一种建立实验犬经皮左心耳封堵途径的方法
2014-06-09张志钢李长永谭洪文储国俊朱玉峰白元许旭东熊文峰黄新苗赵仙先吴弘秦永文
张志钢,李长永,谭洪文,储国俊,朱玉峰,白元,许旭东,熊文峰,黄新苗,赵仙先,吴弘,秦永文
·实验研究Experimental research·
一种建立实验犬经皮左心耳封堵途径的方法
张志钢,李长永,谭洪文,储国俊,朱玉峰,白元,许旭东,熊文峰,黄新苗,赵仙先,吴弘,秦永文
目的验证一种建立实验犬经皮左心耳封堵途径方法的安全性及可行性。方法12只实验犬房间隔穿刺后在不同体位左房造影后测量左心耳颈部直径,沿导丝送入输送封堵器的鞘管至左房中部,沿鞘管送入猪尾导管至左心耳内,沿猪尾导管推送输送长鞘进入左心耳内,退出猪尾导管经长鞘管造影观察鞘管在左心耳内的位置。术后1 h行心电图及经胸超声检查,即刻处死5只实验犬,取心脏观察房间隔穿刺位置、左房及左心耳内损伤情况。其余犬术后1 h及2周经胸超声心动图检查,随访1个月。结果术中1只犬因心脏压塞死亡。8只犬在RAO30°+CRA20°造影可以清楚显示左心耳形态,3只在RAO30°,1只在RAO30°+CAU20°清楚显示左心耳形态,测量左心耳颈部直径为(13.6±5.2)mm,输送长鞘管均成功送入左心耳远端,无气栓、血栓、心脏压塞,2只穿刺点血肿,经加压包扎血肿吸收。术后即刻处死犬取心脏观察心包腔内未见血性液体,1只犬左房后壁轻度血肿,2只左心耳上缘内膜轻度损伤可见血肿,手术操作时间(58±12)min,透视时间(10.1±2.5)min。其余犬术后及2周经胸超声随访无心包积液。随访1个月无猝死、卒中、感染。结论应用本方法可安全有效的建立输送左心耳封堵器至左心耳内的途径。
心房纤颤;左心耳;封堵器;犬
血栓栓塞是心房颤动(房颤)最严重的并发症,导致非瓣膜性房颤患者脑卒中的栓子90%以上来源于左心耳[1]。外科瓣膜手术时结扎左心耳可以降低房颤患者脑卒中风险[2],但是没有外科指征的单纯左心耳结扎会增加致残率和病死率。随着介入器械的发展,近年经皮左心耳封堵术成为预防房颤患者脑卒中的新方法,目前大部分左心耳封堵器械的动物实验都是使用实验犬完成的[3-4],但是关于建立实验犬左心耳封堵器械植入途径方法的报道较少。本课题组探索了一种建立犬经皮左心耳封堵器械途径的方法。
1 材料与方法
1.1 材料
1.1.1 实验动物健康成年杂种犬12只,体质量12~15 kg,雌雄不限;由上海甲干生物科技有限公司提供。
1.1.2 实验器械0.032英寸导引导丝及左房钢丝,8 FSwartz房间隔穿刺鞘管,头端弯曲角度塑形增大的Brockenbrough穿刺针,6 F防漏鞘管及猪尾导管,8~10 F塑形的室间隔输送长鞘管及扩张管,V形实验动物固定架。
1.1.3 实验仪器X线放射成像系统,心电图机,心电压力监测仪及彩色超声诊断仪。
1.1.4 实验药品2.5%戊巴比妥钠,氯胺酮,对比剂(碘普罗胺注射液),青霉素钠粉针。
1.2 方法
1.2.1 房间隔穿刺及左心耳造影实验犬以氯胺酮5 mg/kg肌内注射,麻醉后以特制固定架仰卧固定犬于手术台上,心电监护。常规消毒铺无菌巾单,穿刺股静脉后置入鞘管,以2.5%戊巴比妥钠1m l/kg静脉注射维持麻醉效果。沿鞘管送入0.032英寸导丝至上腔静脉,退出鞘管沿导丝送入Swartz房间隔穿刺鞘管至上腔静脉,按照王胜强等[5]报道的方法进行房间隔穿刺,穿刺时推送穿刺针卵圆窝上部滑动时,按照董建增等[6]报道的方法进行穿刺。穿刺成功后,固定穿刺针推送鞘管超出穿刺针1~2 mm,退出穿刺针,确认无心脏压塞后给予肝素(80~100 u/kg),沿扩张鞘置入0.032英寸左房两圈半钢丝至左房内弯曲2~3圈,固定导丝及扩张鞘,推送Swartz鞘至左房中部,退出扩张鞘,沿导丝经Swartz鞘送入猪尾导管至左心房后,退出导丝于后前位,右前斜30°+头位20°(图1),右前斜30°及右前斜30°+足位20°手推对比剂行左房造影,选择心耳颈部清晰显影的体位测量左心耳颈部直径。

图1 左心房造影(右前斜30°+头位20°)
1.2.2 建立植入经皮左心耳封堵器械途径左心房造影后,沿猪尾导管送入左房钢丝至左房,退出猪尾导管及Swartz鞘管,沿左房钢丝送入经改装的室间隔输送长鞘及扩张鞘至左房中部,固定扩张鞘及导丝推送输送鞘至左房中部,保留左房钢丝退出扩张管。沿左房导丝送入猪尾导管至左房中部,退出导丝。调整猪尾导管进入左心耳内,推注对比剂证实在左心耳内后,固定猪尾导管沿猪尾导管推送输送长鞘管进入左心耳内(图2),轻微调整猪尾导管及输送长鞘的位置使鞘管安全位于左心耳内并且具有良好同轴性,位置理想后回抽猪尾导管内血液,边缓慢退出猪尾导管边向猪尾导管内注射肝素盐水,排除输送鞘管内空气,经鞘管造影观察鞘管在左心耳内的位置(图3)。
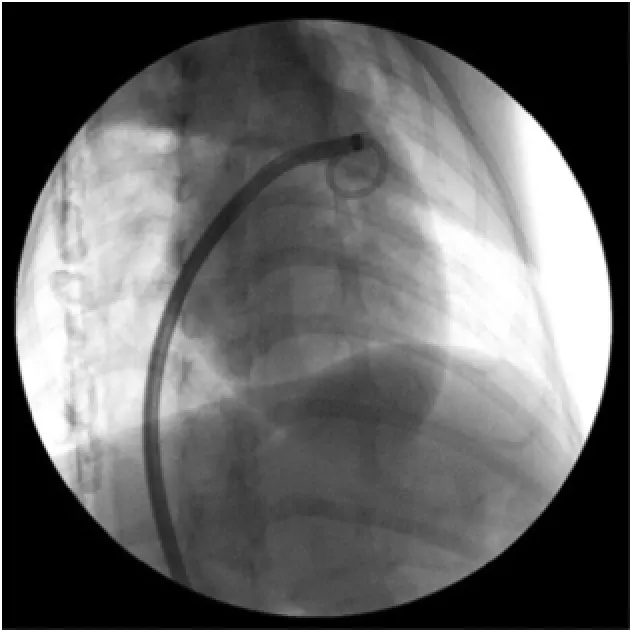
图2 沿猪尾导管推送输送鞘管至左心耳内(右前斜30°+头位20°)
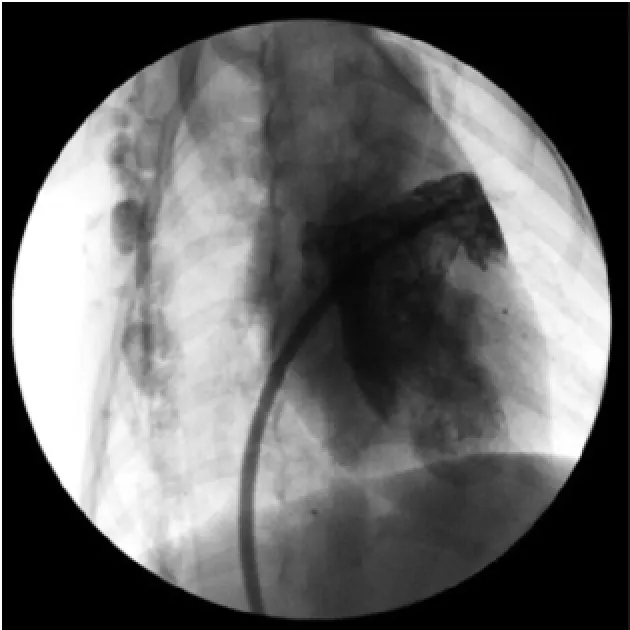
图3 经输送鞘管造影观察鞘管位置(右前斜30°+头位20°)
1.2.3 术后处理及随访术后心电监护1 h后行心电图和心脏超声检查,处死5只实验犬,取心脏标本观察房间隔穿刺位置及左房内损伤情况。其余犬术后经胸超声心动图观察有无心包积液后给予青霉素10万u/kg静脉推注后拔除鞘管,穿刺点”8”字缝合[7]。随访犬术后3 d青霉素肌内注射预防感染,2 d后拆线,2周时行经胸超声心动图检查,随访1个月观察卒中、感染等手术并发症。
2 结果
12只实验犬中9只1次穿刺房间隔成功,1只第2次穿刺房间隔成功,无1只犬房间隔穿刺术中发生心脏压塞,房室传导阻滞。12只犬中8只在RAO30°+CRA20°(图1)、3只在RAO30°,1只在RAO30°+CAU20°投照体位造影可以清楚显示左心耳形态,测量左心耳开口直径为(13.6±5.2)mm,输送鞘管均成功送入左心耳远端,术中无气栓、血栓、心脏压塞。术后处死犬取心脏观察见心包腔内未见血性液体,房间隔穿刺点位于卵圆孔近似中央,周围轻度血肿,边缘整齐无撕裂,距离上腔、下腔静脉,冠状窦及左右房室瓣口距离均>5 mm,1只犬左房后壁轻度血肿,2只左心耳上缘内膜轻度损伤可见血肿,手术操作时间(58±12)min,透视时间(10.1±2.5)min。未处死6只犬2只穿刺点血肿,经加压包扎血肿吸收。术后及2周均无心包积液。随访1个月无猝死、卒中、感染等并发症。
3 讨论
经皮左心耳封堵术是近年发展起来的一种替代华法令预防房颤脑卒中事件的治疗措施,尤其适用于有抗凝禁忌证的高危脑卒中房颤患者[8]。由于犬心耳的解剖形态与人体相似目前经皮左心耳封堵的动物实验均是使用犬完成的[9]。术中房间隔穿刺成功后置入左房0.032英寸导丝,沿导丝置入猪尾导管后进行左心耳造影,左房0.032英寸两圈半钢丝不仅能够提供有效的支撑,其头端弯曲能防止穿刺鞘及导丝从左房滑出以及器械损伤左房及左心耳,是建立经皮左心耳封堵途径的理想工作导丝。左心耳造影是评估左心耳大小及形状的重要方法之一。造影时在房间隔穿刺鞘送入6 F猪尾导管一方面可以避免交换导管时导丝脱出左心房,另一方面,使猪尾导管后端侧孔位于穿刺鞘内,造影时不会造成左右心房同时显影影响观察。左心耳造影时的投照体位文献报道不尽相同[9-10],我们通过观察对比不同体位的影像发现,大多数犬在右前斜30°加头位20°造影可清晰显示左心耳的开口、颈部直径及形态,当该体位造影心耳影像重叠时可在右前斜30°或者右前斜30°加足位20°造影显示左心耳形态。
左心耳封堵术中心脏压塞、气栓等并发症限制了该技术的推广[11],并发症主要发生于房间隔穿刺、操作鞘管、释放及回收封堵器时[12],随着术者经验积累手术并发症可明显降低[13]。Watchman植入过程中将鞘管经导丝经房间隔送入左房后,送入猪尾导管,然后先将猪尾导管送入左上肺静脉再逆时针旋转导管使其进入左心耳内,沿左心耳内猪尾导管送入输送长鞘减少心脏压塞并发症[14],而动物实验通常将输送鞘管直接置入左心耳内[3,15]。我们在动物实验中发现,也可以直接应用左房钢丝将猪尾导管送入左心耳远端,然后沿猪尾导管将输送长鞘送入左心耳远端,利用猪尾导管头端弯曲替代扩张鞘管的尖形头端可减少心耳损伤,并且猪尾导管直径较导丝粗可防止鞘管头端顶在心耳内壁上,有利于安全将鞘管送入左心耳远端。实验中通过术后即刻解剖实验犬发现无心包血性积液,大体观察心脏标本,其中2只动物左心耳上缘轻度血肿,心耳内无穿孔、血栓,其余犬均无心包填塞表现。左心耳壁薄,内有小梁及梳状肌,在调整输送鞘的位置时需要小心操作,尤其是推送鞘管时防止鞘管损伤左心耳上缘导致穿孔。
空气栓塞及血栓是左心耳封堵术中的并发症,操作中经常回抽鞘管并以肝素盐水冲洗可以减少此类并发症[12]。输送长鞘进入左心耳后,由于导管头端与心耳壁接触可造成回抽液体不通畅,无法进行有效排气,在退出其内的猪尾导管时边缓慢回撤导管边向猪尾导管内注射肝素盐水是避免气栓及血栓的方法之一,也可以略微回撤长鞘、回抽液体后常规冲洗排气但是这样可能使鞘管移位。本实验中存活犬随访1个月均无与手术操作相关的并发症。
经皮左心耳封堵术是一项创伤较小,操作相对简单的介入治疗,随着介入器械的发展及手术医师经验的积累,以及更多临床相关研究的进行,该技术将更加完善,其适应证会进一步拓展。目前欧洲指南中将左心耳封堵作为有抗凝禁忌的房颤脑卒中高危患者的一项治疗措施[16]。本研究中探索的方法可以有效的建立输送左心耳封堵器至预定封堵部位的途径,动物实验证实该方法安全、可行由于样本量少,使用经验有限,本法尚需进一步进行验证。
[1]Blackshear JL,Odell JA.Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation[J]. Ann Thorac Surg,1996,61:755-759.
[2]García-Fernández M,Pérez-David E,Quiles J,et al.Role of left atrial appendage obliteration in stroke reduction in patients withmitral valve prosthesis:a transesophageal echocardiographic study[J].JAm Coll Cardiol,2003,42:1253-1258.
[3]Nakai T,Lesh MD,Gerstenfeld EP,et al.Percutaneous left atrial appendage occlusion(PLAATO)for preventing cardioembolism:first experience in caninemodel[J].Circulation,2002,105:2217-2222.
[4]Lam Y-Y,Yan BP,Doshi SK,et al.Preclinical evaluation of a new left atrial appendage occluder(Lifetech LAmbreTMdevice)in a caninemodel[J].Int JCardiol,2013,168:3996-4001.
[5]王胜强,秦永文,胡建强,等.经静脉房间隔穿刺法建立房间隔缺损动物模型[J].介入放射学杂志,2004,13:159-160.
[6]董建增,曹林生,马长生,等.下腔静脉造影指导犬房间隔穿刺术[J].中国介入心脏病学杂志,2005,13:111-113.
[7]Cilingiroglu M,Salinger M,Zhao D,et al.Technique of temporary subcutaneous“Figure-of-Eight”sutures to achieve hemostasis after removal of large-caliber femoral venous sheaths[J].Catheter Cardiovasc Interv,2011,78:155-160.
[8]Horstmann S,Zugck C,Krumsdorf U,et al.Left atrial appendage occlusion in atrial fibrillation after intracranial hemorrhage[J].Neurology,2014,82:135-138.
[9]Bass JL.Transcatheter occlusion of the left atrial appendage—experimental testing of a new Amplatzer device[J].Catheter Cardiovasc Interv,2010,76:181-185.
[10]杨志宏,吴弘,胡建强,等.左心耳造影方法的建立及最佳投照体位的研究[J].介入放射学杂志,2006,15:494-496.
[11]Holmes DR,Reddy VY,Turi ZG,etal.Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation:a randomised noninferiority trial[J].Lancet,2009,374:534-542.
[12]Price MJ.Prevention and Management of Complications of Left Atrial Appendage Closure Devices[J].Intervent Cardiol Clin,2014,3:301-311.
[13]Reddy VY,Holmes D,Doshi SK,et al.Safety of percutaneous left atrial appendage closure:results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF(PROTECT AF)clinical trial and the Continued Access Registry[J].Circulation,2011,123:417-424.
[14]Nakamura M,Kar S.Transcatheter Occlusion of the Left Atrial Appendage[J].Intervent Cardiol Clin,2013,2:225-234.
[15]Cheng Y,Conditt G,Yi G,et al.First in-vivo evaluation of a flexible self-apposing left atrial appendage closure device in the canine model[J].Catheter Cardiovasc Interv,2013,[Epub ahead of print].
[16]Camm AJ,Lip GY,De Caterina R,et al.2012 focused update of the ESC Guidelines for the management of atrial fibrillation:an update of the 2010 ESC Guidelines for the management of atrial fibrillation.Developed with the special contribution of the European Heart Rhythm Association[J].Eur Heart J,2012,33:2719-2747.
Percutaneous occlusion of left atrial appendage in experimental canine models:the establishment of
the delivery pathway ZHANG Zhi-gang,LIChang-yong,TAN Hong-wen,CHU Guo-jun,ZHU Yu-
QINYong-wen,E-mail:qyw2011@126.com
ObjectiveTo evaluate the feasibility and safety of a delivery pathway for the performance of percutaneous left atrial appendage(LAA)occlusion in experimental caninemodels.MethodsTransseptal puncture was performed via femoral vein approach under fluoroscopic and angiographic guidance in 12 experimental dogs.A pigtail catheter was advanced into the left atrium(LA),which was followed by LA angiography.The diameters of the neck of LAA were measured on LAA angiogram obtained in appropriate projection.A fter the delivery sheath was advanced along the wire into LA,a pigtail catheter was inserted into the ostium of the LAA and the sheath was then advanced over the pigtail into the LAA.LAA angiography was then performed through the delivery sheath to confirm the position of the delivery sheath.One hour after the procedure both electrocardiography(ECG)and transthoracic echocardiography(TTE)were carried out in five dogs to check the results,immediately afterwhich the five dogswere sacrificed tomacroscopically observe the damages of the puncture site of inter-atrial septum as well as inside the LA and LAA.One hour and 2 weeks after the procedure TTE was conducted in the remaining 7 dogs and these dogs were followed up for one month.ResultsOne dog died of pericardial tamponade during the operation.In 8 dogs the LAA was clearly displayed in the projection position of right anterior oblique(RAO)30°/cranial(CRA)20°,while in 3 dogs the LAA was well visualized in the projection position of RAO 30°,and in one dog in the projection position of RAO 30°/caudal(CAU)20°.The diameter of LAA neck was(13.6±5.2)mm.The delivery sheath was safely advanced into the LAA along the pigtail catheter in all dogs,and no air embolism,thrombus or pericardial tamponade occurred.Hematoma at puncture point of groin occurred in 2 dogs,which was absorbed through pressure dressing.Macroscopic examination of the heart performed immediately after the operation showed that no bloody pericardial effusion was found,and mild hematoma at posterior wall of LA was seen in one dog and mild damage of the upper-margin intima of LAA was noted in 2 dogs.Themean fluoroscopy timewas(10.1±2.5)minutes and themean operation timewas(58±12)minutes.TEE showed no pericardial effusion 2 weeks after the procedure.During the follow-up period of one month no sudden death,stroke or infection occurred.Conclusion Thismethod of placing the delivery sheath into the LAA is clinically safe and effective,and it can reliably establish a pathway to advance the LAA occluder into LAA.(J Intervent Radiol,2014,23:897-900)
atrial fibrillation;leftatrial appendage;occluder;dog
R541.7
B
1008-794X(2014)-10-0897-04feng,BAIYuan,XU Xu-dong,XIONGWen-feng,HUANG Xin-miao,ZHAO Xian-xian,WU Hong,QIN Yong-wen.Department of Cardiology,Changhai Hospital,Second Military Medical University,Shanghai 200433,China
2014-04-07)
(本文编辑:李欣)
10.3969/j.issn.1008-794X.2014.10.015
上海市自然科学基金BZR1409500;上海市卫生局级科研课题重点课题
200433上海第二军医大学长海医院心内科[张志钢(现在南京军区福州总医院心内科)、李长永、谭洪文、储国俊、朱玉峰、白元、许旭东、黄新苗、赵仙先、吴弘、秦永文],超声科(熊文峰)
秦永文E-mail:qyw2011@126.com