Positive feedback of soil fungi,including arbuscular mycorrhizal fungi,to the invasive weed Ageratina adenophora:evidence from field studies
2014-07-05WenqingYUWanxueLIUWenzhiLIUYujingRENFanghaoWANLiliZHANG00094540078400
Wen-qing YU,Wan-xue LIU,Wen-zhi LIU,Yu-jing REN,Fang-hao WAN,Li-li ZHANG,,, 00094,;,, 54007,;,,,, 8400,
Introduction
Plant invasion,being a multistage process like succession,is characterized by conspicuous spatiotemporal dynamics along the introduction-establishment-naturalization-invasion continuum(Callawayet al.,2004;Shahet al.,2009).Many alien plant species can establish a mutualistic symbiotic relationship with arbuscular mycorrhizal fungi(AMF)in their newly invaded habitats(Fumanalet al.,2006).Therefore,with the overwhelming increase in invasive plants outside their original habitats,how are alien species invasions mediated by mycorrhizal fungi urgently needs to be explored.
The Crofton weed,Ageratina adenophora(Sprengel)King&Robinson(Synonym:Eupatorium adenophorumSprengel)is a noxious perennial weed all over the world that is both allelopathic and toxic to livestock.Native to Mexico and Costa Rica,it has spread to over 30 countries in tropical and subtropical regions of the world.It was first introduced into Yunnan Province of China from Myanmar at the end of the 1940s;from there it rapidly spread to other southern and southwestern provinces of China including Sichuan,Guizhou,Guangxi and Chongqing(Jia,2007).In Yunnan Province alone,A.adenophorahas spread over 2,500,000 hectares,comprising 80%of the province.The weed has invaded woodlands,grasslands,roadsides and farmlands in these provinces,causing serious economic losses to agriculture,forestry,and livestock breeding(Xuet al.,2006).It has out-competing native plants in many areas,rapidly forming monocultures that decrease biodiversity.Its invasion has especially endangered valuable plant resources,and has resulted in the loss of some ecosystem functions(Wanet al.,2010).Previous studies had found thatA.adenophoracreated a favorable soil environment to its own growth by changing the structure of the soil microbial community,including the arbuscular mycorrhizal fungi(AMF)(Liet al.,2009;Niuet al.,2007a,2007b;Yuet al.,2005).
With regard to the roles of mycorrhizal symbiosis in alien plant invasions,some hypotheses have been proposed,among which the resistance hypothesis(Kisaet al.,2007;Mack,1996)suggests that mycorrhizal symbiosis facilitates native plants in resisting alien plant invasion. However, increasing evidence has shown that mycorrhizal symbiosis exhibited positive feedback with alien plant invasion(Reinhart&Callaway,2006;Richardsonet al.,2000),in which alien plant invasion inhibited the mutualistic symbiosis of mycorrhiza with native plants(Callawayet al.,2008;Stinsonet al.,2006)and enhanced the competitive advantage of invading plants over native ones by virtue of mycorrhizal symbiosis(Careyet al.,2004;Marleret al.,1999;Reinhart& Callaway,2004).
Currently,most studies on the feedback between AMF and alien plant invasions have been conducted as greenhouse experiments,with few being carried out as field investigations(Shahet al.,2009).This study was conducted in the field to investigate the effect of soil fungi,including AMF,on the growth and the ability ofA.adenophorato outcompete native plants.
Materials and methods
Study area
The study area was in Kunming,Yunnan Province(24°42'N,102°52'E,altitude 1988 m)intensely invaded byA.adenophora(Liet al.,2009;Niuet al.,2007a,2007b).This region has a subtropical monsoon climate with the average temperature of 19~22℃ in summer and 6~8℃ in winter.The soil type of the test area is the typical red soil in southern China(Hapludult).
Experimental Sites
The experiment sits was in sparse coniferous and broad-leaf mixed forestry,and carried out in two type of communities,one community was still dominated by native plant species with clusters ofA.adenophoraspaced 2~3 m throughout(referred to as partially invaded habitats),while the other community was completely dominated byA.adenophorawith a ten-year invasive history(referred to as invaded habitats),the invasion history was judged by observing the number of plant branchs,we found that the branch ofA.adenophoraincreased one by year.For each type of community(partially invaded habitats and invaded habitats)we established five sites.Each site was about 200 m2,and the distance between adjacent sites was 10~50 m.
Soil sample collection
To exam the differences in soil chemical characteristics between partially invaded habitats and invaded habitats,rhizosphere soil samples at 0~10 cm depth were collected for each location.To exam the differences in soil chemical characteristics duringA.adenophorainvasion,soil samples were collected from sites dominated by native plant species(referred to as uninvaded habitats).Five soil samples were collected from invaded habitats and uninvaded habitats respectively,and ten from partially invaded habitats.We considered that the different distance(from 2 m to 3 m)and the size(from 3 to 6 branch)of the clusters ofA.adenophorain partially invaded habitats might result in difference of soil chemical characteristics more or less,so double soil samples were collected from this type of sites to eliminate the possible difference.The samples were air-dried,then sieved(2 mm mesh),and transferred to plastic bags.
Measurement of soil chemical characteristics
Soil pH,the concentration of soil organic carbon,total nitrogen,available phosphorus and available potassium were measured for each soil sample.Soil pH was measured using a glass electrode(WTW pH 340),soil organic carbon content was determined by the potassium dichromate method(Bao,2000),total nitrogen was quantified using of the Kjeldahl method(Bao,2000),available phosphorus was quantified using the colorimetric Mo-blue-method(Olsen&Sommers,1982),and available potassium was extracted with 1 mol·L-1ammonium acetate and then determined using the burnt-luminosity method(Bao,2000).
Plot treatment and fungicide applicatio n
Five invaded habitats and partially invaded habitats was demarcated respectively,each site was demarcated into 10 plots.Each plot was 3 m ×3 m,at least 2 m from other plots,and 3 ~ 5 m away from trees.Five plots were randomly selected from the ten plots in each site,and had the fungicide benomyl,active ingredient:methyl l-(butylcarbamoyl)benzimidazol-2-ylcarbamate,0.05 g·m-2,1∶1000 dilution,applied as described by Callawayet al.(2004).These plots were termed the"fungicide treatment".As a control,tap water was applied to the other five plots in each site,and these plots were termed the"non-fungicide treatment".Fungicide application was repeated every two weeks,with four applications in total over the 64 days of the experiment.
Measurement of AMF colonization rate
To check the validity of fungicide treatment,for both partially invaded habitats and invaded habitats,Two hundred root segments were randomly selected from the roots ofA.adenophoracollected from each plot including 50 fungicide treatment and 50 non-fungicide treatment plots in invaded habitats and partially invaded habitats,and the colonization rate of AMF in the different treatments was measured.Fresh fibrous 1 cm root segments were soaked in 10% KOH for 25 min,rinsed with 2%hydrochloric acid for 5 min,then stained with 0.01%acid fuchsin-lactic acid-glycerin dye(acid fuchsin 0.1 g,lactic acid 875 mL,glycerin 63 mL,distilled water 300 mL)at 90℃ for 25 min.This was followed by diped in pure lactic acid,after which the percentage of AMF root colonization was scored(http:∥invam.caf.wvu.edu/;Biermann & Linderman,1981;Liu & Chen,2007).
Measurement of photosynthesis and leaf area
Photosynthesis ofA.adenophoraplants was measured on morning after 64 days of the first fungicide application,the day when we measured photosynthesis was sunny and windless,a LI-6400 Portable Photosynthesis System was used.The controlled light intensity of the Photosynthesis System was 600 μmol·m-2·s-1,and the CO2concentration was 400 μmol· mol-1,which determined by the light response curve and CO2response curve respectively.The photosynthesis rates were measured for the top 4th leaves from five plants in each plot.The temperature and atmospheric pressure were also measured at the same time.Subsequent measures of the photosynthesis rate and leaf area were determined using an LI-3000C Portable Leaf Area Meter.
Measurement of nitrogen,phosphorus,potassium,and stable carbon-isotope(δ13C)contents
One leaf samples were collected from per plot,and three of the 5 plots within a treatment were selected.After measuring the photosynthesis rate and leaf area,the upper new leaves(1st~6th leaves)from each selected plant were harvested and brought to the laboratory,where they were cleaned by distilled water and dried at 60℃ for 68 h,and then ground using a carnelian mortar and passed through a 60-mesh sieve.After rapid digestion using the H2SO4-H2O2method,the nitrogen,phosphorus,and potassium levels were measured with the Kjeldahl Nitrogen Analyzer,Mo-Sb calorimetry and the flame photometric method.
To measure the effect of fungicide on the carbon and stable carbon-isotope(δ13C)levels in leaves,a 0.010000 g sample was weighed and bundled using aluminum foil,and subjected to detection with a Thermo Finnigan Elementary Analyzer Flash EA1112(Thermo Finnigan,USA).Reaction temperature was 95℃,7×10-8kPa of the vacuum,Cr2O3/Co3O4oxidation furnace,85 mL·min-1of carrier-He flow rate,80 kPa of Conflo-He pressure and 110 mL·min-1of oxygen injection.The internationally used standard samples(HLY and UREA)were used in the determination of δ13C.
Statistical analyses
SPSS 13.0 software(SPSS Inc.,Chicago,USA)was used for data analysis.Multiple comparison procedures for one-way ANOVA(one-factor analysis of variance)were used to analyze the differences between communities/sites and between the fungicide treatment and non-fungicide treatment.The means were separated using standard error.
Results
The changes in soil chemical properties in A.adenophora-invaded habitats at different invasion stages
The soil pH of invaded habitat was significantly lower than that of partially invaded habitats(P<0.001)and invaded habitats(P<0.001).While the organic carbon content of invaded habitats was 100%and 88.76%higher than that of partially invaded habitats(P< 0.001)and N(P< 0.001),respectively(Table 1).The total nitrogen content of invaded habitats was 75.7%and 61.4%higher than that of partially invaded habitats(P<0.001)and uninvaded habitats(P<0.001),respectively.The available potassium content of invaded habitats was 82.6%and 80.0%higher than that in partially invaded habitats(P<0.001)and uninvaded habtitats(P<0.001),respectively.Whereas,the available phosphorus content of invaded habitats was 27.2%and 29.2%(P=0.008)lower than that of partially invaded habitats(P=0.007)and uninvaded habitats(P=0.006),respectively.No significant difference existed in the all above indicators of soil physico-chemical properties except soil pH between invaded habitats and partially invaded habitats(P=0.026).
Validity of fungicide treatment
The colonization rate of AMF in fungicide-treated and non-treated plots was 19.7%and 93.6% ,respectively,indicating that fungicide treatment significantly inhibited the infection of AMF onA.adenophora.
Effect of fungicide application on the photosynthesis of A.adenophora plants at different invasion stages
In non-fungicide treated plots,the photosynthesis rate ofA.adenophorafrom invaded habitats was significantly higher than that from partially invaded habitats(P=0.007)(Fig.1).However,in fungicide treated plots,no significant difference in photosynthesis ofA.adenophorabetween invaded habitats and partially invaded habitats(P=0.057).While in invaded habitats,fungicide treatment had significant impact on the photosynthesis ofA.adenophora(P=0.004),but not in partially invaded habitats(P=0.676).
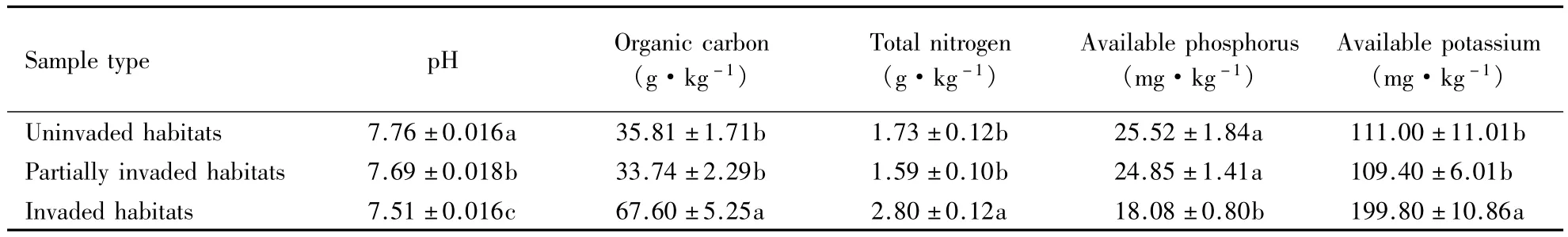
Table 1 Soil chemical contents at different invasion stages of A.adenophora

Fig.1 Photosynthesis rate of A.adenophora
Effect of fungicide treatment on A.adenophora leaf area at different invasion stages
No significant differences was found in the leaf area ofA.adenophorabetween invaded habitats and partially invaded habitats in either non-treated(P=0.224)or treated(P=0.089)plots(Fig.2).However,the leaf area ofA.adenophorain both invaded habitats and partially invaded habitats,was significantly different between fungicide and non-fungicide treated plots(for invaded habitatsP=0.034 and for partially invaded habitats,P=0.043),respectively.

Fig.2 Leaf area of A.adenophora
Effect of fungicide application on carbon,nitrogen,phosphorus and potassium contents in A.adenophora leaves at different invasion stages
In non-fungicide treated plots,the carbon concentration inA.adenophoraleaves from invaded habitats and partially invaded habitats,were not significantly different(P=0.65).The nitrogen and phosphorus content inA.adenophoraleaves from invaded habitats was 15.6%and 32.7%higher than those from partially invaded habitats(for nitrogen:P< 0.001 and for phosphorus:P=0.005),respectively.However,the potassium content inA.adenophoraleaves from invaded habittats was significantly lower than that from partially invaded habitats(P=0.03).In fungicide-trea-ted plots,the levels of carbon,nitrogen,phosphorus,and potassium inA.adenophoraleaves from invaded habitats and partially invaded habitats were 463.1,21.3,1.1 and 21.5 g·kg-1,which were 1.2%(P=0.02),14.1%(P=0.02),22.6%(P<0.001)and 67.5%(P=0.007)higher than that from partially invaded habitats,respectively.For both invaded habitats and partially invaded habitats,fungicide application resulted in a significant decrease in carbon(for invaded habitats:P=0.07;and for partially invaded habitats:P=0.007),nitrogen(for invaded habitats:P=0.02;and for partially invaded habitats:P=0.004),and phosphorus(P< 0.001)levels inA.adenophoraleaves.However,fungicide application resulted in a significant decrease in potassium content inA.adenophoraleaves from partially invaded habitats(P<0.001),but didn't increase the potassium content inA.adenophoraleaves from invaded habitats significantly(P=0.06)(Fig.3).
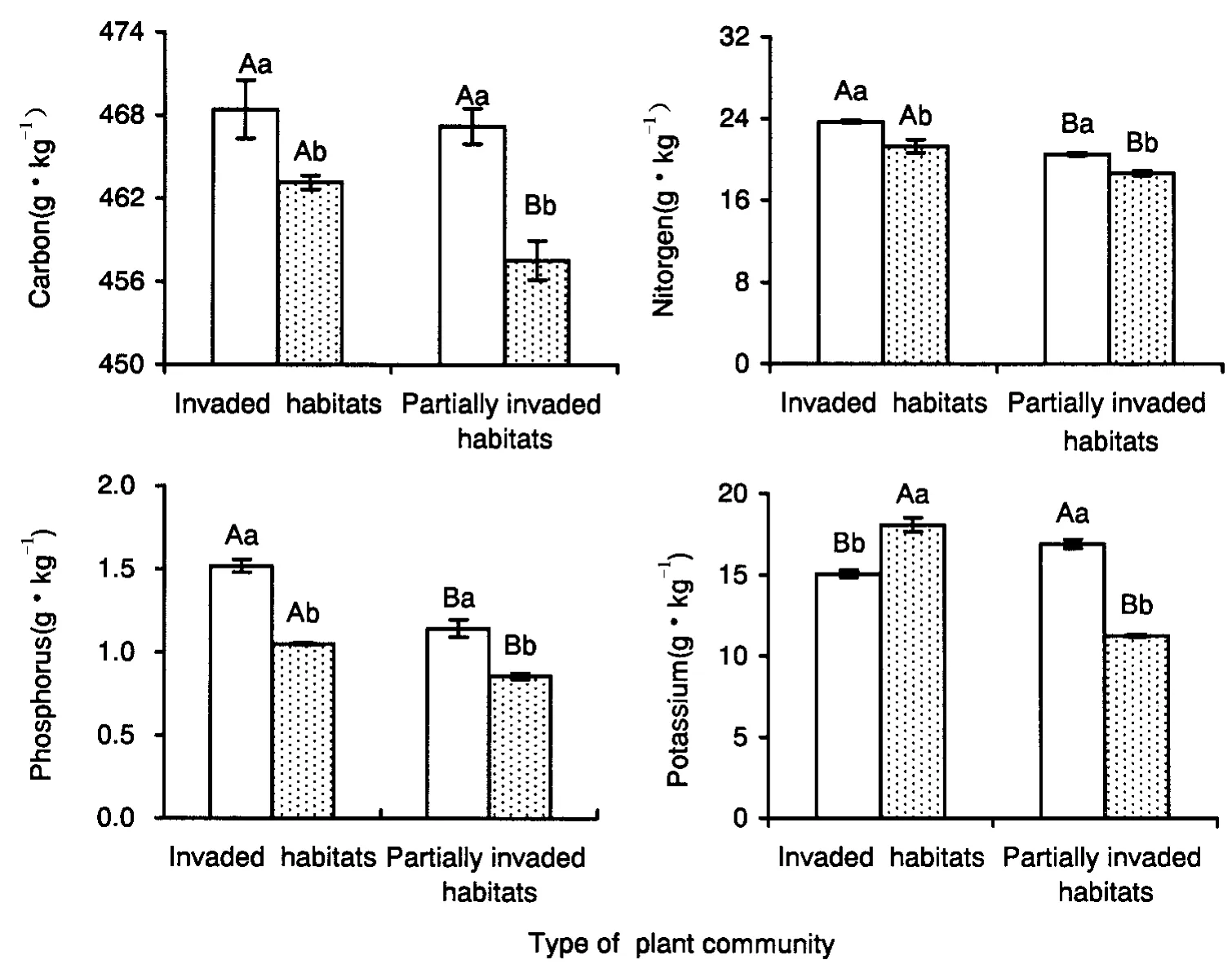
Fig.3 The carbon,nitrogen,phosphorus(P),and potassium(K)contents in A.adenophora leaves
Effect of fungicide application on stable carbon isotope content(δ13C)in A.adenophora leaves at different invasion stages
The stable carbon isotope content(δ13C)inA.adenophoraleaves from partially invaded habitats in non-treated plots was significantly higher than that from non-treated plots in invaded habitats(P=0.001)(Fig.4).In fungicide-treated plots,no significant difference was found in δ13C content between invaded habitats and partially invaded habitats(P=0.25).The δ13C values for both invaded habitats and partially invaded habitats in fungicide-treated plots was significantly lower than values for those same communities in non-treated plots(for invaded habitats:P=0.04 and for partially invaded habitats:P=0.002)respectively.
Effect of fungicide application on the carbon-to-nitrogen ratio in A.adenophora leaves at different invasion stages
The carbon-to-nitrogen ratios inA.adenophoraleaves in non-fungicide treated plots from both invaded habitats and partially invaded habitats were significantly lower than in fungicide treated plots(for invaded habitats A:P=0.04 and partially invaded habitats:P=0.006,respectively)(Fig.5).The carbon-to-nitrogen ratio inA.adenophoraleaves from invaded habitats was significantly lower than the ratio of leaves from partially invaded habitats,in both fungicide-treated and non-treated plots(for non-fungicide treatment:df=1,4;F=220.54,P<0.001 and for fungicide treatment:df=1,4;F=14.65,P=0.02),respectively.
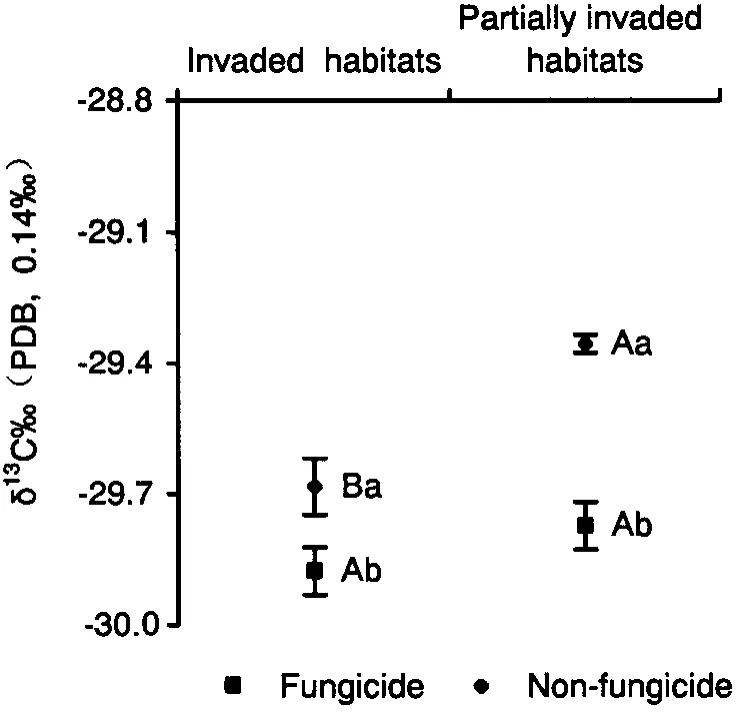
Fig.4 Stable carbon isotope content(δ13C)in A.adenophora leaves

Fig.5 The carbon to nitrogen ratio(C/N)of A.adenophora
Discussion
The feedback of soil nutrients and AMF community on A.adenophora which influenced by its invasion
The invasion ofA.adenophoradisturbed the balance of soil nutrients,which in turn influenced the nutrient status ofA.adenophora.Soil nutrients have a close relationship with the function of soil fungi,including AMF(Corkidiet al.,2002),and invasive plants may change the structure and function of the microbial community through metabolic activity to alter the soil physical-chemical properties and soil ecology(Kourtevet al.,2002;Manglaet al.,2008;Shahet al.,2008a,2008b),thereby creating favorable conditions for invasion(Dudaet al.,2003).
The levels of organic carbon,total carbon,and available potassium in the rhizosphere soil ofA.adenophorafrom invaded habitats were higher than those from partly invaded habitats,indicating that the invasive plant caused AMF to perform beneficial ecological soil-enriching functions,thereby creating a favorable nutrient feedback loop forA.adenophora,and AMF have a capability to degrade organic materials to inorganic components that plants can directly utilize,thereby promotingplantgrowth (Daehler,2003;Hodgeet al.,2001;Leighet al.,2009).
The different types of feedback from soil AMF toA.adenophoraat different invasion stages may result from the alteration of the AMF community structure and biodiversity caused by theA.adenophorainvasion itself(Yuet al.,2011,2012),as Shahet al.(2010)and Wolfe& Klironomos(2005)supported.
Soil fungi transfer carbon for A.adenophora
AMF may influence the way in whichA.adenophora(from partially invaded habitats)obtain carbon in other ways than photosynthesis.Careyet al.(2004)reported the similar conclusion.Evidence thatA.adenophoraobtained carbon from soil via AMF,or from the adjacent plants directly via carbon transfer was stable carbon isotope(δ13C)content inA.adenophoraleaves of different treatments.Soil AMF in invaded habitats promoted carbon accumulation,and enhanced δ13C con-tent.The δ13C content was-25.512‰ to-25.112‰ in red(Hapludult)soil(Qiet al.,2009),which was higher than that inA.adenophoraleaves,indicating thatA.adenophorawas exchanging carbon with soil.Therefore,A.adenophoragrew inA.adenophoradominated communities obtained carbon not only via photosynthesis,but also from soil organic substances.
The contribution of soil AMF in partially invaded habitats to the increase of carbon content was greater than that in invaded habitats.Therefore,soil AMF in partially invaded habitats transferred more carbon than that in invaded habitats.The contribution of soil AMF in partially invaded habitats to the increase of δ13C content was greater than that in invaded habitats,suggesting that soil AMF in partially invaded habitats may transfer carbon with a higher proportion of δ13C,which is possibly from adjacent C4plants.Our field investigation showed that most native plants adjacent toA.adenophora-invaded habitats were C4plants(such asSetaria faberiiHerrm and ferns).Careyet al.(2004)provided indirect evidence that AMF transferred carbon from the native plantFestuca idahoensisElmer toCentaurea cyanusL.using stable isotope and physiological methods.Giovannettiet al.(2006)also supported the view that mycorrhiza regulated carbon transfer from native to alien plants,tipping the competitive balance toward alien plants.
Soil fungi,including AMF,mainly created feedback beneficial toA.adenophora.Soil fungi can also transfer carbon from adjacent native plants toA.adenophora.As the feedback favorable toA.adenophoraestablished via a decrease in soil pH,an increase in organic carbon,total nitrogen and available potassium contents through soil fungi and the carbon transfer from soil or native plants via fungi,these may be important mechanisms ofA.adenophorainvasion.
Bao S D.2000.Soil and Agricultural Chemistry Analysis.Beijing:Chinese Agricultural Press,100-109(In Chinese).
Biermann B and Linderman R G.1981.Quantifying verculararbuscular mycorrhizas:a proposed method towards standardization.New Phytologist,87:63-67.
Callaway R M,Thelen G C,Barth S,Ramsey P W and Gannon J E.2004.Soil fungi alter interactions between the invaderCentaurea maculosaand North American natives.Ecology,85:1062-1071.
Callaway R M,Cipollini D,Barto K,Thelen G C,Hallett S G,Prati D,Stinson K and Klironomos J N.2008.Novel weapons:invasive plant suppresses fungal mutualists in America but not in its native Europe.Ecology,89:1043-1055.
Carey E V,Marler M J and Callaway R M.2004.Mycorrhizae transfer carbon from a native grass to an invasive weed:evidence from stable isotopes and physiology.Plant Ecology,172:133-141.
Corkidi,Rowland D,Johnson N C and Allen E B.2002.Nitrogen fertilization alters the functioning of arbuscular mycorrhizas at two semiarid grassland.Plant and Soil,240:299-310.
Daehler C C.2003.Performance comparisons of co-occurring native and alien invasive plants:implications for conservation and restoration.Annual Review of Ecology,Evolution,and Systematics,34:183-211.
Duda J J,Freeman C D,Emlen J M,Belnap J,Kitchen S G,Zak J C,Sobek E,Tracy M and Montante J.2003.Differences in native soil ecology associated with invasion of the exotic annual chenopod,Halogeton glomeratus.Biology and Fertility of Soils,38:72-77.
Fumanal B,Plenchette C,Chauvel B and Bretagnolle F.2006.Which role can arbuscular mycorrhizal fungi play in the facilitation ofAmbrosia artemisiifoliaL.invasion in France?.Mycorrhiza,17(1):25-35.
Giovannetti M,Avio L,Fortuna P,Pellegrino E,Sbrana C and Strani P.2006.At the root of the wood wide web:self recognition and nonself incompatibility in mycorrhizal networks.Plant Signaling&Behavior,1:1-5.
Hodge A,Campbell C D and Fitter H.2001.An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material.Nature,413:297-299.
Jia G K.2007.Distribution and harmfulness of alien invasive speciesEupatorium adenophorumin Guangxi.Journal of Baise University,20(3):90-95.
Kisa M,Sanon A,Thioulouse J,Assigbetse K,Sylla S,Spichiger R,Dieng L,Berthelin J,Prin Y,Galiana A,Lepage M and Duponnois R.2007.Arbuscular mycorrhizal symbiosis can counter balance the negative influence of the exotic tree speciesEucalyptus camaldulensison the structure and functioning of soil microbial communities in a sahelian soil.FEMS Microbiology Ecology,62:32-44.
Kourtev P S,Ehrenfeld J G and Häggblom M.2002.Exotic plant species alter the microbial community structure and function in the soil.Ecology,83:3152-3166.
Leigh J,Hodge A and Fitter A H.2009.Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material.New Phytologist,181:199-207.
Li H N,Liu W X,Dai L,Wan F H and Cao Y Y.2009.Invasive impacts ofAgeratina adenophora(Asteraceae)on the changes of microbial community structure,enzyme activity and fertility in soil ecosystem.Scientia Agricultura Sinica,42:3964-3971(In Chinese).
Liu R J and Chen Y L.2007.Myxorrhizology.Beijing:Science Press(In Chinese).
Mack R N.1996.Biotic barriers to plant naturalization∥Moran V C and Hoffman J H.Proceedings of the9th International Symposium on Biological Control of Weeds.Stellenbosch,South Africa:University of Cape Town,39-46.
Mangla S and Callaway R M.2008.Exotic invasive plant accumulates native soil pathogens which inhibit native plants.Journal of Ecology,96:58-67.
Marler M J,Zabinski C A and Callaway R M.1999.Myvorrhizae indirectly enhance competitive effects of an invasive forb on a native bunchgrass.Ecology,80:1180-1186.
Nijjer S,Rogers W E and Siemann E.2004.The effect of mycorrhizal inoculum on the growth of five native tree species and the invasive Chinese Tallow tree(Sapium sebiferum).Texas Journal of Science,56:357-368.
Niu H B,Liu W X,Wan F H and Li B.2007a.An invasive aster(Ageratina adenophora)invades and dominates forest understories in China:altered soil microbial communities facilitate the invader and inhibit natives.Plant and Soil,294:73-85.
Niu H B,Liu W X and Wan F H.2007b.Invasive effects ofAgeratina adenophoraSprengel(Asteraceae)on soil microbial community and physical and chemical properties.Acta Ecologica Sinica,27:3051-3060(In Chinese).
Olsen S R and Sommers L E.1982.Phosphorus//Page A L,Miller R H and Keeney D R.Methods of Soil Analysis,Part2.Madison,Wisconsin:Soil Sci.Soc.Am,Am Soc Agron Press,403-430.
Qi B,Ding L L,Cui J H and Wang Y H.2009.Measurement of organic carbon stable isotope composition of different soil types by EA-IRMS system.Journal of Nuclear Agricultural Sciences,23:492-496(In Chinese).
Reinhart K O and Callaway R M.2004.Soil biota facilitate exoticAcerinvasions in Europe and North America.Ecological Applications,14:1737-1745.
Reinhart K O and Callaway R M.2006.Soil biota and invasive plants.New Phytologist,170:445-457.
Richardson D M,Allsopp N,D'Antonio C M,Milton S J and Rejmánek M.2000.Plant invasions the role of mutualisms.Biological Reviews,75:65-93.
Shah M A,Reshi Z and Rashid I.2008a.Mycorrhizal source and neighbour identity differently influenceAnthemis cotulaL.invasion in the Kashmir Himalaya,India.Applied Soil E-cology,40:330-337.
Shah M A,Reshi Z and Rashid I.2008b.Mycorrhizosphere mediated Mayweed Chamomile invasion in the Kashmir Himalaya,India.Plant and Soil,312:219-225.
Shah M A,Reshi Z A and Khasa D P.2009.Arbuscular mycorrhizas:drivers or passengers of alien plant invasion.Botanical Review,75:397-417.
Shah M A,Reshi Z A and Rasool N.2010.Plant invasions induce a shift in Glomalean spore diversity.Tropical Ecology,51:317-323.
Stinson K A,Campbell S A,Powell J R,Wolfe B E,Callaway R M,Thelen G C,Hallett S G,Prati D and Klironomos J N.2006.Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms.PLoS Biology,4:727-731.
Wan F H,Liu W X,Guo J Y,Qiang S,Li B P,Wang J,Yang G Q,Niu H B,Gui F R,Huang W K,Jiang Z L and Wang W Q.2010.Invasive mechanism and control strategy ofAgeratina adenophora(Sprengel).Science China Life Sciences,53:1291-1298.
Wolfe B E and Klironomos J N.2005.Breaking new ground:soil communities and exotic plant invasion.Bioscience,55:477-487.
Xu H G,Ding H,Li M Y,Qiang X,Guo J Y,Han Z M,Huang Z G,Sun H Y,He S P,Wu H R and Wan F H.2006.The distribution and economic losses of alien species invasion to China.Biological Invasions,8:1495-1500.
Yu X J,Yu D,Lu Z J and Ma K P.2005.A new mechanism of invader success:exotic plant inhibits natural vegetation restoration by changing soil microbe community.Chinese Science Bulletin,50:1105-1112.
Yu W Q,Liu W X and Wan F H.2011.Effects of exotic plantAgeratina adenophorainvasion on mycorrhizal fungal community.Chinese Journal of Eco-Agriculture,19:883-889(In Chinese).
Yu W Q,Liu W X,Gui F R,Liu W Z,Wan F H and Zang L L.2012.The effects of exoticAgeratina adenophoraSprengel invasion on soil physical and chemical characteristics and arbuscular mycorrhizal fungi.Acta Ecologica Sinica,32:7027-7035(In Chinese).
