, Desalination, 230, 305-313 (2008).
8 Song, Y.L., Dong, B.Z., Gao, N.Y., Xia, S.J., “Huangpu River water treatment by microfiltration with ozone pretreatment”, Desalination, 250 (1), 71-75 (2010).
9 Li, M., Wu, G., Guan, Y., Zhang, X., “Treatment of river water by a hybrid coagulation and ceramic membrane process”, Desalination, 280, 114-119 (2011).
10 Muhammad, N., Sinha, R., Krishnan, E.R., Patterson, C.L., “Ceramic filter for small system drinking water treatment: evaluation of membrane pore size and importance of integrity monitoring”, J. Environ. Eng. ASCE, 135 (11), 1181-1191 (2009).
11 Hofs, B., Ogier, J., Vries, D., Beerendonk, E.F., Cornelissen, E.R.,“Comparison of ceramic and polymeric membrane permeability and fouling using surface water”, Sep. Purif. Technol., 79 (3), 365-374 (2011).
12 Cui, Z., Xing, W., Fan, Y., Xu, N., “Pilot study on the ceramic membrane pre-treatment for seawater desalination with reverse osmosis in Tianjin Bohai Bay”, Desalination, 279, 190-194 (2011).
13 Liu, Z., Chu, H., Dong, B., Liu, H., “Characterization of natural organic foulants removed by microfiltration”, Desalination, 277, 370-376 (2011).
14 Barbot, E., Moustier, S., Bottero, J.Y., Moulin, P., “Coagulation and ultrafiltration: Understanding of the key parameters of the hybrid process”, J. Membr. Sci., 325 (2), 520-527 (2008).
15 Liu, T., Chen, Z.L., Yu, W.Z., Shen, J. M., Gregory, J., “Effect of two-stage coagulant addition on coagulation-ultrafiltration process for treatment of humic-rich water”, Water Research, 45 (14), 4260-4268 (2011).
16 Meyn, T., Altmann, J., Leiknes, T., “In-line coagulation prior to ceramic microfiltration for surface water treatment-minimisation of flocculation pre-treatment”, Desalination and Water Treatment, 42, 163-176 (2012).
17 Lerch, A., Panglisch, S., Buchta, P., Tomitac, Y., Yonekawa, H., Hattori, K., Gimbel, R., “Direct river water treatment using coagulation/ceramic membrane microfiltration”, Desalination, 179, 41-50 (2005).
18 Li, W.X., Zhou, L.Y., Xing, W.H., Xu, N.P., “Coagulation-microfiltration for lake water purification using ceramic membranes”, Desalination and Water Treatment, 18, 239-244 (2010).
19 Hermia, J., “Constant pressure blocking filtration laws-application to power-lawnon-newtonian fluids”, Transactions of the Institution of Chemical Engineers, 60 (3), 193-187 (1982).
20 Tian, Y., Chen, L., Zhang, S., Zhang, S., “A systematic study of soluble microbial products and their fouling impacts in membrane bioreactors”, Chem. Eng. J., 168 (3), 1093-1102 (2011).
21 Jacob, J., Prádanos, P., Calvo, J.I., Hernández, A., Jonsson, G.,“Fouling kinetics and associated dynamics of structural modifications”, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 138, 173-183 (1998).
22 Yuan, W., Zydney, A.L., “Humic acid fouling during microfiltration”, J. Membr. Sci., 157 (1), 51-62 (1999).
23 Zydney, A.L., Yuan, W., Kocic, A., “Analysis of humic acid fouling during microfiltration using a pore blockage-cake filtration model”, J. Membr. Sci., 198 (1), 51-62 (2002).
24 Salahi, A., Abbasi, M., Mohammadi, T., “Permeate flux decline during UF of oily wastewater: Experimental and modeling”, Desalination, 251, 153-160 (2010).
25 Beatriz, C.M., Rene, R., Castro, C.A., Torrico, J.S., Riquelme, M.L.,“Analysis of the membrane fouling mechanisms involved in clarified grape juice ultrafiltration using statistical tools”, Ind. Eng. Chem. Res., 51 (10), 4017-4024 (2012).
26 Li, M., Zhao, Y., Zhou, S., Xing, W., Wong, F.S., “Resistance analysis for ceramic membrane microfiltration of raw soy sauce”, J. Membr. Sci., 299, 122-129 (2007).
27 Wang, L., Wang, X.D., Fukushi, K.I., “Effects of operational conditions on ultrafiltration membrane fouling”, Desalination, 229, 181-191 (2008).
28 Lee, J.D., Lee, S.H., Jo, M.H., Park, P.K., Lee, C.H., Kwak, J.W.,“Effect of coagulation conditions on membrane filtration characteristics in coagulation-microfiltration process for water treatment”, Environ. Sci. Technol., 34 (17), 3780-3788 (2000).
29 Konieczny, K., Bodzek, M., Rajca, M., “A coagulation-MF system for water treatment using ceramic membranes”, Desalination, 198, 92-101 (2006).
30 Costa, A.R., Pinho, M.N., Elimelech, M., “Mechanisms of colloidal natural organic matter fouling in ultrafiltration”, J. Membr. Sci., 281, 716-725 (2006).
10.1016/S1004-9541(14)60014-8
2012-12-06, accepted 2013-02-26.
* Supported by the National Natural Science Foundation of China (21276124, 21125629,21076102), Research Project of Natural Science for Universities Affiliated with Jiangsu Province (10KJB530002), Key Projects in the National Science & Technology Pillar Program (2011BAE07B09-3), Jiangsu Province Industrial Supporting Project (BE2011831).
** To whom correspondence should be addressed. E-mail: xingwh@njut.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Steam Reforming of Dimethyl Ether by Gliding Arc Gas Discharge Plasma for Hydrogen Production*
- Interaction Analysis and Decomposition Principle for Control Structure Design of Large-scale Systems*
- Influence of Design Margin on Operation Optimization and Control Performance of Chemical Processes*
- Photocatalytical Inactivation of Enterococcus faecalis from Water Using Functional Materials Based on Natural Zeolite and Titanium Dioxide*
- Enhancing Structural Stability and Pervaporation Performance of Composite Membranes by Coating Gelatin onto Hydrophilically Modified Support Layer*
- Measurement and Modeling for the Solubility of Hydrogen Sulfide in Primene JM-T*
