Changes in motor function in the unaffected hand of stroke patients should not be ignored
2014-06-01LingliZhangPeihongLiZhibangMaoXiangQiJunZouZhushengYu
Lingli Zhang, Peihong Li, Zhibang Mao, Xiang Qi, Jun Zou, Zhusheng Yu
Shanghai University of Sport, Shanghai 200438, China
Changes in motor function in the unaffected hand of stroke patients should not be ignored
Lingli Zhang, Peihong Li, Zhibang Mao, Xiang Qi, Jun Zou, Zhusheng Yu
Shanghai University of Sport, Shanghai 200438, China
Motor function changes in the unaffected hand of stroke patients with hemiplegia. These changes are often ignored by clinicians owing to the extent of motor disability of the affected hand. Finger tapping frequency and Lind-mark hand function score showed that the motor function of unaffected hands in stroke patients was poorer than that of a healthy control hand. After 2 weeks of rehabilitation treatment, motor function of the unaffected hand of stroke patients was obviously improved. Therefore, attention should also be paid to motor function in the unaffected hand of stroke patients with hemiplegia during rehabilitation.
nerve regeneration; stroke; unaffected hand; dominant hand; right hand; finger tapping; Lind-mark hand function assessment; rehabilitation training; neural regeneration
Zhang LL, Li PH, Mao ZB, Qi X, Zou J, Yu ZS. Changes in motor function in the unaffected hand of stroke patients should not be ignored. Neural Regen Res. 2014;9(13):1323-1328.
Introduction
Hands play an important role in people’s daily life, with fi ne movements including percussion, swing, pinching and grasping. Rapid fi nger tapping is a time-honored task often used for assessment of motor skills (Heuer and Schulna, 2002), and can be used as a method of detection, control, evaluation and validation to assess the integrity of the neuromuscular system. It has been studied, for example, in research on handedness (Peters, 1980), on individual differences in skill acquisition (Ackerman and Cianciolo, 2000), and on neurobehavioral effects of toxic agents (Baker et al., 1985), as well as in clinical neurological examinations (Shimoyama et al., 1990).
Finger tapping has the advantage of being a relatively pure neurologically driven motor task because the inertial and intersegmental interactions are so small that biomechanical in fl uences on movement are reduced (Liu et al., 2006). Finger tapping occurs via a single joint movement. Time, amplitude and frequency are three important characteristics of this action.
With effective rehabilitation, stroke patients can partially regain their motor control and continue their activities of daily living (Ang et al., 2011). As the patient’s unaffected hand plays a significant role in everyday life, whether the function of the unaffected hand following stroke is affected is directly relative to the patient’s viability and living quality, and is therefore an important question to answer. Whether the function of the unaffected hand in stroke patients is changed is still inconclusive in the clinical setting.
In this study, we quantified the subjects’ finger tapping frequency (finger tapping), compared the finger tapping frequency with the traditional evaluation hand function scales pre- and post-treatment, and analyzed the correlation between these parameters. These studies provided a reference about tapping frequency applications in hand function assessment.
Subjects and Methods
Part one
Stroke patients
Thirty stroke patients with right cerebral damage and hemiplegia (18 males and 12 females) who were admitted to Yangpu District Old Hospital, China and the Neurological Rehabilitation Department of Hospital of Shanghai University of Sport, China from February 1 to March 31, 2012 were included in this study. These patients included 9 cases of cerebral hemorrhage, 17 cases of cerebral infarction and 4 cases of cerebral trauma.
Inclusion criteria: (1) stroke patients with right cerebral damage who presented with limb hemiplegia; (2) conformation to the diagnosis standard of the 4thNational Cerebrovascular Disease Academic Conference (Journal of Neuroscience and Neurosurgery Society, 1996) and examinations of CT and MRI of skull; (3) conscious, completely normal ability of listening and understanding (Liao and Zhu, 1996), stable vital signs within 48 hours, and actively cooperated with treatment and inspection; (4) fi rst onset stroke, course within 13-month duration; (5) right-handed; (6) able to maintain normal sitting position or posture with normal level of help. Exclusion criteria: (1) visually impaired; (2) consciousness disorder or serious cognitive dysfunction; (3) unable to maintain the normal sitting position or to swing the unaffected fore fi nger.
Healthy subjects
Thirty-eight healthy right-handed subjects were selectedfrom patients’ families as the control group.

Table 1 Comparison of subject gender and age

Table 2 Basic clinical information of patients

Table 3 Lind-mark hand function assessment criteria

Table 4 Lind-mark hand function assessment criteria
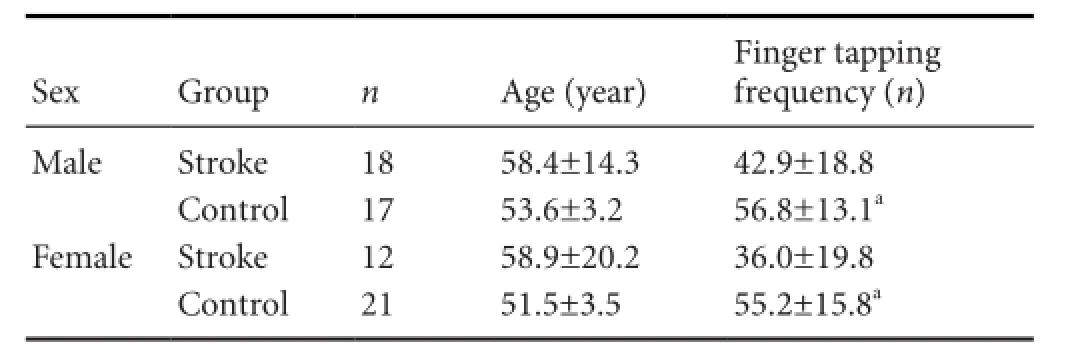
Table 5 Finger tapping comparison between males and females

Table 6 Changes in finger tapping frequency and Lind-mark scores pre-and post-treatment
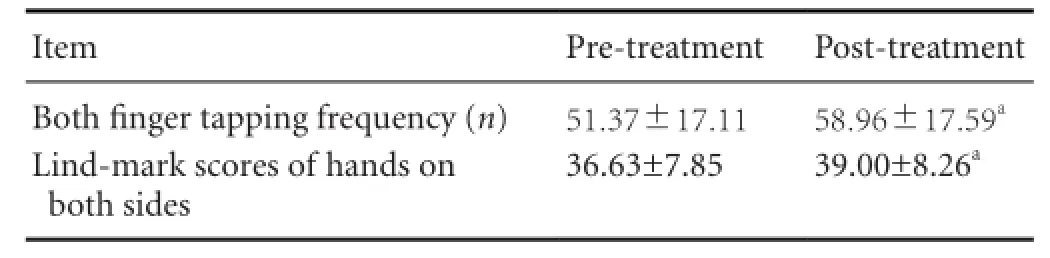
Table 7 Finger tapping frequency and Lind-mark scores pre- and post-treatment
The basic clinical information of all participants is shown inTable 1. Informed consent was obtained from all participants. The study protocol was approved by the Ethics Committee of Shanghai University of Sport, China.
Part two
Stroke patients
Based on case records and questionnaires, twenty-seven stroke patients who were admitted to the Neurological Rehabilitation Departments of Shanghai Tianshan Hospital and the Shanghai 7thPeople’s Hospital, China from July 2013 to March 2014 were also included in this study. The basic clinical information of these patients is shown inTable 2.
All the cases conformed to the diagnosis standard of the 4thNational Cerebrovascular Disease Academic Conference and examinations of CT and MRI of skull.
Inclusion criteria:(1) First onset; (2) conscious, completely normal ability of listening and understanding, and can actively cooperate with treatment and inspection; (3) right-handed before the onset of this disease; (4) able to maintain a sitting position or posture with normal level of help; (5) hand function assessment above Ii in Brunnstrom, which means the finger showed slight flexion (Yoo et al., 2014). Exclusion criteria: Consciousness disorder or serious cognitive dysfunction.
All patients underwent 2 weeks of rehabilitation training, including beam position, stretching exercises, mat exercises, inverse technique, position control, sitting to standing up, walking up and down the stairs, upper limb movement training, muscle strength training, endurance training, and auxiliary functional electrical stimulation. Before and after treatment, all subjects were subjected to 8-second index finger tapping frequency and Lind-mark hand function assessment by trained physicians. The study protocol was approved by the Ethics Committee of Shanghai University of Sport in China.
Finger tapping test
The fi nger tapping test, a method of measuring the speed reaction of the human body through the frequency and speed of fi nger tapping (Figure 1; Yu et al., 2010; Invention patent No. 200410017340.1), was used to reflect nervous system frequency and distribution of impulse through the information from finger tapping frequency. The test uses infrared photoelectric sensors to detect finger tapping movements. The signal was detected through a serial input Microsoft computer by controlling and recording time accuracy up to a millisecond, and results were fi nally displayed on the computer screen in Microsoft Excel 2010 database (Figure 2).
Lind-mark hand function assessment
The Lind-mark scale was created based on the Fugl-Meyer-Assessment (FMA) scale by the Swedish scholar Birgitta (Arya et al., 2014). The Lind-mark scores involve more details than the FMA scale. The assessment contents are (1) fi nger fl exion; (2) fi nger extension; (3) pointed thumb and index fi nger; (4) hook grip: grip a stick, with metacarpophalangeal joint extension and interphalangeal joint fl exion; (5) side grip: clip a piece of paper between the thumb and forefinger (thumb should be in straight adduction); (6) pinch grip: grip a pen with thumb and index fi nger; (7) cylindrical grip: using the thumb and fore fi nger to hold a cup; (8) spherical grip: hold a tennis ball with fi ngers apart. Each index was used in each of abovementioned eight movements. A total score of 24 points was possible. Scoring criteria are shown inTable 3.
Design and procedure
The medical history of subjects was reviewed with permission from the hospital and the selected eligible patients. Patients and their families were contacted, and the purpose, procedure and relative points for attention were explained to them. All the patients signed the informed consent. Medical staff explained the questionnaire covering central nerve injury. We arranged speci fi c test times after the consultation with patients and family members. Patients cooperated to actively and voluntarily complete the test.
The unified testing time was set in software before the test. In high-intensity and fast-paced sport, the energy supply system is mainly composed of ATP creatine phosphate and glycolysis. To reduce the effect of fatigue on the speed of index fi nger tapping, the test time was set to 8 seconds. The swing of index fi nger can comparatively eliminate factors of muscle strength, speed endurance and strength endurance and correctly measure neural response speed and movement frequency (Liu et al., 2006).
Subjects were asked to sit in a normal posture during the test (head straight, eyes staring in front of the swing frame), metacarpophalangeal joint arch, palm heel and three lateral fingers touching the desktop, and index finger stretched into the swing frame. Different subjects have different fi nger lengths, so swing angles differed to achieve the same oscillation amplitude. To reduce the in fl uence on test results, subjects were asked to put on a uni fi ed set of 8-cm-long light extender to adjust the index fi nger length and index fi nger amplitude. Angle of swinging was controlled up to 30°. A swing frame to limit height of finger tapping was used. If the swing amplitude of the index finger extender was not up to the height of the frame, the result was distinguished automatically by the instrument as error data.
Subjects can process formal tests after one or two exercises. The tester pressed the “start” button when the subject was ready. The subject heard the starting gun as soon as his fi nger swung rapidly, and at the same time, the instrument automatically started timing. When setting time was over, the data were recorded into the computer. The frequency from hearing the sound of the pistol to starting to swing the index fi nger was within 8 seconds.
Statistical analysis
SPSS 13.0 software (SPSS, Chicago, IL, USA) was used to analyze data, which were expressed as mean ± SEM. Inter-group statistical differences were compared using two-sample t-test, and paired samples t-test was used for intra-group comparison. Correlation between fi nger tapping frequency and Lindmark scores was analyzed using Pearson’s correlation analysis. P values < 0.05 were considered statistically signi fi cant.
Results
Finger tapping comparison between stroke group and control group
Judging from the group only and excluding the influence of age and gender, finger tapping frequency of the control group’s dominant hand was significantly higher than the stroke group’s right hand (P < 0.05;Table 4).
By gender, fi nger tapping frequency of dominant hands in the males and females in control group was signi fi cantly higher than the stoke group (P < 0.05;Table 5).
Changes in patients’ fi nger tapping frequency and Lindmark scores pre- and post-treatment
After 2-week rehabilitation treatment, the affected finger tapping frequency and Lind-mark scores were both signi ficantly improved compared with before treatment (P < 0.01;Table 6).
After 2-week rehabilitation, the sum of both fi nger tapping frequency and Lind-mark scores were signi fi cantly improved compared with that before treatment (P < 0.01;Table 7).
The sum of fi nger tapping frequency correlated positively with Lind-mark scores pre- and post-treatment
As shown inFigure 3, there was a positive correlation between sum of finger tapping frequency and Lind-mark scores pre- and post-treatment (r = 0.460, 0.469, P < 0.05).
Discussion
One method of assessing the integrity of the neuromuscular system and examining motor control issues is to measure an individual’s ability to tap their fingers. Finger tapping has the advantage of being a relatively pure neurologically driven motor task because the inertial and intersegmentalinteractions are so small that biomechanical influences on movement are reduced (Liu et al., 2006).
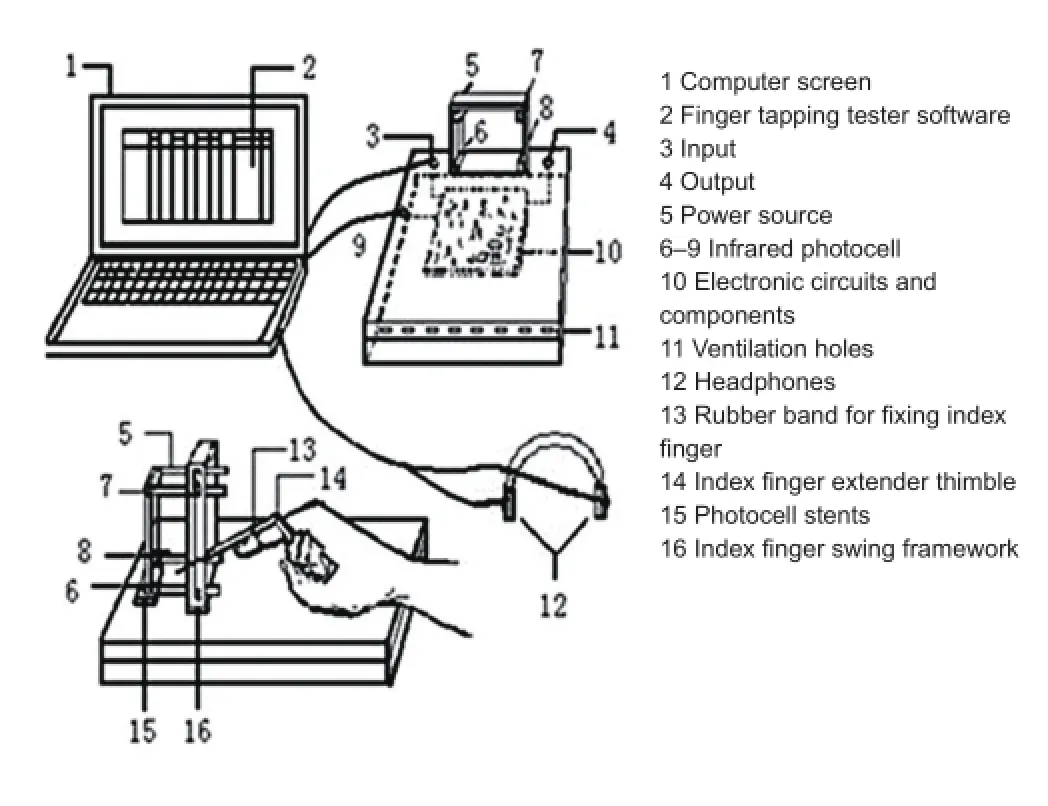
Figure 1 Representation illustrating the fi nger tapping tester.

Figure 2 Representation illustrating the fi nger tapping tester.

Figure 3 The sum of fi nger tapping frequency correlated positively with Lind-mark scores pre- and post-treatment in patients (Pearson’s correlation analysis).
In rehabilitation, improvements in motor learning are achieved through goal-directed repetitive exercise. Repetitive exercise itself can increase motor learning (Lee et al., 2012). Finger tapping is applied in the fi eld of rehabilitation, which mainly includes the disease assessment and prognostic evaluation of patients with cerebral palsy, cerebrovascular accident and craniocerebral injury (Werhahn et al., 2003; Gebruers et al., 2010), and Parkinson’s disease (Bernatzky et al., 2004; Ellis et al., 2008; Lou et al., 2009; Kano et al., 2013; Keitel et al., 2013; Miller et al., 2013; Robles-García et al., 2013; Ayán et al., 2014; Khan et al., 2014). Finger tapping can also aid in the detection of corticospinal excitability in combination with transcranial magnetic stimulation (Ameli et al., 2009; Roosink and Zijdewind, 2010; Lee et al., 2012; Orosz et al., 2012), developmental coordination disorder (Whitall et al., 2008; Roche et al., 2011), complex regional pain syndrome (Ben-Pazi et al., 2006; Scharoun et al., 2013), attention deficit hyperactivity disorder (Maihöfner et al., 2007), and evaluation of the piano (Loehr and Palmer, 2007; Szirmi, 2010; Slater et al., 2013).
The finger tapping frequency of the unaffected hand of the stroke patients was evidently lower than that of the dominant hand of the healthy group, which illustrates that the hemiplegic patients’ healthy hand function and fi ne activities have been weakened. The disability of the ipsilateral (unaffected) limb often is hidden by the contralateral side’s hemiplegia and sensation disorders (Weng et al., 2003).
This phenomenon occurs because, on one hand, ipsilat-eral cerebral stroke affects the contralateral limb function, as 80% of nerves from the cerebral cortex precentral gyrus cross to the contralateral side and control the opposite limb, while the remaining uncrossing nerves go directly through the anterior corticospinal tract to control the ipsilateral limb. Therefore, when a cerebral hemisphere is damaged by stroke, the patients’ unaffected upper limb and hand function might be influenced because of the existence of the uncrossed nerve fi bers (Yin, 2005). The right and left hemispheres are connected by the corpus callosum The damaged hemisphere affects the function of the intact hemisphere, thus affecting limb motion control, and thus the unaffected cerebral hemisphere is used to strengthen and compensate for the inadequacy of the function of the affected side.
By contrast, clinically, the use frequency of the unaffected hand has been shown to decrease during daily life. When patients struggled to complete an action, family members would do it for them. The excess help deprived the patient of opportunities and desire to participate in daily activities, especially with actions involving dif fi cult fi ne motor and coordinated motor function. This lack of participation would make patients gradually rely on others (Dou and Qiu, 1997). All the above would only exacerbate the condition, decreasing functional recovery.
Different opinions exist about whether motor function on the unaffected side in hemiplegic patients’ actually decreases. Some doctors and therapists consider that the sensation in the unaffected limb and motor function in hemiparalysis patients may decrease to varying degrees. Other practitioners think that motor function of the hand after damage will be more fl exible because of increased use of the unaffected limb in daily life. Our results have indicated that function of the unaffected hand in hemiparalysis patients is worse than the healthy group’s dominant hand, which is consistent with the ideas put forward by Dou and Qiu (1997).
Following stroke, motor disability and rehabilitation of the unaffected upper limbs in hemiparalysis patients is often ignored, thereby precluding an accurate and comprehensive assessment and treatment regime for the patient. In the case of hemiplegic patients, where function in the affected hand is not likely to recover, encouragement to use the contralateral hand for compensation should be an area of rehabilitation focus. Our results show that emphasis on exercising the patient’s unaffected side would indirectly affect the rehabilitation of the contralateral side, even if only by a small margin.
Gu et al. (1999) found that there is a high correlation between the Lind-mark score on balance function and FMA score on balance function, which means Lind-mark has a good validity in the evaluation of hemiplegic rehabilitation. The results of the present study showed that after treatment, the Lind-mark scale scores were signi fi cantly changed, indicating that patients achieved a good therapeutic effect.
We selected the fi nger tapping tester as an instrument in this experiment, and analyzed the fi nger tapping frequency under controlled conditions, which made assessments more accurate by excluding the impact of subjectivity on the experiment and reducing experimental error. Finger tapping in unaffected hands in the stroke group was significantly worse than that in the dominant hands in the control group. Therefore, rehabilitation therapists should intensify treatment targeted at the unaffected upper limb’s fine motor activities and coordination. Finger tapping frequency can refl ect treatment effect and be used to evaluate hand function in stroke.
Acknowledgments:We thank the patients and families who contributed their time to this study and Zhu YL for her assistance with the project. We also thank the Rehabilitation Department of the Hospital of Shanghai University of Sport, Yangpu District Old Hospital, Shanghai Fudan University affiliated Huashan Hospital, and Shanghai Changning District Tianshan Hospital for supporting this study.
Author contributions:Yu ZS and Zhang LL designed this study. Zhang LL and Mao ZB performed experiments and analyzed experimental data. Zhang LL, Li PH and Qi X were responsible for manuscript writing. Zou J revised the manuscript. All authors approved the final version of this manuscript.
Con fl icts of interest:None declared.
Ackerman PL, Cianciolo AT (2000) Cognitive, perceptual-speed, and psychomotor determinants of individual differences during skill acquisition. J Exp Psychol Appl 6:259-290.
Ameli M, Grefkes C, Kemper F, Riegg FP, Rehme AK, Karbe H, Fink GR, Nowak DA (2009) Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol 66:298-309.
Ang KK, Guan C, Chua KS, Ang BT, Kuah CW, Wang C, Phua KS, Chin ZY, Zhang H (2011) A large clinical study on the ability of stroke patients to use an EEG-based motor imagery brain-computer interface. Clin EEG Neurosci 42:253-258.
Ayán C, Cancela JM, Gutiérrez-Santiago A, Prieto I (2014) Effects of two different exercise programs on gait parameters in individuals with Parkinson’s disease: a pilot study. Gait Posture 39:648-651.
Baker EL, Letz R, Fidler A (1985) A computer-administered neurobehavioral evaluation system for occupational and environmental epidemiology. Rationale, methodology, and pilot study results. J Occup Med 27:206-212.
Ben-Pazi H, Shalev RS, Gross-Tsur V, Bergman H (2006) Age and medication effects on rhythmic responses in ADHD: possible oscillatory mechanisms? 44:412-416.
Bernatzky G, Bernatzky P, Hesse HP, Staffen W, Ladurner G (2004) Stimulating music increases motor coordination in patients af fl icted with Morbus Parkinson. Neurosci Lett 361:4-8.
Dou ZL, Qiu WH (1997) An evaluation about effect of stroke on the sensorimotor performance of the unaffected upper extremity. Zhongguo Kangfu Lilun yu Shijian 3:164-167.
Ellis T, Katz DI, White DK, DePiero TJ, Hohler AD, Saint-Hilaire M (2008) Effectiveness of an inpatient multidisciplinary rehabilitation program for people with Parkinson disease. Phys Ther 88:812-819.
Gebruers N, Vanroy C, Truijen S, Engelborghs S, De Deyn PP (2010) Monitoring of physical activity after stroke: a systematic review of accelerometry-based measures. Arch Phys Med Rehabil 91:288-297.
Gu XD, Li JH, Ye XJ, et al. (1999) Study on application of assessment of lindmark balance function in rehabilitation for hemiplegia. Zhonghua Wuli Yixue yu Kangfu Zazhi 2:76-78.
Journal of Neuroscience, Neurosurgery Society (1996) Various cerebrovascular disease diagnostic criteria. J Neurol 29:379-380.
Kano O, Okonogi S, Hanashiro S, Miura K, Ikeda K, Iwasaki Y (2013) Levodopa-responsive benign tremulous parkinsonism. Case Rep Neurol 5:139-142.
Keitel A, Ferrea S, Südmeyer M, Schnitzler A, Wojtecki L (2013) Expectation modulates the effect of deep brain stimulation on motor and cognitive function in tremor-dominant Parkinson’s disease. PLoS One 8:e81878.
Khan T, Nyholm D, Westin J, Dougherty M (2014) A computer vision framework for fi nger-tapping evaluation in Parkinson’s disease. Artif Intell Med 60:27-40.
Lee HJ, Park YW, Jeong DH, Jung HY (2012) Effects of night sleep on motor learning using transcranial magnetic stimulation. Ann Rehabil Med 36:226-232.
Liao HS, Zhu YL (1996) Rehabilitation Assessment and Treatment of Stroke. Beijing: Huaxia Publishing House.
Liu W, Forrester L, Whitall J (2006) A note on time-frequency analysis of fi nger tapping. J Mot Behav 38:18-28.
Loehr JD, Palmer C (2007) Cognitive and biomechanical in fl uences in pianists’ fi nger tapping. Exp Brain Res 178:518-528.
Lou JS, Dimitrova DM, Park BS, Johnson SC, Eaton R, Arnold G, Nutt JG (2009) Using modafinil to treat fatigue in Parkinson disease: a double-blind, placebo-controlled pilot study. Clin Neuropharmacol 32:305-310.
Maihöfner C, Baron R, DeCol R, Binder A, Birklein F, Deuschl G, Handwerker HO, Schattschneider J (2007) The motor system shows adaptive changes in complex regional pain syndrome. Brain 130:2671-2687.
Miller NS, Kwak Y, Bohnen NI, Müller ML, Dayalu P, Seidler RD (2013) The pattern of striatal dopaminergic denervation explains sensorimotor synchronization accuracy in Parkinson’s disease. Behav Brain Res 257:100-110.
Heuer H, Schulna R (2002) Phasing of muscle activity during rapid fi nger oscillations. J Mot Behav 34:277-289.
Orosz A, Jann K, Wirth M, Wiest R, Dierks T, Federspiel A (2012) Theta burst TMS increases cerebral blood fl ow in the primary motor cortex during motor performance as assessed by arterial spin labeling (ASL). Neuroimage 61:599-605.
Peters M (1980) Why the preferred hand taps more quickly than the non-preferred hand: Three experiments on handedness. Can J Psychol 34:62-71.
Robles-García V, Arias P, Sanmartín G, Espinosa N, Flores J, Grieve KL, Cudeiro J (2013) Motor facilitation during real-time movement imitation in Parkinson’s disease: a virtual reality study. Parkinsonism Relat Disord 19:1123-1129.
Roche R, Wilms-Floet AM, Clark JE, Whitalla J (2011) Auditory and visual information do not affect self-paced bilateral fi nger tapping in children with DCD. Hum Mov Sci 30:658-671.
Roosink M, Zijdewind I (2010) Corticospinal excitability during observation and imagery of simple and complex hand tasks: Implications for motor rehabilitation. Behav Brain Res 213:35-41.
Scharoun SM, Bryden PJ, Otipkova Z, Musalek M, Lejcarova A (2013) Motor skills in Czech children with attention-deficit/hyperactivity disorder and their neurotypical counterparts. Res Dev Disabil 34:4142-4153.
Shimoyama I, Ninchoji T, Uemura K (1990) The fi nger-tapping test. A quantitative analysis. Arch Neurol 47:681-684.
Slater J, Tierney A, Kraus N (2013) At-risk elementary school children with one year of classroom music instruction are better at keeping a beat. PLoS One 8:e77250.
Szirmi I (2010) How does the brain create rhythms? Ideggyogy Sz 63:13-23.
Weng CS,Gao HM, Xu J, et al. (2003) A study on motor control de fi cits in the ipsilesional upper extremity of patients with hemiplegia after stroke. Zhongguo Kangfu Yixue Zazhi 18:85-87.
Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG (2003) Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol 54:464-472.
Whitall J, Chang TY, Horn CL, Jung-Potter J, McMenamin S, Wilms-Floet A, Clark JE (2008) Auditory-motor coupling of bilateral fi nger tapping in children with and without DCD compared to adults. Hum Mov Sci 27:914-931.
Yin K (2005) Analysis of the unaffected hand function of hemiplegia after stroke. Jilin Yixue 26:1277.
Yu ZS, Ouyang X, Cai G, Shen XZ, Yang YB, Wang WG (2010) Application of “Finger Oscillation Frequency Test” in selection of athletes. Shanghai Tiyu Xueyuan Xuebao 34:61-63.
Copyedited by Robens J, Apricò K, Li CH, Song LP, Zhao M
10.4103/1673-5374.137581
Zhusheng Yu, Department of Kinesiology, Shanghai University of Sport, Shanghai 200438, China, yuzhusheng5707@hotmail.com.
http://www.nrronline.org/
Accepted: 2014-06-12
杂志排行
中国神经再生研究(英文版)的其它文章
- Stem cell therapy for central nerve system injuries: glial cells hold the key
- Beyond taxol: microtubule-based strategies for promoting nerve regeneration after injury
- Neuroprotective effect of the traditional Chinese herbal formula Tongxinluo: a PET imaging study in rats
- Neuroprotective effects of Asiaticoside
- Treating Alzheimer’s disease with Yizhijiannao granules by regulating expression of multiple proteins in temporal lobe
- Autophagy activation aggravates neuronal injury in the hippocampus of vascular dementia rats
