Stem cell therapy for central nerve system injuries: glial cells hold the key
2014-06-01LiXiaoChikakoSaikiRyojiIde
Li Xiao, Chikako Saiki, Ryoji Ide
1 Pharmacology Department, The Nippon Dental University, School of Life Dentistry at Tokyo, Tokyo, Japan
2 Physiology Department, The Nippon Dental University, School of Life Dentistry at Tokyo, Tokyo, Japan
Stem cell therapy for central nerve system injuries: glial cells hold the key
Li Xiao1, Chikako Saiki2, Ryoji Ide2
1 Pharmacology Department, The Nippon Dental University, School of Life Dentistry at Tokyo, Tokyo, Japan
2 Physiology Department, The Nippon Dental University, School of Life Dentistry at Tokyo, Tokyo, Japan
Li Xiao, Ph.D., Pharmacology
Department, The Nippon Dental
University, School of Life Dentistry at
Tokyo, 1-9-20 Fujimi, Chiyoda-ku, Tokyo 102-8159, Japan, xiaoli@tky.ndu.ac.jp.
Mammalian adult central nerve system (CNS) injuries are devastating because of the intrinsic dif fi culties for effective neuronal regeneration. The greatest problem to be overcome for CNS recovery is the poor regeneration of neurons and myelin-forming cells, oligodendrocytes. Endogenous neural progenitors and transplanted exogenous neuronal stem cells can be the source for neuronal regeneration. However, because of the harsh local microenvironment, they usually have very low ef fi cacy for functional neural regeneration which cannot compensate for the loss of neurons and oligodendrocytes. Glial cells (including astrocytes, microglia, oligodendrocytes and NG2 glia) are the majority of cells in CNS that provide support and protection for neurons. Inside the local microenvironment, glial cells largely in fl uence local and transplanted neural stem cells survival and fates. This review critically analyzes current fi nding of the roles of glial cells in CNS regeneration, and highlights strategies for regulating glial cells’ behavior to create a permissive microenvironment for neuronal stem cells.
Neuron regeneration; stem cell therapy; glial cells; microenvironment; oligodendrocyte regeneration; CNS injury
Funding: This work was supported in part by the Nippon Dental University Research Project 4 Grant and Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scienti fi c Research (26861689).
Xiao L, Saiki C, Ide R. Stem cell therapy for central nerve system injuries: glial cells hold the key. Neural Regen Res. 2014;9(13):1253-1260.
Introduction
Adult mammalian central nerve system (CNS) has a difficulty in repairing injuries because it lacks the regenerative power to replace damaged neuronal cells and reconstruct dendritic connections (Bregman et al., 1995; Horner and Gage, 2000). During the past two decades, two basic strategies have been established for neuronal regeneration: endogenous self-repair and exogenous cellular replacement. Self-repair requires endogenous neural stem cells and cellular replacement needs stem cell transplantation (Cheng et al., 1996; Stichel et al., 1998a; Horner and Gage, 2000; Bradbury and McMahon, 2006; Okano, 2009; Trueman et al., 2013). It has been demonstrated that neural stem cells (NSCs) exist in the subventricular zone (SVZ), and hippocampus of brain and the central canal of the spinal cord (Temple, 1989; Reynolds and Weiss, 1992). They can migrate to the lesion site to repair the injured tissue. These endogenous NSCs could be a regenerative source for the damaged neural cells (Gage et al., 1995, 2000; Gensert and Goldman, 1997). Nevertheless, because their number and regenerative ability are limited, they cannot fully repair the damaged tissue in CNS (Picard-Riera et al., 2004). Exogenous neuronal stem cells then become an expected source for neurogenesis. Stem cell [such as embryonic stem cells (ES cells) and induced pluripotent stem cells (iPS cells)] transplantation can offer a large number of neural progenitors for introducing new neuronal cells to the damaged CNS tissue (McDonald et al., 1999; Tsuji et al., 2010; Chen et al., 2011). Moreover bone marrow mesenchymal stem cells and dental pulp stem cells are able to be differentiated into neuronal lineages both in vivo and in vitro (Jiang et al., 2002; Jin et al., 2002; Xiao and Tsutsui, 2013). However, no matter how experimental studies showed that exogenous NSCs can be effectively differentiated into mature neuronal cells in vitro, in most in vivo experiments they only showed modest recovery of the injured CNS (Tetzlaff et al., 2011; Mothe and Tator, 2012). The reason for this is considered to be that the damaged microenvironment prevents neuronal regeneration (neurogenesis) (Neumann, 2000; Imitola et al., 2006; Yiu and He, 2006; Charil and Filippi, 2007). Factors present in the injured microenvironment (such as in fl ammatory mediators) influence survival, self-renewal, migration and neuronal differentiation of both endogenous NSCs and transplanted exogenous stem cells (Watanabe et al., 2007; Singhal et al., 2008; Kim et al., 2012; Dooley et al., 2014). As a result, endogenous NSCs cannot efficiently perform self-repair (Singhal et al., 2008), and exogenous stem cells have difficulty to differentiate into functional neurons, but do contribute to glial scar formation (Suhonen et al., 1996). To obtain better therapeutic result for traumatic injuries ofCNS, it is essential to create a permissive microenvironment for both endogenous and exogenous neuronal stem cells and direct them to differentiate towards functional neurons and oligodendrocytes. Glial cells play key role in the maintenance and homeostasis of the local microenvironment (Walz, 1989; Gourine et al., 2010). The interaction between glial cells and neuronal stem cells is essential for functional neuronal regeneration. Here we summarize recent fi ndings about the role of glial cells in CNS injuries as well as the possible methods to control their behavior for stem cell-based neuroregeneration.
Local microenvironment of neurons
CNS neurons are the functional cells which form neural networks using their axons to process and transmit information through synapses. There are four types of glial cells in the local microenvironment surrounding neurons: astrocyte, oligodendrocyte, microglia and NG2 glia (polydendrocytes, or oligodendrocyte progenitor cells). Astrocytes are the supporting cells which form blood-brain-barrier (BBB) or blood-spinal cord barrier (BSCB) with endothelial cells, provide nutrients to neuron, and control synapse formation and function (Abbott, 2002; Bartanusz et al., 2011). Microglia are the defensive cells which fi rst and mainly respond to injuries. Oligodendrocytes also act as the supporting cells which provide myelin (the insulating sheath) around the axons of neurons. NG2 glia generate oligodendrocytes, astrocytes and microglia in CNS and serve as the primary source of remyelinating cells in demyelinated lesions (Raff et al., 1983; Nishiyama et al., 2009). These four types of cells are embedded in the extracellular matrix (ECM) and work together through cell-cell or cell-ECM interactions to maintain the function of neurons (Figure 1).
Although the pathologies among the three types of traumatic CNS injuries, traumatic brain injury (TBI), spinal cord injury (SCI) and stroke, are quite different, they all have two injury phases, primary and secondary, and the cellular and molecular responses in the local microenvironment are similar. The common cellular and molecular responses are cell death (apoptosis and necrosis), in fl ammation, glial cells’ activation, excitotoxicity, increase in free radicals, accumulation of acid and toxic products, demyelination, axonal degeneration and glial scar formation (Hausmann, 2003; Buga et al., 2008; Veenith et al., 2009; Mothe et al., 2012; Huang et al., 2014). Particularly, after injuries neurons and oligodendrocytes die and undergo demyelination, whereas astrocytes and microglia are activated and proliferate (Vela et al., 2002; Lalancette-Hébert et al., 2007). Microglia are the fi rst glial cells to be activated by traumatic injury. They release chemical mediators and reactive oxygen species (ROS) that promote peripheral macrophages infiltration through the BBB. Microglia and in fi ltrated macrophages build a defensive system to prevent pathogens and neurotoxins, and clean up the debris of dead cells (El Khoury et al., 2007). At the same time, they also produce in fl ammatory factors that are neurotoxic (Dheen et al., 2007). NG2 glia are then stimulated by signals from activated microglia. They proliferate to replace the dying oligodendrocytes, provide a favorable substrate for axon growth, and contribute to the reconstruction of neuroglial network (Nishiyama et al., 2005; Yang et al., 2006; Wigley et al., 2007; 2009; Wu et al., 2010). Astrocytes are the last glial cells to respond after being activated by micraoglia. The in fl ammatory products of microglia play important roles for activating astrocytes (such as IL-1, IL-6, and TNF-α) (Lee et al., 1993; Röhl et al., 2006; Zhang et al., 2010; Erta et al., 2012). Astrocytes rapidly proliferate and then form a glial scar to make a border between the damaged and the healthy tissues. The glial scar [which is a complex of astrocytes, NG2 glia, extracellular matrix (ECM) and other cellular compounds] reestablishes the physical and chemical integrity of the CNS (Silver and Miller, 2004). However, it also produces many inhibitory molecules (such as myelin inhibitors) that prevent the migration of endogenous NSCs as well as inhibit signi fi cant axonal regeneration (McKeon et al., 1999; Silver and Miller, 2004). As a result, the local microenvironment is reconstructed and produces various factors to influence neuronal regeneration (Fitch and Silver, 2008) (Figure 2). Therefore, to make a welcoming microenvironment for stem cells-based neuronal regeneration in traumatic CNS injuries, we must 1) control glial scar formation and diminish the production of harmful factors; 2) regulate microglia’s behaviors; and 3) fi ll up NG2 glia to generate remyelinating oligodendrocytes.
Chronic neurodegenerative diseases (such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis and amyotrophic lateral sclerosis) share similar cellular and molecular changes with traumatic CNS injuries, but at different levels. The differences between chronic neurodegenerative diseases and traumatic CNS are summarized in Table 1. Although the detailed mechanism for chronic neurodegenerative disorders is not understood, a rough outline emerged as 1) The key feature is chronic in fl ammation, in particular of microglia activation (Nguyen et al., 2002; Amor et al., 2010). 2) Immunological challenges might be aetiological factors (Nguyen et al., 2002). 3) The permeability of the BBB is increased which allows for the entry of immune elements (Zlokovic, 2008). 4) Astrocytes are activated and release both excitotoxic and neural protective molecules (Maragakis et al., 2006). 5) Glial scar formation usually is not observed because the moderate injury severity (Sofroniew, 2009). 6) Neurons are the only victims. They undergo apoptotic cell death. 7) Olidendrocytes undergo apoptosis in multiple sclerosis whereas they are complement-activated in Alzheimer’s disease and Parkinson’s disease (Peferoen et al., 2014). 8) NG2 glia are speci fi cally involved in myelin repair (Richardson et al., 2011). For these reasons, a complex regulation of glial cells might be required for preventing chronic neurodegeneration.
Directing the glial scar towards functional neuronal cells

Figure 1 Glial cells and neurons in the healthy central nerve system.
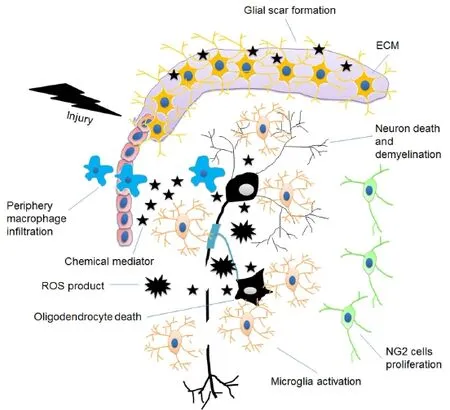
Figure 2 Damaged central nerve system microenvironment.

Table 1 Differences of the microenvironment between traumaticcentral nerve system injuries and chronic neurodegenerative diseases
Astrocytes have been characterized as neural stem cells in speci fi c regions of adult CNS. Doetsch et al (1999) demonstrated that in the subventricular zone (SVZ) of mouse brain, a subpopulation of astrocytes could give rise to immature precursors and neuroblasts both in vivo and in vitro. Later, Sanai et al. (2004) showed that SVZ astrocytes in the adult human brain are clonal precursors of self-renewing multipotent neurospheres and can be differentiated into neurons in the absence of exogenous growth factors. However, in the vast majority of the CNS, such as the cortical regions, astrocytes apparently cannot produce new neurons but form glial scar (the main barrier of axonal regeneration) after an injury (Silver and Miller, 2004; Kriegstein et al., 2009). In the lesion? sit, astrocytes play a crucial role in the healing process. They are activated by the migrated microglia through the integral membrane proteins (Volterra and Meldolesi, 2005) and offer tissue repair just like the fi broblasts in the non-CNS tissue. To repair the damaged tissue, astrocytes first proliferate and then wall off the wounded areas to form glial scar that reduces the spread and persistence of inflammatory cells. Although the glial scar serves to repair the BBB, prevent an overwhelming inflammatory response and decrease neuronal loss and demyelination, it does block axon guidance and inhibits neural regeneration (Stichel and Müller, 1998b; Sofroniew, 2005; Yiu and He, 2006; Barres, 2008). Therefore, inhibitory treatments of glial scar have been considered useful for neuronal regeneration. In vivo treatment with peptide amphiphile could reduce glial scar formation, increase the number of oligodendroglia at the site of spinal cord injury and resulted in signi fi cant behavioral improvements (Tysseling-Mattiace et al., 2008). IFN-β-encoding could help transplanted NSCs to inhibit glial scar formation in spinal cord injury through the stimulation of TLR4 signaling (Nishimura et al., 2013). However, as we described before, glial scar can offer wound healing, limits the inflammation and protects the healthy tissue. Inhibition of glial scar might have harmful effects. Therefore, directing astrocytes in the glial scar towards functional neurons becomes an ideal strategy for CNS regeneration. Postnatal astrocytes can be redirected toward neurons by forced expression of Pax6 or pro-neural transcription factor neurogenin-2 in vitro (Heins et al., 2002; Berninger et al., 2007). Astrocytes isolated from an adult cortex, reprogrammed with the pCAG-Neurog2-containing retroviral vector, could give rise to synapse-forming glutamatergic neurons (Heinrich et al., 2010). Reprogramming astrocytes with individual stem transcription factors OCT4, SOX2, or NANOG could generate NSCs. These astrocyte-derived NSCs were able to generate mature neurons in vivo with positive expression of synaptic proteins and neurotransmitters (Corti et al., 2012). However, since transplanted stem cells in vivo usually differentiate into glial cells instead of functional neurons, it is possible that these in vitro reprogrammed astrocyte-NSCs will have the same problems. A recent study made a breakthrough in resolving this impasse. Guo et al (2014) demonstrated that reactive glial cells, including both astrocytes and NG2 glia, in the glial scar can be reprogrammed into functional neurons in the adult mouse cortex when infected with a retrovirus-encoded single transcription factor, NeuroD1 (which plays an important role during embryonic brain development and adult neurogenesis). Astrocytes were mainly reprogrammed into glutamatergic neurons whereas NG2 glia were reprogrammed into both glutamatergic and GABAergic neurons. Although safety issues need to be resolved, this technology does open the door to functional recovery for CNS injuries.
Moreover, as the main component of glial scar, chondroitin sulphate proteoglycans (CSPGs) (a major component of the ECM) have been considered as inhibitors for neurite outgrowth (Hynds and Snow, 1999; Tan et al., 2005). Conversely, evidence has emerged that CSPGs promote proliferation and functional differentiation of neural stem cells in vitro (Sirko et al., 2007; Tham et al., 2010). An in vivo study con fi rmed that CSPGs have either beneficial or destructive effects in spinal cord repair. The data showed that in mice SCI model, there was an inhibition of CSPGs synthesis immediately after SCI impaired functional motor recovery and increased tissue loss. In contrast, 2 days after SCI, CSPGs inhibition improved recovery (Rolls et al., 2008). The underlying mechanism of the duo faces of CSPGs is far from being clear. Based on the above-mentioned evidence, it emerges that CSPGs are important molecules for CNS repair and endogenous neural stem cells proliferation and differentiation in the acute phase in CNS injuries, but become a barrier in the chronic phase. Since the best period for stem cell transplantation for SCI is about 2 weeks after the trauma, simultaneously using CSPGs inhibitors might be bene fi cial.
Microglia is a double edged sword: either exacerbating degeneration or promoting repair
In non-CNS tissues the common inflammatory response includes invasion of leucocytes, activation of fi broblasts and release of chemical mediators. However, CNS was regarded as an “immune privileged” organ, because it has intrinsic immune cells to respond to the in fl ammatory insults. Concretely, after injury the first and mainly activated immune cells in CNS are microglia instead of leucocytes. Microglia (the resident macrophages) proliferate and migrate to the lesion site. They produce inflammatory mediators such as IL-1, IL-6, TNF-alpha, ROS and nitric oxide (NO) (Yao et al., 1992; Erta et al., 2012; Qin and Crews, 2012; Guadagno et al., 2013). These in fl ammatory mediators not only act on local cells, but also recruit peripheral leukocytes to pass the BBB through the interactions between endothelial cells and astrocytes (Engelhardt and Ransohoff, 2005). Activated microglia and peripheral leukocytes eliminate pathogens and clean up the debris caused by physical trauma and in fl ammatory response. Cross-talk between microglia and neural stem cells (NSCs) plays a key role in neuronal regeneration (Kokaia et al., 2012). Activated microglia produce a plethora of chemical mediators ranging from neurotoxic triggers to neurontrophic factors which can impair NSCs survival and neuronal differentiation as well as bene fi cial effects on neu-rogenesis. In chronic neurodegenerative diseases activated microglia can kill neurons and oligodendrocytes through the phagocyte NADPH oxidase (PHOX) and the inducible nitric oxide synthase (iNOS) (Brown and Neher, 2010; de Pablos et al., 2014). Lipopolysaccharide (LPS) has been used for triggering chronic in fl ammation and exacerbating neurodegeneration in animal models of chronic neurodegenerative diseases (Qin et al., 2007; Pott Godoy et al., 2008; Murray et al., 2011). LPS-activated microglia ef fi ciently triggered apoptosis in mouse NSCs via TNF-α-involved mitochondrial pathway (Guadagno et al., 2013). This activation is likely controlled by caspase signaling. Knockdown or chemical inhibition of caspase signaling hindered microglia activation and consequently reduced neurotoxicity (Burguillos et al., 2011). As a tetracycline antibiotic, minocycline can permeate through the BBB or BSCB (Aronson, 1980). Minocycline is known to inhibit microglial activation. An in vitro study showed that minocycline signi fi cantly decreased excitotoxins (glutamate and kainite) induced neuron death (Tikka et al., 2001). In a variety of experimental models of neuron degeneration minocycline protected neurons by inhibiting microglial activation-associated chronic inflammation suggesting minocycline is a potential therapeutic reagent for chronic neurodegenerative diseases (Fan et al., 2007; Plane et al., 2010; Kobayashi et al., 2013). In an experimental rat stroke model, minocycline presented positive effects on endogenous neural stem cells’ survival and activation (Rueger et al., 2012). A similar study showed that minocycline-preconditioned exogenous neural stem cells enhanced neuroprotection in a rat ischemic stroke model after transplantation (Sakata et al., 2012). These reports suggest minocycline treatment is also beneficial to both endogenous and exogenous neural stem cells. However, in some animal models (such as Parkinson’s Disease), minocycline increased neuron death (Plane et al., 2010). Moreover, in a phase III randomised trial minocycline treatment showed a harmful effect on patients with amyotrophic lateral sclerosis (ALS) indicating this drug needs to be carefully investigated (Gordon et al., 2007). Natural products and hydrogen sulfide have been used to inhibit neurotoxic microglia activation that consequently achieves neuroprotection (Choi et al., 2011; Zhang et al., 2014). On the other hand, in traumatic CNS injuries activated microglia are favorable. They provide immune-related requirements for CNS self-repair. Franzen et al. (1998) demonstrated that grafted macrophages could contribute to axonal regeneration in an animal model of spinal cord injury suggesting that microglia (the local microphage in CNS) might be beneficial for neuronal regeneration. It has been demonstrated that CNS injury-activated microglia induce neuronal stem proliferation and promote functional neuronal and oligodendritic differentiation in vitro (Deierborg et al., 2010). Activated microglia promote the generation of neurons from white matter cells by secreting trypsinogen PRSS2 (Nikolakopoulou et al., 2013). Microglia interacted with transplanted ES cell-derived neural stem cells by fusion. The fused microglia-neural stem cells showed improvement on the functional recovery of CNS (Schwartz, 2003; Cusulin et al., 2012). These evidences suggest that for traumatic CNS injuries, such as spinal cord injury, co-transplanting microglia with neuronal stem cells might be a promising approach.
Oligodendrocyte regeneration
Oligodendrocytes are vulnerable to both physical trauma and inflammatory response after CNS injury, duo to they are the end products of neuronal lineage and have high metabolic activity, high levels of intracellular iron and low concentration of antioxidants, such as glutathione (Thorburne and Juurlink, 1996; Bradl and Lassmann, 2010; Amaral et al., 2013). In a rodent model of spinal cord injury, massive cell death of oligodendrocytes is triggered by proteolytic enzymes, chemical mediators and oxidative stress (which were produced by microglia and in fi ltrated macrophages) (Frei et al., 2000; Dong et al., 2003). Death of oligodendrocytes causes demyelination which impairs axon function and consequently induces neuron death (Almad et al., 2011). Moreover, fully differentiated oligodendrocytes are postmitotic and cannot repair myelin in the presence of demyelinated axons in adult CNS (Keirstead et al., 1997). Therefore, remyelination requires oligodendrocyte progenitor cells. As progenitors of oligodendrocyte, NG2 glia give rise to the majority of oligodendrocytes in CNS during development (Polito and Reynolds, 2005). NG2 glia also can produce astrocytes and neurons depending on environmental conditions (Trotter et al., 2010). In adult CNS, when the injury causes demyelination or generalized tissue destruction, NG2 glia rapidly proliferate, migrate to the lesion site (Franklin et al., 1997), and differentiate into oligodendrocytes which remyelinate neurons (Islam et al., 2009; Nishiyama et al., 2009). NG2 glia also provide an adhesive substrate for axonal growth cones and promote their growth (Yang et al., 2006). Moreover, it has been reported that NG2 glia stabilize the regenerating front of dystrophic axons in the inhibitory environment of the glial scar after spinal cord injury (Busch et al., 2010). However, evidence showed that local NG2 glia have limited ability to proliferate (Payne et al., 2013) and their population cannot be completely restored (Keirstead et al., 1998; Richardson et al., 2011). It has been demonstrated that the pleiotropic cytokine, leukemia inhibitory factor (LIF) potentiates the differentiation and survival of oligodendrocyte precursors and prevent oligodendrocyte apoptosis in response to either growth factor removal or cytotoxic challenge in vitro (Kerr and Patterson, 2005). In vivo studies con fi rmed that endogenous LIF production limits autoimmune demyelination and oligodendrocyte loss (Butzkueven et al., 2006). Delivery of exogenous LIF stimulates oligodendrocyte progenitor cell proliferation and enhances hippocampal remyelination (Bauer and Patterson, 2006; Deverman et al., 2012). Furthermore, LIF expands the pool of adult neural stem cells by promoting their self-renewal, thereby preventing the emergence of more differentiated progeny, both in vivo and in vitro (Pitman et al., 2004; Bauer and Patterson, 2006). LIF also promoted proliferation, survival, and differentiation in cultivated ES cells-derived neuron progenitors (Majumder et al., 2012). Hence, a combination of exogenous LIF supply and stem cell transplantation might be bene fi cial for remyelination and neurogenesis.
Conclusion
Regeneration of the nervous system requires not only en-dogenous and exogenous neuronal stem cells but also an appropriate microenvironment. Glia cells are the cellular comportments of the microenvironment surrounding neurons. Microglia, astrocytes and NG2 glia play crucial roles on neuronal regeneration. Microglia have dual effects on neuronal regeneration. In chronic neurodegenerative diseases it is necessary to suppress microglial activation. Minocycline is a strong inhibitor of microglial activation that showed neuronal protection in experimental animal models of neurodegenerative diseases. But its potential harmful effects need to be carefully examined. Natural products and hydrogen sul fi de might be potential therapeutic reagents for controlling microglial activation-mediated chronic in fl ammation in CNS. In traumatic injuries microglia are beneficial for neuronal stem cells. Co-transplanting microglia with neuronal stem cells might be a promising approach. Astrocytes produce glial scar in the lesion the sites in CNS. They also have stem cell properties. Inhibition of glial scar formation (such as peptide amphiphile treatment and IFN-β-encoding) could help neuronal regeneration. Since glial scar also has pro fi table effects for repair, the inhibition of glial scar might have side effects. Technologies for converting harmful glial scar into functional neurons have provided innovative approaches for the potential regeneration of CNS. NG2 glia are the progenitors of oligodendrocyte so their numbers need to be increased in CNS injured tissue. Exogenous LIF supply can maintain the rate of proliferation of NG2 glia, and promote proliferation, survival, and differentiation of neural stem cells. A combination of LIF treatment and stem cell transplantation might be permissive for remyelination and neurogenesis.
In conclusion, appropriate control of glial cells behavior can improve the local microenvironment and consequently help survival and functional differentiation of neuronal stem cells. Thus, glial cells can be an important target of future research for the treatment of CNS injuries and diseases.
Acknowledgments: We would like to thank Mr. Nathaniel Green for proofreading.
Con fl icts of interest: None declared.
Abbott NJ (2002) Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat 200:629-638.
Almad A, Sahinkaya FR, McTigue DM (2011) Oligodendrocyte fate after spinal cord injury. Neurotherapeutics 8:262-273.
Amaral AI, Meisingset TW, Kotter MR, Sonnewald U (2013) Metabolic aspects of neuron-oligodendrocyte-astrocyte interactions. Front Endocrinol (Lausanne) doi: 10.3389/fendo.2013.00054
Amor S, Puentes F, Baker D, van der Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129:154-169.
Aronson AL (1980) Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc 176: 1061-1068.
Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60:430-440.
Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M (2011) The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol 70:194-206.
Bauer S, Patterson PH (2006) Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci 26:12089-12099.
Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Götz M (2007) Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci 27:8654-8664.
Bradbury EJ, McMahon SB (2006) Spinal cord repair strategies: why do they work? Nat Rev Neurosci 7:644-653.
Bradl M, Lassmann H (2010) Oligodendrocytes: biology and pathology. Acta Neuropathol 119:37-53.
Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME (1995) Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 378:498-501.
Brown GC, Neher JJ (2010) In fl ammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 41:242-247.
Buga AM, Dunoiu C, Bălşeanu A, Popa-Wagner A (2008) Cellular and molecular mechanisms underlying neurorehabilitation after stroke in aged subjects. Rom J Morphol Embryol 49:279-302.
Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B (2011) Caspase signalling controls microglia activation and neurotoxicity. Nature 472:319-324.
Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH, Silver J (2010) Adult NG2+cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci 30:255-265.
Butzkueven H, Emery B, Cipriani T, Marriott MP, Kilpatrick TJ (2006) Endogenous leukemia inhibitory factor production limits autoimmune demyelination and oligodendrocyte loss. Glia 53:696-703.
Charil A, Filippi M (2007) In fl ammatory demyelination and neurodegeneration in early multiple sclerosis. J Neurol Sci 259:7-15.
Chen LW, Kuang F, Wei LC, Ding YX, Yung KK, Chan YS (2011) Potential application of induced pluripotent stem cells in cell replacement therapy for Parkinson’s disease. CNS Neurol Disord Drug Targets 10:449-458.
Cheng H, Cao Y, Olson L (1996) Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science 273:510-513.
Choi DK, Koppula S, Suk K (2011) Inhibitors of Microglial Neurotoxicity: Focus on Natural Products. Molecules 16:1021-1043.
Corti S, Nizzardo M, Simone C, Falcone M, Donadoni C, Salani S, Rizzo F, Nardini M, Riboldi G, Magri F, Zanetta C, Faravelli I, Bresolin N, Comi GP (2012) Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp Cell Res 318:1528-1541.
Cusulin C, Monni E, Ahlenius H, Wood J, Brune JC, Lindvall O, Kokaia Z (2012) Embryonic stem cell-derived neural stem cells fuse with microglia and mature neurons. Stem Cells 30:2657-2671.
de Pablos RM, Herrera AJ, Espinosa-Oliva AM, Sarmiento M, Muñoz MF, Machado A, Venero JL (2014) Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of in fl ammation. J Neuroin fl ammation 11:34.
Deierborg T, Roybon L, Inacio AR, Pesic J, Brundin P (2010) Brain injury activates microglia that induce neural stem cell proliferation ex vivo and promote differentiation of neurosphere-derived cells into neurons and oligodendrocytes. Neuroscience 171:1386-1396.
Deverman BE, Patterson PH (2012) Exogenous leukemia inhibitory factor stimulates oligodendrocyte progenitor cell proliferation and enhances hippocampal remyelination. J Neurosci 32:2100-2109.
Dheen ST, Kaur C, Ling EA (2007) Microglial activation and its implications in the brain diseases. Curr Med Chem 14:1189-1197.
Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97:703-716.
Dong H, Fazzaro A, Xiang C, Korsmeyer SJ, Jacquin MF, McDonald JW (2003) Enhanced oligodendrocyte survival after spinal cord injury in Bax-de fi cient mice and mice with delayed Wallerian degeneration. J Neurosci 23:8682-8691.
Dooley D, Vidal P, Hendrix S (2014) Immunopharmacological intervention for successful neural stem cell therapy: New perspectives in CNS neurogenesis and repair. Pharmacol Ther 141:21-31.
El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD (2007) Ccr2 de fi ciency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 13:432-438.
Engelhardt B, Ransohoff RM (2005) The ins and outs of T-lymphocyte traf fi cking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol 26:485-495.
Erta M, Quintana A, Hidalgo J (2012) Interleukin-6, a Major Cytokine in the Central Nervous System. Int J Biol Sci 8:1254-1266.
Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, Van Nostrand WE (2007) Minocycline reduces microglial activation and improves behavioral de fi cits in a transgenic model of cerebral microvascular amyloid. J Neurosci 27:3057-3063.
Fitch MT, Silver J (2008) CNS injury, glial scars, and in fl ammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol 209:294-301.
Franklin RJ, Gilson JM, Blakemore WF (1997) Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J Neurosci Res 50:337-344.
Franzen R, Schoenen J, Leprince P, Joosten E, Moonen G, Martin D (1998) Effects of macrophage transplantation in the injured adult rat spinal cord: a combined immunocytochemical and biochemical study. J Neurosci Res 51:316-327.
Frei E, Klusman I, Schnell L, Schwab ME (2000) Reactions of oligodendrocytes to spinal cord injury: cell survival and myelin repair. Exp Neurol 163:373-380.
Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J (1995) Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 92:11879-11883.
Gage FH (2000) Mammalian neural stem cells. Science 287:1433-1438.
Gensert JM, Goldman JE (1997) Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron 19:197-203.
Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, Hilton JF, Spitalny GM, MacArthur RB, Mitsumoto H, Neville HE, Boylan K, Mozaffar T, Belsh JM, Ravits J, Bedlack RS, Graves MC, McCluskey LF, Barohn RJ, Tandan R; Western ALS Study Group (2007) Ef fi cacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 6:1045-1053.
Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S (2010) Astrocytes control breathing through pH-dependent release of ATP. Science 329:571-575.
Guadagno J, Xu X, Karajgikar M, Brown A, Cregan SP (2013) Microglia-derived TNFα induces apoptosis in neural precursor cells via transcriptional activation of the Bcl-2 family member Puma. Cell Death Dis 4:e538.
Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G (2014) In Vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 14:188-202.
Hausmann ON (2003) Post-traumatic in fl ammation following spinal cord injury. Spinal Cord 41:369-378.
Heinrich C, Blum R, Gascón S, Masserdotti G, Tripathi P, Sánchez R, Tiedt S, Schroeder T, Götz M, Berninger B (2010) Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol 8:e1000373.
Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Götz M (2002) Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci 5:308-315.
Horner PJ, Gage FH (2000) Regenerating the damaged central nervous system. Nature 407:963-970.
Huang L, Wu ZB, Zhuge Q, Zheng W, Shao B, Wang B, Sun F, Jin K (2014) Glial scar formation occurs in the human brain after ischemic stroke. Int J Med Sci 11:344-348.
Hynds DL, Snow DM (1999) Neurite outgrowth inhibition by chondroitin sulfate proteoglycan: stalling/stopping exceeds turning in human neuroblastoma growth cones. Exp Neurol 160:244-255.
Imitola J, Chitnis T, Khoury SJ (2006) Insights into the molecular pathogenesis of progression in multiple sclerosis: potential implications for future therapies. Arch Neurol 63:25-33.
Islam MS, Tatsumi K, Okuda H, Shiosaka S, Wanaka A (2009) Olig2-expressing progenitor cells preferentially differentiate into oligodendrocytes in cuprizone-induced demyelinated lesions. Neurochem Int 54:192-198.
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41-49.
Jin HK, Carter JE, Huntley GW, Schuchman EH (2002) Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase-de fi cient mice delays the onset of neurological abnormalities and extends their life span. J Clin Invest 109:1183-1191.
Keirstead HS, Blakemore WF (1997) Identi fi cation of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol 56:1191-1201.
Keirstead HS, Levine JM, Blakemore WF (1998) Response of the oligodendrocyte progenitor cell population (de fi ned by NG2 labelling) to demyelination of the adult spinal cord. Glia 22:161-170.
Kerr BJ, Patterson PH (2005) Leukemia inhibitory factor promotes oligodendrocyte survival after spinal cord injury. Glia 51:73-79.
Kim H, Cooke MJ, Shoichet MS (2012) Creating permissive microenvironments for stem cell transplantation into the central nervous system. Trends Biotechnol 30:55-63.
Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K (2013) Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4:e525.
Kokaia Z, Martino G, Schwartz M, Lindvall O (2012) Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nat Neurosci 15:1078-1087.
Kriegstein A, Alvarez-Buylla A (2009) The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149-84.
Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J (2007) Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci 27:2596-2605.
Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW (1993) Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol 150:2659-2667.
Majumder A, Banerjee S, Harrill JA, Machacek DW, Mohamad O, Bacanamwo M, Mundy WR, Wei L, Dhara SK, Stice SL (2012) Neurotrophic effects of leukemia inhibitory factor on neural cells derived from human embryonic stem cells. Stem Cells 30:2387-2399.
Maragakis NJ, Rothstein JD (2006) Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol 2:679-689.
McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW (1999) Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 5:1410-1412.
McKeon RJ, Jurynec MJ, Buck CR (1999) The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci 19:10778-10788.
Mothe AJ, Tator CH (2012) Advances in stem cell therapy for spinal cord injury. J Clin Invest 122:3824-3834.
Murray CL, Skelly DT, Cunningham C (2011) Exacerbation of CNS in fl ammation and neurodegeneration by systemic LPS treatment is independent of circulating IL-1β and IL-6. J Neuroin fl ammation doi: 10.1186/1742-2094-8-50.
Neumann H (2000) The immunological microenvironment in the CNS: implications on neuronal cell death and survival. J Neural Transm Suppl 59:59-68.
Nguyen MD, Julien JP, Rivest S (2002) Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci 3:216-227.
Nikolakopoulou AM, Dutta R, Chen Z, Miller RH, Trapp BD (2013) Activated microglia enhance neurogenesis via trypsinogen secretion. Proc Natl Acad Sci U S A 110:8714-8719.
Nishimura Y, Natsume A, Ito M, Hara M, Motomura K, Fukuyama R, Sumiyoshi N, Aoki I, Saga T, Lee HJ, Wakabayashi T, Kim SU (2013) Interferon-β delivery via human neural stem cell abates glial scar formation in spinal cord injury. Cell Transplant 22:2187-2201.
Nishiyama A, Yang Z, Butt A (2005) Astrocytes and NG2-glia: what’s in a name? J Anat 207:687-693.
Nishiyama A, Komitova M, Suzuki R, Zhu X (2009) Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10:9-22.
Okano H (2009) Strategies toward CNS-regeneration using induced pluripotent stem cells. Genome Inform 23:217-220.
Payne SC, Bartlett CA, Savigni DL, Harvey AR, Dunlop SA, Fitzgerald M (2013) Early proliferation does not prevent the loss of oligodendrocyte progenitor cells during the chronic phase of secondary degeneration in a CNS white matter tract. PLoS One 8:e65710. doi: 10.1371/journal.pone.0065710.
Peferoen L, Kipp M, van der Valk P, van Noort JM, Amor S (2014) Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 141:302-313.
Picard-Riera N, Nait-Oumesmar B, Baron-Van Evercooren A (2004) Endogenous adult neural stem cells: limits and potential to repair the injured central nervous system. J Neurosci Res 76:223-231.
Pitman M, Emery B, Binder M, Wang S, Butzkueven H, Kilpatrick TJ (2004) LIF receptor signaling modulates neural stem cell renewal. Mol Cell Neurosci 27:255-266.
Plane JM, Shen Y, Pleasure DE, Deng W (2010) Prospects for minocycline neuroprotection. Arch Neurol 67:1442-1448.
Polito A, Reynolds R (2005) NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat 207:707-716.
Pott Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ (2008) Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain 131:1880-1894.
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroin fl ammation and progressive neurodegeneration. Glia 55:453-462.
Qin L, Crews FT (2012) NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroin fl ammation doi: 10.1186/1742-2094-9-5.
Raff MC, Miller RH, Noble M (1983) A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303:390-396.
Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707-1710.
Richardson WD, Young KM, Tripathi RB, McKenzie I (2011) NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron 70:661-673.
Röhl C, Lucius R, Sievers J (2006) The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res 1129:43-52.
Rolls A, Shechter R, London A, Segev Y, Jacob-Hirsch J, Amariglio N, Rechavi G, Schwartz M (2008) Two Faces of Chondroitin Sulfate Proteoglycan in Spinal Cord Repair: A Role in Microglia/Macrophage Activation. PLoS Med 5: e171.
Rueger MA, Muesken S, Walberer M, Jantzen SU, Schnakenburg K, Backes H, Graf R, Neumaier B, Hoehn M, Fink GR, Schroeter M (2012) Effects of minocycline on endogenous neural stem cells after experimental stroke. Neuroscience 215:174-183.
Sakata H, Niizuma K, Yoshioka H, Kim GS, Jung JE, Katsu M, Narasimhan P, Maier CM, Nishiyama Y, Chan PH (2012) Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci 32:3462-3473.
Sanai N, Tramontin AD, Quiñones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-García Verdugo J, Berger MS, Alvarez-Buylla A (2004) Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427:740-744.
Schwartz M (2003) Macrophages and microglia in central nervous system injury: are they helpful or harmful? J Cereb Blood Flow Metab 23:385-394.
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146-156.
Singhal S, Lawrence JM, Bhatia B, Ellis JS, Kwan AS, Macneil A, Luthert PJ, Fawcett JW, Perez MT, Khaw PT, Limb GA (2008) Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Müller stem cells into degenerating retina. Stem Cells 26:1074-1082.
Sirko S, von Holst A, Wizenmann A, Götz M, Faissner A (2007) Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development 134:2727-2738.
Sofroniew MV (2005) Reactive astrocytes in neural repair and protection. Neuroscientist 11:400-407.
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638-647.
Stichel CC, Müller HW (1998a) Experimental strategies to promote axonal regeneration after traumatic central nervous system injury. Prog Neurobiol 56:119-148.
Stichel CC, Müller HW (1998b) The CNS lesion scar: new vistas on an old regeneration barrier. Cell Tissue Res 294:1-9.
Suhonen JO, Peterson DA, Ray J, Gage FH (1996) Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature 383: 624-627.
Tan AM, Zhang W, Levine JM (2005) NG2: a component of the glial scar that inhibits axon growth. J Anat 207:717-725.
Temple S (1989) Division and differentiation of isolated CNS blast cells in microculture. Nature 340:471-473.
Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK (2011) A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma 28:1611-1682.
Tham M, Ramasamy S, Gan HT, Ramachandran A, Poonepalli A, Yu YH, Ahmed S (2010) CSPG is a secreted factor that stimulates neural stem cell survival possibly by enhanced EGFR signaling. PLoS One 5:e15341.
Thorburne SK, Juurlink BH (1996) Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem 67:1014-1022.
Tikka T, Fiebich BL, Goldsteins G, Keinanen R, and Koistinaho J (2001) Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation, proliferation of microglia. J Neurosci 21: 2580-2588.
Trotter J, Karram K, Nishiyama A (2010) NG2 cells: Properties, progeny and origin. Brain Res Rev 63:72-82.
Trueman RC, Klein A, Lindgren HS, Lelos MJ, Dunnett SB (2013) Repair of the CNS using endogenous and transplanted neural stem cells. Curr Top Behav Neurosci 15:357-398.
Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, Higashi H, Nagai T, Katoh H, Kohda K, Matsuzaki Y, Yuzaki M, Ikeda E, Toyama Y, Nakamura M, Yamanaka S, Okano H (2010) Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A 107:12704-12709.
Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA (2008) Self-assembling nano fi bers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci 28:3814-3823.
Veenith T, Goon SSh, Burnstein RM (2009) Molecular mechanisms of traumatic brain injury: the missing link in management. World J Emerg Surg 4:7.
Vela JM, Yáñez A, González B, Castellano B (2002) Time course of proliferation and elimination of microglia/macrophages in different neurodegenerative conditions. J Neurotrauma 19:1503-1520.
Volterra A, Meldolesi J (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6:626-640.
Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y (2007) A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25:681-686.
Walz W (1989) Role of glial cells in the regulation of the brain ion microenvironment. Prog Neurobiol 33:309-333.
Wigley R, Hamilton N, Nishiyama A, Kirchhoff F, Butt AM (2007) Morphological and physiological interactions of NG2-glia with astrocytes and neurons. J Anat 210:661-670.
Wigley R, Butt AM (2009) Integration of NG2-glia (synantocytes) into the neuroglial network. Neuron Glia Biol 5:21-28.
Wu J, Yoo S, Wilcock D, Lytle JM, Leung PY, Colton CA, Wrathall JR (2010) Interaction of NG2(+)glial progenitors and microglia/macrophages from the injured spinal cord. Glia 58:410-422.
Xiao L, Tsutsui T (2013) Human dental mesenchymal stem cells and neural regeneration. Hum Cell 26:91-96.
Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A (2006) NG2 glial cells provide a favorable substrate for growing axons. J Neurosci 26:3829-3839.
Yao J, Keri JE, Taffs RE, Colton CA (1992) Characterization of interleukin-1 production by microglia in culture. Brain Res 591:88-93.
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617-627.
Zhang D, Hu X, Qian L, O’Callaghan JP, Hong JS (2010) Astrogliosis in CNS pathologies: is there a role for microglia? Mol Neurobiol 41:232-241.
Zhang Q, Yuan L, Liu D, Wang J, Wang S, Zhang Q, Gong Y, Liu H, Hao A, Wang Z (2014) Hydrogen sul fi de attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol Res 84C:32-44.
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178-201.
10.4103/1673-5374.137570
http://www.nrronline.org/
Accepted: 2014-07-01
杂志排行
中国神经再生研究(英文版)的其它文章
- Beyond taxol: microtubule-based strategies for promoting nerve regeneration after injury
- Neuroprotective effect of the traditional Chinese herbal formula Tongxinluo: a PET imaging study in rats
- Neuroprotective effects of Asiaticoside
- Treating Alzheimer’s disease with Yizhijiannao granules by regulating expression of multiple proteins in temporal lobe
- Autophagy activation aggravates neuronal injury in the hippocampus of vascular dementia rats
- Role of Notch-1 signaling pathway in PC12 cell apoptosis induced by amyloid beta-peptide (25-35)
