不同方式注射骨髓间充质干细胞对溃疡性结肠炎大鼠模型的疗效
2014-07-07安徽医科大学海军临床学院北京00048海军总医院消化内科北京00048
孙 辉,李 毅,孙 涛,安徽医科大学海军临床学院,北京 00048;海军总医院 消化内科,北京 00048
不同方式注射骨髓间充质干细胞对溃疡性结肠炎大鼠模型的疗效
孙 辉1,李 毅2,孙 涛1,2
1安徽医科大学海军临床学院,北京 100048;2海军总医院 消化内科,北京 100048
目的对比心脏注射和鼠尾静脉注射骨髓间充质干细胞(bone marrow mesenchymal stromal cells,BMSCs)对溃疡性结肠炎(ulcerative colitis,UC)大鼠模型的疗效,并分析可能的治疗机制。方法体外扩增培养雄性SD大鼠的BMSCs,用5%三硝基苯磺酸(trinitrobenzenesulfonic acid,TNBS)/乙醇灌肠建立UC大鼠模型,并建立正常对照组D。造模后24 h,A组由心脏注射BMSCs悬液0.5 ml(含1×106个干细胞),B组由鼠尾静脉注射BMSCs悬液0.5 ml(含1×106个干细胞),C组由鼠尾静脉注射0.5 ml 0.9%氯化钠注射液,计算各组大鼠的疾病活动指数(disease activity index,DAI)。移植后7 d处死大鼠,光镜下观察结肠病理变化,及人性别确定区Y蛋白(human sex-determining region Y protein,SRY蛋白)、自杀相关因子(factor associated suicide,FAS)、G-蛋白偶联受体5(leucine-rich repeat containing g protein coupled receptor 5,Lgr5)的表达。结果A组较B、C组DAI评分低(F=90.603,P<0.05),但A、B组间差异无统计学意义。干细胞移植组大鼠均检测到SRY蛋白,A组优于B组;C组Fas表达高于D组(t=9.494,P<0.05),A、B组Fas表达较C组下调,且A组低于B组,3组差异有统计学意义(F=38.6,P<0.05);A、B组LGR-5较C组表达明显增加,且A组较B组高,3组差异有统计学意义(F=24.7,P<0.05)。结论心脏注射BMSCs对UC的疗效优于鼠尾静脉注射;BMSCs可通过减轻肠道细胞凋亡、促进肠道干细胞再生起到对UC的治疗作用。
骨髓间充质干细胞;移植;溃疡性结肠炎;大鼠
溃疡性结肠炎(ulcerative colitis,UC)是一种病因尚不十分清楚的慢性非特异性结直肠炎症性疾病,难治愈,易复发,被世界卫生组织列为现代难治病之一。目前治疗该病药物有氨基水杨酸制剂、糖皮质激素、免疫抑制剂等,严重者行手术治疗。这些方法虽然可缓解症状,但难以解决UC易复发的难题。近年来提出的干细胞疗法为治疗UC提供了新方向。本实验对比心脏注射及鼠尾静脉注射两种方式对UC的疗效,并分析研究可能的治疗机制。
材料和方法
1 实验动物 健康清洁级Sprague-Dawley(SD)大鼠,雌性(6 ~ 8周龄)40只,雄性(3 ~ 4周龄)5只,购自军事医学科学院实验动物中心,饲养于海军总医院实验动物中心SPF级动物实验室。实验前,动物在实验条件下适应环境1周,动物房温度18 ~24℃,相对湿度50% ~ 55%,自然光照周期,予标准配方的普通饲料喂养,自由摄食和饮水。
2 主要试剂 DMEM/F12培养基(Thermo,USA),特级胎牛血清(Invitrogen,USA),胰蛋白酶(Solarbio),2,4,6-三硝基苯磺酸[trinitrobenzenesulfonic acid (TNBS),Sigma];PE标记小鼠抗大鼠CD34、CD44、CD45、CD90抗体;兔抗SRY-FITC、兔抗Fas单克隆抗体、兔抗LGR-5单克隆抗体、SP试剂盒和DAB染色剂:北京博奥森生物技术有限公司。
3 骨髓间充质干细胞(bone marrow mesenchymal stromal cells,BMSCs)的分离培养及鉴定 将雄性(3 ~4周龄)5只SD大鼠颈椎脱臼处死后,置于75%乙醇中浸泡约5 min,于无菌条件下分离出大鼠的股骨和胫骨,用注射器冲出骨髓细胞后,离心1 000 r/min,5 min,弃上清液,加入DMEM/F12培养基重悬细胞,于37℃,5% CO2饱和湿度孵箱内培养,并利用流式细胞仪检测P3代细胞CD34、CD44、CD45、CD90的表达情况。
4 UC模型的建立及实验动物分组 用三硝基苯磺酸/乙醇复合法建立UC大鼠模型[1]。大鼠造模前禁食不禁水48 h,禁食24 h后提起鼠尾将其悬空,使挣扎以排出大肠远端的大便,共2次,每次间隔6 h以上,排空大便后以10%水合氯醛腹腔注射麻醉大鼠,用外径为2 mm的硅胶管插入大鼠肛内约8 cm,经管内注入溶液0.6 ml(100 mg/kg TNBS + 50%乙醇),约10 s灌注完毕。将造模成功的30只雌性SD大鼠随机分为3组,A组(心脏注射干细胞移植)10只,B组(鼠尾静脉注射干细胞移植)10只,C组(鼠尾静脉注射0.9%氯化钠注射液)10只,以及D组(正常对照)10只。
5 疾病活动指数(disease activity index,DAI)评分[2]造模后观察大鼠的体质量变化、粪便性状及便血情况。DAI=(体质量下降分数+粪便性状分数+隐血分数)/3。DAI评分标准:无体质量下降、粪便性状正常、无便血记为0分;体质量下降1% ~ 5%记为1分;体质量下降5% ~ 10%、稀便、粪便隐血(+)记为2分;体质量下降11% ~ 15%记为3分;体质量下降>15%、腹泻、便血记为4分(粪便性状正常是成形粪便;稀便是糊便或者半成形粪便,但不黏附肛门;腹泻是水样便并且黏附肛门)。
6 BMSCs移植及取材 造模后24 h,将含1×106个BMSCs的悬浮液0.5 ml经心脏注射于A组大鼠体内,B组于鼠尾静脉注射等量BMSCs,C组于鼠尾静脉注射等量0.9%氯化钠注射液。观察记录大鼠毛发、体质量、粪便性状及便血情况,7 d时处死大鼠,取结肠组织分别经液氮、4%甲醛固定,用于HE染色、SRY蛋白免疫荧光染色、Fas、LGR-5免疫组化染色。
7 免疫组化法评分 免疫组化采用SP三步法,具体操作步骤参见试剂盒说明书。高倍镜下抽取5处评分,以胞质或胞膜出现棕黄色或棕褐色颗粒状物者为阳性细胞,分别根据染色强度和阳性细胞比例进行评分,阳性细胞占同类细胞数<5%为0分;5% ~ 25%为1分;26% ~ 50%为2分;51% ~ 75%为3分;>75%为4分。胞质染色阴性为0分;浅黄色为1分;棕黄色为2分;棕褐色为3分。最后将两个分数相加。
8 统计学处理 采用SPSS16.0统计软件,计量资料以±s表示,两组或多组定量资料比较采用t检验或χ2分析,服从正态分布且方差齐性的计量资料,多样本均数间两两比较采用Student-Newman-Keuls检验,P<0.05为差异有统计学意义。
结 果
1 BMSCs培养及鉴定 原代培养的BMSCs约12 h开始贴壁,72 h后大量贴壁,呈短梭形,传代纯化后大部分呈长梭形,漩涡状生长(图1)。取3代细胞行流式细胞术检测,BMSCs表面标记物CD34阳性率为0.78%、CD44阳性率99.38%、CD45阳性率0.43%、CD90阳性率为98.78%(图2)。
2 DAI评分 造模前大鼠饮食、活动及粪便性状正常,毛色光泽,体质量正常。造模后1 d大鼠开始出现懒动,厌食,粪便次数增多,软便或质稀,隐血试验阳性或黏液脓血便等现象,3 d后症状达到高峰,大鼠毛发无光泽、变脆,懒动扎堆,粪便完全为稀便。之后大鼠饮食、活动、体质量逐渐增加,毛发逐渐恢复光泽,粪便性状逐渐恢复,对照组大鼠粪便无变化,体质量略增加。7 d时A组DAI评分(1.53±0.28)较B组(1.66±0.22)、C组(2.93±0.26)均低,3组差异有统计学意义(F=90.603,P<0.05),但A、B组差异无统计学意义。
3 组织病理学观察 取结肠组织行HE染色,A组结肠镜下见上皮破损范围较小,黏膜及黏膜下层见炎性细胞浸润,B组镜下上皮破损范围较大,黏膜及黏膜下大量炎性细胞浸润,C组镜下可见巨大溃疡,大量炎性细胞浸润;D组镜下见结肠上皮完整,固有层腺体形态正常排列整齐,黏膜及黏膜下层可见少量炎症细胞浸润,未见糜烂及溃疡。见图3。
4 SRY蛋白免疫荧光检查 移植组大鼠结肠组织均可检测到SRY荧光蛋白,部分阳性细胞呈簇排列,模型组大鼠未能在组织中检测到SRY蛋白。见图4。
5 Fas免疫组化检查 C组Fas大量表达,正常对照组仅有少量Fas表达(t=9.494,P<0.05);A、B组Fas表达较C组下调,且A组表达较B组低,3组差异有统计学意义(F=38.6,P<0.05)。见表1,图5。
6 LGR-5免疫组化检查 正常对照组LGR-5有少量表达,A、B组较C组表达明显增加,且A组含量较B组高,3组差异有统计学意义(F=24.7,P<0.05)。见表1,图6。
表1 各组免疫组化评分Tab. 1 Immunohistochemical score of rats in 4 groups (±s)

表1 各组免疫组化评分Tab. 1 Immunohistochemical score of rats in 4 groups (±s)
aP<0.05, vs group B;aP<0.05, vs group C;bP<0.05, vs group C;cP<0.05, vs group D
Group (n=10)FasLGR-5 A 2.2±0.45a5.4±0.55aB 4.0±0.71b4.0±1.00bC 5.8±0.84c1.8±0.84 D 0.4±0.550.8±0.84
图 1 培养3 d的P3-BMSCs (×100)Fig. 1 P3-BMSCs cultured for 3 days (×100)
讨 论
UC是一种慢性非特异性炎症性疾病,其发病机制是多方面的,目前认为其可能与免疫、遗传、环境等因素有关[3-7]。目前,治疗UC的方法疗效有限,且不良反应难以避免,因此探究一种新的治疗方法相当必要。
BMSCs跨胚层横向分化的可塑性、强大的分化潜能及低免疫原性使其在细胞再生和免疫调节方面有广泛的应用前景[8-10]。近年,已有大量实验证明,BMSCs可促进实验性结肠炎模型肠道修复[11-16]。在干细胞移植的基础实验中,尾静脉注射是主要途径,但尾静脉注射的BMSCs大部分聚集于肺、肾、肝等器官,仅有少量出现在结肠,因此,探究新的注射方式就尤为必要。随着介入技术在临床的应用,通过动脉系统注射药物越来越受到重视,其可使药物快速到达靶器官,减少转运途中的损失。但在动物实验中,技术、仪器有限,因此,本实验通过心脏注射和鼠尾静脉注射两种方式注射BMSCs到大鼠体内,对比疗效,并从BMSCs促进肠道干细胞增殖、减轻肠道细胞凋亡方面探讨BMSCs治疗UC的可能机制。
移植BMSCs后,观察大鼠体质量变化,粪便性状及便血情况,7 d时A组较B组、C组DAI评分均低(F=90.603,P<0.05),但A、B组间无明显差异,HE染色发现A、B组结肠病理差异明显。因此我们推测心脏注射BMSCs可减少其在肺部的损失,到达肠道的细胞量多于鼠尾静脉注射的量,可更好改善UC大鼠病理损伤,但因治疗时间不足,临床改善不明显。通过检测肠道干细胞标记物LGR-5,发现A、B组肠道黏膜上皮LGR-5高于C组,且A组高于B组,表明移植明显促进受损肠道黏膜组织内肠道干细胞增殖,这与Nishida等[17]的研究一致。因此我们推测心脏注射干细胞的效果优于鼠尾静脉注射,BMSCs可以促进肠道干细胞的增殖,但其是否分化为结肠黏膜上皮,尚需进一步研究。Fas是细胞凋亡相关蛋白之一,其配体Fas Ligand主要存在于被炎症介质激活的T淋巴细胞(尤其是T辅助细胞Th1)和自然杀伤(natural killer,NK)细胞上,已有研究证实BMSCs具有免疫调节功能,可通过调节树突状细胞的增殖、活化,间接或直接影响T细胞、B细胞和NK细胞等细胞的增殖、活化及细胞因子的分泌[18]。本实验研究表明,A、B组Fas免疫组化评分低于C组,且A组低于B组,表明心脏注射优于鼠尾静脉注射,BMSCs可能通过对免疫炎症细胞的阻断从而减轻细胞凋亡。但也有研究表明关于BMSCs抗凋亡的机制可能与其分泌胰岛素样生长因子、血管内皮生长因子、肝细胞生长因子等有关[19-20]。

图 2 流式细胞术检测大鼠第3代BMSCs表面CD抗原的表达Fig. 2 Expression of markers CD34, CD44, CD45 and CD90 of BMSCs in rats tested by flow cytometry
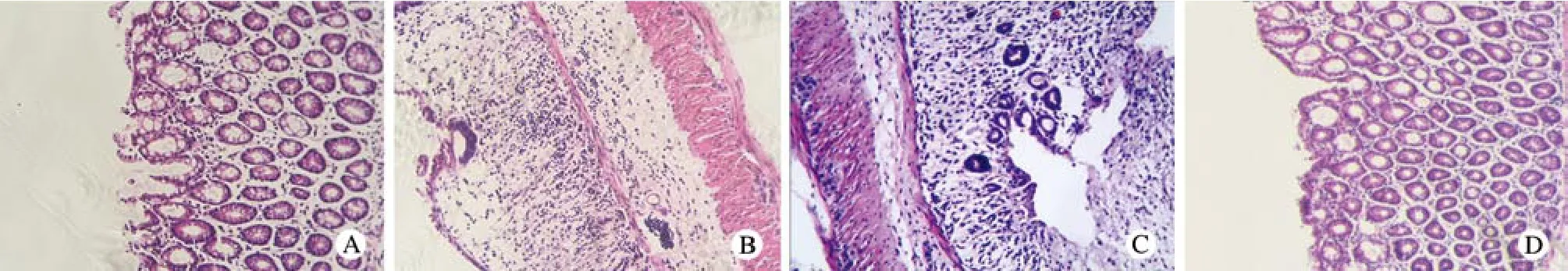
图 3 大鼠结肠组织病理改变(HE×200) A: 心脏注射组; B: 鼠尾静脉注射组; C: 模型对照组; D: 正常对照组Fig. 3 Pathological-changes in colon of rats (HE staining×200) A: Intracardiac injection group; B: Intravenous injection group; C: Model control group; D: Normal control group

图 4 大鼠结肠组织SRY蛋白的表达(免疫荧光×200) A: 心脏注射组; B: 鼠尾静脉注射组; C: 模型对照组; D: 正常对照组Fig. 4 Expression of SRY protein in colon of rats (immunofluorescence staining×200) A: Intracardiac injection group; B: Intravenous injection group; C: Model control group; D: Normal control group
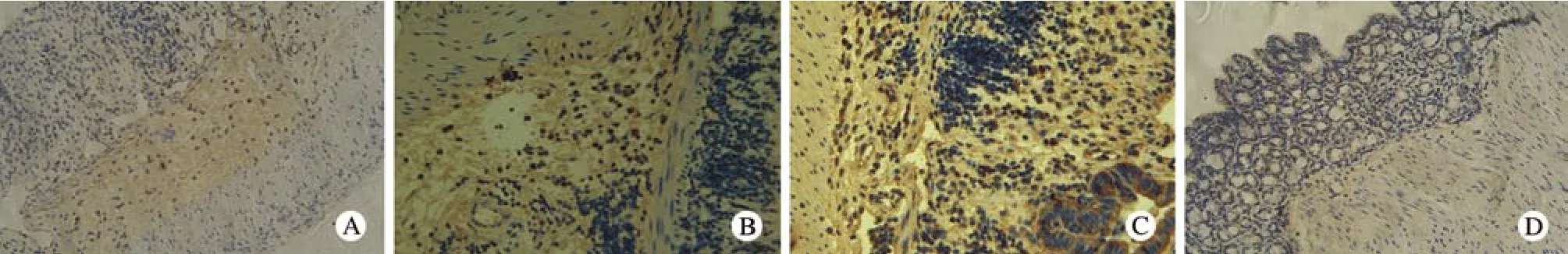
图 5 光镜下大鼠结肠组织Fas的表达(免疫组化×200) A:心脏注射组; B: 鼠尾静脉注射组; C: 模型对照组; D: 正常对照组Fig. 5 Expression of Fas in colon of rats (immunohistochemical staining×200) A: Intracardiac injection group; B: Intravenous injection group; C: Model control group; D: Normal control group

图 6 大鼠结肠组织LGR-5的表达(免疫组化×200) A:心脏注射组; B: 鼠尾静脉注射组; C: 模型对照组; D: 正常对照组Fig. 6 Expression of LGR-5 in colon of rats (immunohistochemical staining×200) A: Intracardiac injection group; B: Intravenous injection group; C: Model control group; D: Normal control group
综上所述,心脏注射BMSCs可减少其在大鼠肺部的损失,增加其向受损肠道的聚集,参与肠道组织损伤的修复,促进肠道干细胞增殖,减轻肠道细胞凋亡,加速肠道修复,较鼠尾静脉注射的疗效更优。但其是否可分化成肠道干细胞,将在后续研究中予以关注。
1 Fan H, Shen L, Tang Q, et al. Effect of Wumeiwan on cytokines TNF-alpha, IL-6, IL-8, IL-10 and expression of NF-kappaBp65 in rats with ulcerative colitis[J]. J Huazhong Univ Sci Technolog Med Sci, 2009, 29(5):650-654.
2 Murano M, Maemura K, Hirata I, et al. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate Sodium (DSS)-induced colitis[J]. Clin Exp Immunol, 2000, 120(1): 51-58.
3 Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease[J]. Nature, 2011, 474(7351):307-317.
4 Cooney R, Baker J, Brain O, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation[J]. Nat Med, 2010, 16(1): 90-97.
5 Hundorfean G, Neurath MF, Mudter J. Functional relevance of T helper 17 (Th17) cells and the IL-17 cytokine family in inflammatory bowel disease[J]. Inflamm Bowel Dis, 2012, 18(1): 180-186.
6 Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47[J]. Nat Genet, 2011, 43(3): 246-252.
7 Lochner M, Ohnmacht C, Presley L, et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells[J]. J Exp Med, 2011, 208(1):125-134.
8 Faghihi F, Baghaban Eslaminejad M. The effect of nano-scale topography on osteogenic differentiation of mesenchymal stem cells[J]. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub,2014, 158(1): 5-16.
9 Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression[J]. Stem Cells, 2009, 27(8): 1954-1962.
10 Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation[J]. Curr Mol Med, 2013, 13(5): 856-867.
11 张夏梦,寿折星,石月萍,等.骨髓间充质干细胞对溃疡性结肠炎大鼠结肠组织血管内皮的修复作用[J] .世界华人消化杂志,2013,21(28):2908-2914.
12 熊轩轩,吴克俭,费素娟,等.骨髓间充质干细胞治疗大鼠溃疡性结肠炎的作用及机制研究[J].中华临床医师杂志:电子版,2013,7(7):3029-3035.
13 Del Fattore A, Luciano R, Fierabracci A, et al. Mesenchymal stem/ stromal cell-derived microparticles show anti-inflammatory activity in an animal model of ulcerative colitis[J]. Cytotherapy, 2014, 16(4,Supplement): S25.
14 He XW, He XS, Lian L, et al. Systemic infusion of bone marrowderived mesenchymal stem cells for treatment of experimental colitis in mice[J]. Dig Dis Sci, 2012, 57(12): 3136-3144.
15 Tanaka H, Arimura Y, Yabana T, et al. Myogenic lineage differentiated mesenchymal stem cells enhance recovery from dextran sulfate sodium-induced colitis in the rat[J]. J Gastroenterol,2011, 46(2): 143-152.
16 Zhang Q, Shi S, Liu Y, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis[J]. J Immunol, 2009, 183(12): 7787-7798.
17 Nishida T, Tsuji S, Tsujii M, et al. Cultured bone marrow cell local implantation accelerates healing of ulcers in mice[J]. J Gastroenterol, 2008, 43(2): 124-135.
18 Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications[J]. Arch Pharm Res,2012, 35(2): 213-221.
19 Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice[J]. Stem Cells, 2008, 26(8): 2075-2082.
20 Xu YX, Chen L, Wang R, et al. Mesenchymal stem cell therapy for diabetes through paracrine mechanisms[J]. Med Hypotheses,2008, 71(3): 390-393.
Comparison of therapeutic effects in different ways of bone marrow mesenchymal stromal cells injection in rats with ulcerative colitis
SUN Hui1, LI Yi2, SUN Tao1,2
1Affiliated Clinical Faculty of Navy General Hospital, Anhui Medical University, Beijing 100048, China;2Department of Gastroenterology, PLA Navy General Hospital, Beijing 100048, China
SUN Tao. Email: drsuntao1@163.com
ObjectiveTo compare the efficacy of intracardiac injection and intravenous injection of bone marrow mesenchymal stromal cells (BMSCs) in rats with ulcerative colitis (UC) and analyze the therapeutic mechanism.MethodsBMSCs from SD male rats were in vitro cultured. The rat model of UC was established by 5% trinitrobenzenesulfonic acid (TNBS)/ethanol enema. Group D was the control group. And 24 hours later, rats of group A were injected with 0.5 ml of BMSCs suspension via the heart (1×106). Rats of group B were injected with 0.5 ml of BMSCs suspension via tail vein (1×106). Rats of group C were injected with equal volume of normal saline via tail vein. Disease activity index (DAI) of rats in every group was observed and calculated. The rats were sacrificed at day 7 after injection. The pathological changes of the colon and the expression of human sex-determining region Y protein (SRY protein), factor associated suicide (FAS), leucine-rich repeat containing g protein coupled receptor 5 (Lgr5) were observed under microscope.ResultsDAI of group A was lower than that of group B and C (F=90.603, P<0.05) but there were no differences between group A and B. The repairing of injured colon in group A was much better than in group B. The SRY protein could be detected in transplantation group. Compared with the control group, the expression of Fas in group C increased (t=9.494, P<0.05). Compared with group C, the expression of Fas in treatment group decreased. And the expression in group A was lower than in group B. There were significant difference among the three groups (F=38.6, P<0.05). Compared with group C, the expression of LGR-5 in treatment group was significantly increased. And the expression in group A was higher than in group B. There were significant difference among the three groups (F=24.7, P<0.05).ConclusionThe efficacy of intracardiac injection of BMSCs is much better than the efficacy of intravenous injection in rats with UC. BMSCs plays a therapeutic role in rats with UC by reducing intestinal cells apoptosis and promoting intestinal stem cell regeneration.
bone marrow mesenchymal stromal cells; transplantation; ulcerative colitis; rats
R 574.1;R 332
A
2095-5227(2014)11-1155-05
10.3969/j.issn.2095-5227.2014.11.020
时间:2014-07-18 10:15 网络出版地址:http://www.cnki.net/kcms/detail/11.3275.R.20140718.1015.001.html
2014-05-05
首都医学发展科研基金项目(2009-3083)
Supported by Capital Medical Development and Research Foundation of Beijing(2009-3083)
孙辉,女,在读硕士。研究方向:炎症性肠病。Email: sunhui5625@126.com
孙涛,男,博士,主任医师,副院长。Email: drsuntao1@163.com
