Targeted thrombolysis strategies for neuroprotective effect
2014-06-01JunpingZhangGuoxingMaZhiminLvYuZhouChunguangWenYaqingWuRuianXu
Junping Zhang, Guoxing Ma, Zhimin Lv, Yu Zhou, Chunguang Wen, Yaqing Wu, Ruian Xu
School of Biomedical Sciences, Huaqiao University & Engineering Research Center of Molicular Medicine, Ministry of Education, Xiamen, Fujian Province, China
Targeted thrombolysis strategies for neuroprotective effect
Junping Zhang, Guoxing Ma, Zhimin Lv, Yu Zhou, Chunguang Wen, Yaqing Wu, Ruian Xu
School of Biomedical Sciences, Huaqiao University & Engineering Research Center of Molicular Medicine, Ministry of Education, Xiamen, Fujian Province, China
Stroke is usually treated by systemic thrombolytic therapy if the patient presents within an appropriate time window. There is also widespread interest in the development of thrombolytic agents that can be used in cases of delayed presentation. Current agents that can be used in cases of delayed presentation of nerve damage by thrombus. Current systemic thrombolytic therapy is associated with adverse effects such as fi brinogenolysis and bleeding. In an attempt to increase the ef fi cacy, safety, and speci fi city of thrombolytic therapy, a number of targeted thrombolytic agents have been studied in recent years. This review focuses on the concepts underlying targeted thrombolytic therapy and describes recent drug developments in this fi eld.
nerve regeneration; review; thrombolytic agent; fibrinolytic system; stroke; cerebrovascular disease; neuroprotective drug; fibrinolytic mechanism; fibrin targeting; platelet targeting; red blood cell targeting; cerebral hemorrhage; NSFC grant; neural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 81271692.
Zhang JP, Ma GX, Lv ZM, Zhou Y, Wen CG, Wu YQ, Xu RA. Targeted thrombolysis strategies for neuroprotective effect. Neural Regen Res. 2014;9(13):1316-1322.
Introduction
Cardiovascular diseases including pulmonary embolism, atherosclerosis, coronary heart disease, acute myocardial infarction, and stroke are major causes of morbidity and mortality worldwide (Capstick and Henry, 2005; Prasad et al., 2006; van der Worp and van Gijn, 2007; White and Chew, 2008; Jones et al., 2010; Collart et al., 2012; Siddiqui et al., 2013; Starmans et al., 2013). These diseases result from obstruction of blood fl ow by thrombus, and the most effective method of preventing morbidity and mortality associated with these diseases is to prevent thrombus formation. When thrombus has already formed, the best treatment strategy is to achieve rapid recanalization of the occluded vessel by angioplasty, surgery, or thrombolysis/ fi brinolysis to remove the thrombus and prevent further propagation (Absar et al., 2013). Stroke is usually treated by thrombolytic therapy, which works by interacting with the body’s intrinsic fibrinolytic system (Kowalski et al., 2009). Many thrombolytic agents have been found to effectively dissolve thrombus, including streptokinase, urokinase, and tissue-type plasminogen activator. These agents comprise proteolytic components of the blood clotting cascade, and as they are circulated throughout the cardiovascular system they do not selectively target speci fi c organs or tissues. The systemic side effects of these agents such as fi brinogenolysis and bleeding (Kowalski et al., 2009; Absar et al., 2013) result in unavoidable clinical dif fi culties
(Oyama et al., 2013). Liu et al. (2006) reported that addition of a neuroprotective agent can increase the effectiveness of thrombolytic therapy, increase the therapeutic time window, and reduce cerebral ischemia-reperfusion injury. It is hoped that thrombolytic agents with neuroprotective effects can be developed for clinical use.
Mechanism of thrombolysis
Intravascular thrombus formation is a complex physiological process that involves interactions among many factors. When a blood vessel is injured, local accumulation of platelets and fi brin results in thrombus formation to prevent blood loss. Thrombus formation may also occur without vessel injury under some conditions. After thrombosis, plasminogen activator cleaves the sensitive Arg561-Val562 peptide bond of plasminogen (Lijnen and Collen, 2000; Lijnen, 2001; Oyama et al., 2013), thereby activating plasminogen to form plasmin (Gabriel et al., 1992; Castellino and Ploplis, 2005; Kunamneni et al., 2007; Baumer et al., 2013; Gomaraschi et al., 2013), which triggers the body’s mechanisms for dissolving thrombus into soluble fi brin degradation products and restores the blood fl ow (Guyatt et al., 2012) (Figure 1).
Classes of thrombolytic drugs
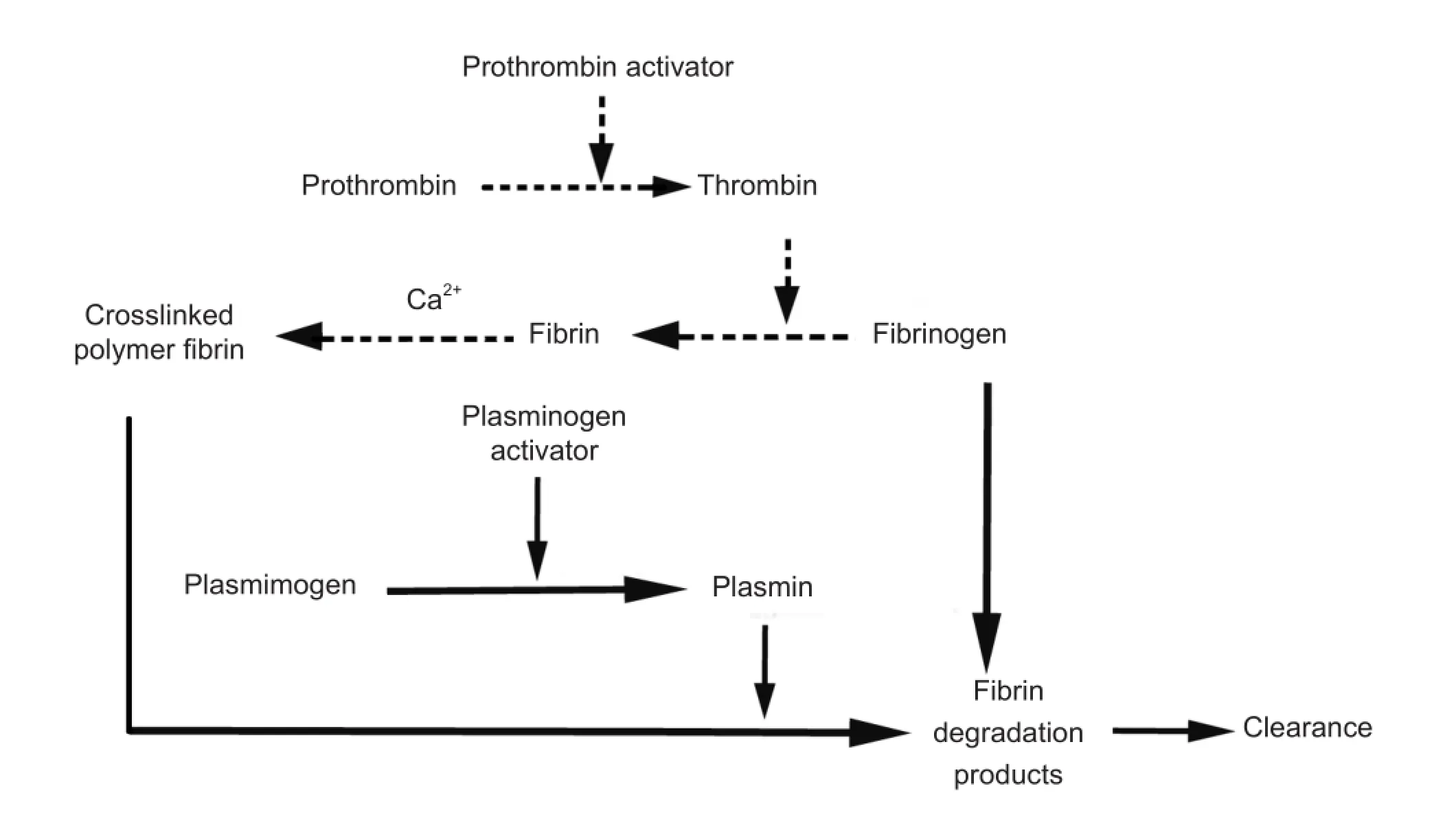
Figure 1 Thrombosis and thrombolysis pathways.
In an attempt to increase the ef fi cacy and safety of thrombolytic therapy, recent research has focused on the development of targeted thrombolytic agents. Thrombolytic agents have been used with the aim of recanalizing the occluded vessel since streptokinase was first used in patients with acute myocardial infarction (Meyer et al., 1965). Four generations of thrombolytic agents have subsequently been used in clinical trials for the treatment of various clotting disorders (Kirmani et al., 2012). However, thrombolytic therapy is still associated with many problems. The fi rst-generation fi brinolytic agents are effective for thrombolysis, but are not fi brin speci fi c (Bentley and Sharma, 2005) and they may induce immunological responses resulting in drug resistance, fever, and allergic reactions (Verstraete, 2000). The second-generation fi brinolytic agents are more fi brin speci fi c, do not induce adverse immunological responses (Balami et al., 2013), and have a shorter half-life (Collen and Lijnen, 1991; Epplera et al., 1998; Kim et al., 2009). The majority of third- and fourth-generation fi brinolytic agents have advantages over second-generation agents, but are currently only available in clinical trials (Longstaff et al., 2008). There is a need to improve the specificity, efficacy, and safety of the drugs available for clinical use (Toombs, 2001). Current research is focused on enhancing the ability to target the site of the thrombus and reduce adverse effects and complications (Table 1).
Targeted thrombolysis
Thrombus is mainly composed of a mesh of fi brin and platelets (Cadroy and Hanson, 1990; Varin et al., 2013; Wadajkar et al., 2013) and may also include red blood cells (RBCs). Precise targeting of the thrombus site has been attempted by targeting these components.
Fibrin targeting
Fibrin (also called Factor Ia) is a fi brous, non-globular, insoluble protein that is produced in response to bleeding, and is the main protein component of thrombus (Ghasemi et al., 2012). There is a high concentration of fi brin in all types of thrombus, including acute and chronic, and arterial and venous thrombus (Sirol et al., 2005). Fibrin is a tough protein substance arranged in long fi brous chains that form from fibrinogen during blood coagulation (Mosesson, 2005). When tissue damage results in bleeding, fi brinogen in the wound is converted to fi brin monomers by the action of thrombin (Lord, 2007; Ariens, 2013). The fibrin monomers combine to form long fi brin threads that entangle platelets to build a spongy mass that gradually hardens and contracts, resulting in thrombus formation. Several proteins can bind to fi brinogen and/or fi brin and can thereby in fl uence thrombus formation, structure, and degradation.
Carboxypeptidase N (CPN) is an enzyme that cleaves C-terminal arginine residues from bradykinin, and belongs to the same family of zinc metallocarboxy-peptidases as thrombin-activatable fi brinolysis inhibitor (Walker et al., 2008). Talens et al. studied and identi fi ed CPN as a novelthrombus component with possible anti fi brinolytic properties (Talens et al., 2012a), but could not prove the presence of CPN in thrombus (Talens et al., 2012b). CPN should therefore be investigated further to determine whether it binds directly to fi brin or fi brinogen. The above-mentioned studies used surface plasmon resonance to determine that thrombus-bound CPN has the same molecular forms as CPN in the plasma, and that CPN may bind to fi brinogen and fi brin.
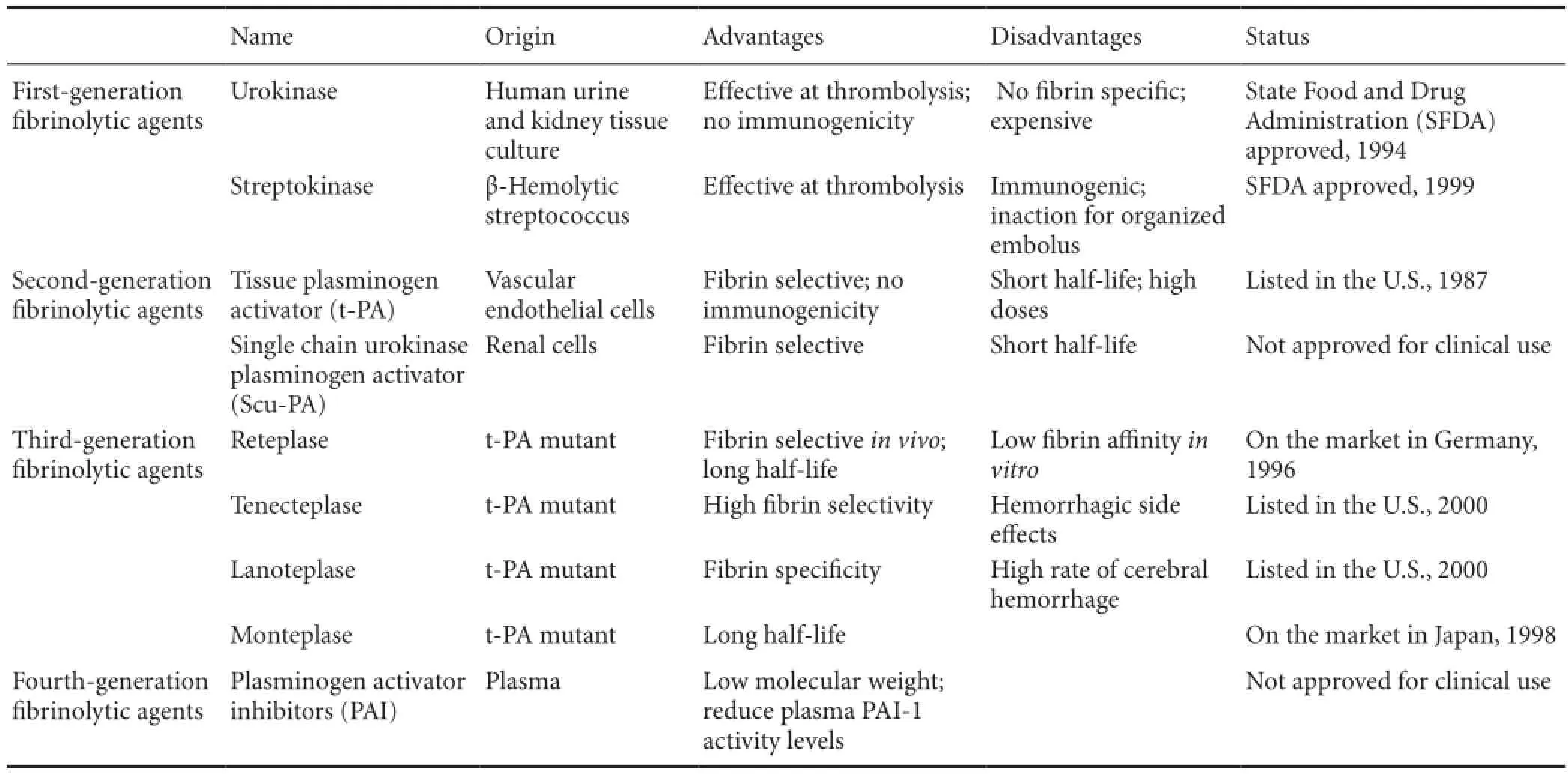
Table 1 Classes of thrombolytic drugs
Fibrin has been considered as a molecular target for the selective delivery of thrombolytic agents to the thrombus. Recently, ultrasound-assisted drug delivery has been investigated as a method of targeting a speci fi c area (Klibanov et al., 2010), and intrinsically echogenic liposomes have been used as a vehicle to achieve ultrasound-triggered controlled drug release (Huang, 2008; Greineder et al., 2013). Ultrasound was found to improve the effectiveness of tissue plasminogen activator (tPA), but was associated with hemorrhagic side effects (Datta et al., 2006; Holland et al., 2008; Meunier et al., 2009). Use of tPA-loaded intrinsically echogenic liposomes was found to be similarly effective to other treatment methods, while offering the advantages of ultrasound monitoring and enhanced thrombolysis with site-speci fi c delivery (Shaw et al., 2009; Laing et al., 2012).
The fi brin-speci fi city of thrombolytic agents may be improved by conjugating them with fi brin-speci fi c monoclonal antibodies (Vaidya et al., 2012). A hybrid molecule conjugated with tPA and the fi brin-speci fi c monoclonal antibody 59D8 by a disul fi de bond (Runge et al., 1987) has a 10-fold higher af fi nity for fi brin than urokinase and a 100-fold higher affinity for fibrin than tPA. Furthermore, experimental use of a hybrid recombinant plasminogen activator, antifibrin antibody 59D8-low-molecular-weight single-chain urokinase-type plasminogen activator, for antibody targeting of fi brin increased the thrombolytic and antithrombotic potency with less impairment of hemostasis compared with recombinant tPA and recombinant single-chain urokinase-type plasminogen activator (Runge et al., 1996).
Current strategies for site-speci fi c delivery are focused primarily on the local release of therapeutic agents by drug-eluting stents. However, this technique is expensive and can only be used in limited situations (Muni and Gross, 2004; Huang et al., 2008). Nanoparticles have recently attracted attention as potential vehicles for targeted drug therapy, and have been shown to increase therapeutic effectiveness (Cyrus et al., 2008; Tsuruta et al., 2009; Gu et al., 2012; McCarthy et al., 2012). Yurko et al. (2009) used in vitro fi brinolysis assays to show that use of 40-nm polystyrene-latex nanoparticles covalently conjugated to tPA and anti- fi brin antibody could deliver tPA directly to the site of the thrombus, thereby lowering the risk of hemorrhage. Since then, a thrombolytic agent that conjugates an anti fi brin monoclonal antibody and urokinase to a perfluorocarbon nanoparticle has been developed, and its effectiveness for targeted thrombolysis has been evaluated (Marsh et al., 2007, 2011). In animal studies, the maximum lytic effect was achieved with an enzyme load of 100-400 per nanoparticle.
Platelet targeting
Platelets (also called thrombocytes) are small, disk-shaped, clear, anuclear cell fragments, 2-3 μm in diameter, which are derived from fragmentation of precursor megakaryocytes. Platelets circulate in the blood of mammals and play a vital role in the process of thrombus formation to maintain hemostasis (Langer and Gawaz, 2008). Under pathophysiological conditions, platelet activation can also play a critical rolein various thromboembolic diseases (Harrison, 2000). Platelet membrane glycoprotein IIb/IIIa (GPIIb/IIIa) receptor activation is the fi nal common pathway of platelet aggregation (Kulkarni et al., 2000; ten Berg et al., 2001; Davi and Patrono, 2007; Badimon and Vilahur, 2008; Gladding et al., 2008). The GPIIb/IIIa receptor is the most abundant protein on the platelet membrane (Jennings, 2009; Vaidya et al., 2011), and is a potential target for novel thrombolytic agents. L-arginine-glycine-aspartic acid peptide (RGD) is a GPIIb/IIIa antagonist that binds to activated GPIIb/IIIa receptors speci fi cally on aggregated platelets (Meyer et al., 2006), and has been used to develop targeted thrombolytic drugs. Under normal conditions, the spatial configuration of GPIIb/IIIa receptors is stable, and the platelet is inactive. When platelet agonists such as thrombin, collagen, adenosine diphosphate, and thromboxane A2 bind with their receptors on the platelet membrane, GPIIb/IIIa forms a functional dimer complex that exposes the platelet membrane (Huang et al., 2008), resulting in binding with RGD. Conversely, the GPIIb/IIIa receptors are hidden on the unactivated platelet membrane, and cannot bind with RGD. RGD therefore binds only with activated platelets in the thrombus, and has no effect on circulating platelets. The hexapeptide H-Pro-Ser-Nva-Gly-Asp-Trp-OH also binds to the GPIIb/IIIa receptor on activated platelets, and development of thrombus-targeted microbubbles has been attempted by binding this hexapeptide to microbubbles (Zhou et al., 2011). Binding of the hexapeptide to the GPIIb/IIIa receptor delivers the microbubbles speci fically to the thrombus (Wang et al., 2006). Platelet-targeted microbubbles have also been investigated for the prevention of thrombus recurrence. The microbubbles were found to be good carriers of thrombolytic drugs, and to be bene fi cial for preventing thrombus recurrence in vivo. GPIIb/IIIa receptors have been used as a target for the delivery of thrombolytic agents by conjugation with a monoclonal antibody modifi ed with N-succinimidyl-3-(2-pyridyldithio) propionate at 7E3 Fab’ (Bates et al., 1991) to selectively bind urokinase to GPIIb/IIIa on the platelet membrane. The conjugated urokinase was found to have higher thrombolytic activity than unconjugated urokinase (Bode et al., 1991). Platelet activation also exposes phosphatidylserine on the platelet membrane. Annexin V has a high af fi nity for phosphatidylserine, and binding of annexin V to the B chain of urokinase-type plasminogen activator by a disul fi de bond was found to increase the thrombolytic activity of the plasminogen activator in in vitro tests (Okabayashi et al., 1996).
RBC targeting
In most cases, labile and complex biotherapeutic agents such as enzymes require precise delivery to the target site. The best way to achieve this goal is to use coupling drugs such as synthetic or natural polymers with various geometric confi gurations, phospholipid liposomes, albumin, antibodies, or other biological molecules as carriers (Simone et al., 2008). RBCs (also called erythrocytes) are anuclear, biconcave, discshaped cells with a diameter of 7-8 μm, thickness of 2-3 μm, and membrane surface area of about 160 μm2, and are the most common type of blood cell (Pierige et al., 2008). RBCs have many features that make them ideal carriers for drugs in the bloodstream (Magnani et al., 2002; Danchin et al., 2008), especially when sustained action is needed (Bax et al., 1999; Millan et al., 2004; Muzykantov, 2010; Greineder et al., 2013; Muzykantov, 2013). RBCs can transport many substances, and the RBC membrane is supported by a complex cytoskeleton comprising a hexagonal lattice of actin-spectrin filaments interconnected by anchoring integral plasmalemmal proteins via numerous structural and connector proteins (Muzykantov, 2010; Luo et al., 2012). Ineffective delivery of plasminogen activators to the thrombus site limits their therapeutic effectiveness. This problem cannot be solved by increasing the dose because of the associated risk of adverse effects (Ganguly et al., 2006). However, the use of RBCs as drug carriers may help to solve this problem by enabling the use of plasminogen activators as thromboprophylactic agents (Ganguly et al., 2005). RBCs have recently been used as intravascular carriers for targeted drug delivery (Sera fi ni et al., 2004; Rossi et al., 2005).
Once thrombus is established, it becomes progressively more impermeable to RBCs, and RBC carriers of plasminogen activators can therefore potentially prevent vascular occlusion in patients at imminent risk of thrombosis without lysing hemostatic clots. Murciano et al. (2003) hypothesized that tPA conjugated to RBCs would dissolve nascent clots while having minimal effects on preexisting hemostatic clots or extravascular tissues. This RBC-based drug delivery method alters the fi brinolytic pro fi le of tPA, thereby permitting prophylactic fibrinolytic therapy. Conjugating of tPA to RBCs also reduces its central nervous system toxicity by spatially con fi ning the drug to the vascular system. Administration of RBC-tPA before or after cerebral hypoxia/ischemia may preserve the responses to cerebral vasodilators and prevent neuronal injury mediated through the extracellular signal-related kinase (mitogen-activated protein kinase) pathway, indicating that use of RBC-tPA may increase the bene fi t/risk ratio of thrombolytic therapy (Armstead et al., 2009).
Plasminogen activators are currently not used for thromboprophylaxis because of their rapid clearance, associated risk of bleeding, and extravascular toxicity. Zaitsev et al. (2006) conjugated tPA to a monoclonal antibody against complement receptor type 1 expressed primarily on human RBCs, and found that tPA bound rapidly to RBCs in the bloodstream and circulated safely for many hours after injection in mice, providing prophylactic thrombolysis without hemorrhagic side effects, similar to use of preformed RBC-tPA. This approach provided rapid and tight binding of tPA to RBCs, which markedly prolonged the circulation of tPA, accelerated lysis of venous and occlusive arterial thrombus that formed subsequent to injection, and reduced bleeding from preexisting hemostatic clots. Subsequently, a single-chain antibody fragment-tissue type plasminogen activator fusion targeted to RBC glycophorin-A related antigen was developed that bound safely to circulating RBCs and had anti-thrombotic effects in a mouse model of thrombosisthat were qualitatively similar to RBC-tPA and superior to tPA (Zaitzev et al., 2010).
Conclusion
Currently, the ability to dissolve thrombus using conventional thrombolytic therapy is limited. Pharmacological agents have generally targeted the transformation of plasminogen and plasmin, thereby facilitating the natural process of fibrinolysis. However, these agents do not discriminate between healthy and at-risk vasculature, and are widely distributed in the circulation. Development of newer targeted thrombolytic agents should enable selective delivery to speci fi c organs, tissues, or cells to enhance targeting of the thrombus and reduce adverse effects, thereby achieving superior ef fi cacy and safety compared with existing therapeutic options.
Several approaches have been proposed for the targeted delivery of plasminogen activators, including conjugation with specific monoclonal antibodies, oligopeptides, and nanoparticles. Conjugation with fi brin-speci fi c immunoconjugates seems to be a less promising approach because of the poor speci fi city of antibody binding to fi brin, and the inability to distinguish between hemostatic clots and occlusive thrombus. The effects of platelet-speci fi c agents are obvious, but their safety and effectiveness need to be further studied. Strategies based on conjugating thrombolytic agents to RBCs are less practical. Use of a single-chain antibody fragment-tissue type plasminogen activator fusion targeted to RBC glycophorin-A related antigen appears to be at least as promising as use of tPA-loaded nanoparticles for the prevention of both venous and arterial thrombus formation. Effective clearance of preexisting thrombus using methods such as ultrasound (Uesugi et al., 2010) and enhancement of the effectiveness of thrombolytic agents may provide a good approach to the treatment of stroke.
Acknowledgments:We thank the School of Biomedical Sciences, Huaqiao University, China for providing reference information resources.
Author contributions:Zhang JP and Ma GX wrote the manuscript. Lv ZM and Wen CG performed the literature search. Zhou Y revised the manuscript. Wu YQ and Xu RA obtained funding. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Absar S, Nahar K, Kwon YM, Ahsan F (2013) Thrombus-targeted nanocarrier attenuates bleeding complications associated with conventional thrombolytic therapy. Pharm Res 30:1663-1676.
Ariëns RA (2013) Fibrin(ogen) and thrombotic disease. J Thromb Haemost 11 Suppl 1:294-305.
Armstead WM, Ganguly K, Kiessling JW, Chen XH, Smith DH, Higazi AA, Cines DB, Bdeir K, Zaitsev S, Muzykantov VR (2009) Red blood cells-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia/ischemia through inhibition of ERK MAPK activation. J Cereb Blood Flow Metab 29:1463-1474.
Badimon L, Vilahur G (2008) Coronary atherothrombotic disease: progress in antiplatelet therapy. Rev Esp Cardiol 61:501-513.
Balami JS, Chen R, Sutherland BA, Buchan AM (2013) Thrombolytic agents for acute ischaemic stroke treatment: the past, present and future. CNS Neurol Disord Drug Targets 12:145-154.
Bates ER, McGillem MJ, Mickelson JK, Pitt B, Mancini GB (1991) A monoclonal antibody against the platelet glycoprotein IIb/IIIa receptor complex prevents platelet aggregation and thrombosis in a canine model of coronary angioplasty. Circulation 84:2463-2469.
Bäumer W, Herrling GM, Feige K (2013) Pharmacokinetics and thrombolytic effects of the recombinant tissue-type plasminogen activator in horses. BMC Vet Res 9:158.
Bax BE, Bain MD, Talbot PJ, Parker-Williams EJ, Chalmers RA (1999) Survival of human carrier erythrocytes in vivo. Clin Sci 96:171-178.
Bentley P, Sharma P (2005) Pharmacological treatment of ischemic stroke. Pharmacol Ther 108:334-352.
Bode C, Meinhardt G, Runge MS, Freitag M, Nordt T, Arens M, Newell JB, Kubler W, Haber E (1991) Platelet-targeted fi brinolysis enhances clot lysis and inhibits platelet aggregation. Circulation 84:805-813.
Cadroy Y, Hanson SR (1990) Effects of red blood cell concentration on hemostasis and thrombus formation in a primate model. Blood 75:2185-2193.
Capstick T, Henry MT (2005) Efficacy of thrombolytic agents in the treatment of pulmonary embolism. Eur Respir J 26:864-874.
Castellino FJ, Ploplis VA (2005) Structure and function of the plasminogen/plasmin system. Thromb Haemost 93:647-654.
Collart P, Coppieters Y, Levêque A (2012) Trends in acute myocardial infarction treatment between 1998 and 2007 in a Belgian area (Charleroi). Eur J Prev Cardiol 19:738-745.
Collen D, Lijnen HR (1991) Basic and clinical aspects of fibrinolysis and thrombolysis. Blood 78:3114-3124.
Cyrus T, Zhang H, Allen JS, Williams TA, Hu G, Caruthers SD, Wickline SA, Lanza GM (2008) Intramural delivery of rapamycin with alphavbeta3-targeted paramagnetic nanoparticles inhibits stenosis after balloon injury. Arterioscler Thromb Vasc Biol 28:820-826.
Danchin N, Coste P, Ferrières J, Steg PG, Cottin Y, Blanchard D, Belle L, Ritz B, Kirkorian G, Angioi M, Sans P, Charbonnier B, Eltchaninoff H, Guéret P, Khalife K, Asseman P, Puel J, Goldstein P, Cambou JP, Simon T, et al. (2008) Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the french registry on acute ST-elevation myocardial infarction (FAST-MI). Circulation 118:268-276.
Datta S, Coussios CC, McAdory LE, Tan J, Porter T, De Courten-Myers G, Holland CK (2006) Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol 32:1257-1267.
Davì G, Patrono C (2007) Platelet activation and atherothrombosis. N Engl J Med 357:2482-2494.
Eppler S, Senn T, Gilkerson E, Modi NB (1998) Pharmacokinetics and pharmacodynamics of recombinant tissue-type plasminogen activator following intravenous administration in rabbits: a comparison of three dosing regimens. Biopharm Drug Dispos 19:31-38.
Gabriel DA, Muga K, Boothroyd EM (1992) The effect of fi brin structure on fi brinolysis. J Biol Chem 267:24259-24263.
Ganguly K, Krasik T, Medinilla S, Bdeir K, Cines DB, Muzykantov VR, Murciano JC (2005) Blood clearance and activity of erythrocyte-coupled fi brinolytics. J Pharmacol Exp Ther 312:1106-1113.
Ganguly K, Goel MS, Krasik T, Bdeir K, Diamond SL, Cines DB, Muzykantov VR, Murciano JC (2006) Fibrin affinity of erythrocyte-coupled tissue-type plasminogen activators endures hemodynamic forces and enhances fi brinolysis in vivo. J Pharmacol Exp Ther 316:1130-1136.
Ghasemi Y, Dabbagh F, Ghasemian A (2012) Cloning of a fi brinolytic enzyme (Subtilisin) gene from bacillus subtilis in Escherichia coli. Mol Biotechnol 52:1-7.
Gladding P, Webster M, Ormiston J, Olsen S, White H (2008) Antiplatelet drug nonresponsiveness. Am Heart J 155:591-599.
Gomaraschi M, Ossoli A, Vitali C, Pozzi S, Serdoz LV, Pitzorno C, Sinagra G, Franceschini G, Calabresi L (2013) Off-target effects of thrombolytic drugs: apolipoprotein A-I proteolysis by alteplase and tenecteplase. Biochem Pharmacol 85:525-530.
Greineder CF, Howard MD, Carnemolla R, Cines DB, Muzykantov VR (2013) Advanced drug delivery systems for antithrombotic agents. Blood 122:1565-1575.
Gu Z, Rolfe BE, Xu ZP, Campbell JH, Lu GQ, Thomas AC (2012) Antibody-targeted drug delivery to injured arteries using layered double hydroxide nanoparticles. Adv Healthc Mater 1:669-673.
Guyatt GH, Norris SL, Schulman S, Hirsh J, Eckman MH, Akl EA, Crowther M, Vandvik PO, Eikelboom JW, McDonagh MS, Lewis SZ, Gutterman DD, Cook DJ, Schünemann HJ; American College of Chest Physicians (2012) Methodology for the development of antithrombotic therapy and prevention of thrombosis guidelines: Antithrombotic Therapy and Prevention of Thrombosis, 9thed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141:53S-70S.
Harrison P (2000) Progress in the assessment of platelet function. Br J Haematol 111:733-744.
Holland CK, Vaidya SS, Datta S, Coussios CC, Shaw GJ (2008) Ultrasound-enhanced tissue plasminogen activator thrombolysis in an in vitro porcine clot model. Thromb Res 121:663-673.
Huang GF, Zhou ZM, Srinivasan R, Penn MS, Kottke-Marchant K, Marchant RE, Gupta AS (2008) Affinity manipulation of surface-conjugated RGD peptide to modulate binding of liposomes to activated platelets. Biomaterials 29:1676-1685.
Huang SL (2008) Liposomes in ultrasonic drug and gene delivery. Adv Drug Deliver Rev 60:1167-1176.
Jennings LK (2009) Role of platelets in atherothrombosis. Am J Cardiol 103:4A-10A.
Kim JY, Kim JK, Park JS, Byun Y, Kim CK (2009) The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials 30:5751-5756.
Kirmani JF, Alkawi A, Panezai S, Gizzi M (2012) Advances in thrombolytics for treatment of acute ischemic stroke. Neurology 79:S119-125.
Klibanov AL, Shevchenko TI, Raju BI, Seip R, Chin CT (2010) Ultrasound-triggered release of materials entrapped in microbubble-liposome constructs: a tool for targeted drug delivery. J Control Release 148:13-17.
Kowalski M, Brown G, Bieniasz M, Oszajca K, Chabielska E, Pietras T, Szemraj Z, Makandjou-Ola E, Bartkowiak J, Szemraj J (2009) Cloning and expression of a new recombinant thrombolytic and anthithrombotic agent - a staphylokinase variant. Acta Biochim Pol 56:41-53.
Kulkarni S, Dopheide SM, Yap CL, Ravanat C, Freund M, Mangin P, Heel KA, Street A, Harper IS, Lanza F, Jackson SP (2000) A revised model of platelet aggregation. J Clin Invest 105:783-791.
Kunamneni A, Abdelghani TT, Ellaiah P (2007) Streptokinase-the drug of choice for thrombolytic therapy. J Thromb Thrombolysis 23:9-23.
Laing ST, Moody MR, Kim H, Smulevitz B, Huang SL, Holland CK, McPherson DD, Klegerman ME (2012) Thrombolytic efficacy of tissue plasminogen activator-loaded echogenic liposomes in a rabbit thrombus model. Thromb Res 130:629-635.
Langer HF, Gawaz M (2008) Platelet-vessel wall interactions in atherosclerotic disease. Thromb Haemost 99:480-486.
Lijnen HR (2001) Elements of the fi brinolytic system. Ann Ny Acad Sci 936:226-236.
Lijnen HR, Collen D (2000) Molecular basis of thrombolytic therapy. J Nucl Cardiol 7:373-381.
Liu H, Jia X, Yang J, Liu Z, Wang G, Li L (2006) Neuroprotective effect of Shenfu injection after intra-arterial thrombolysis. Neural Regen Res 1:428-431.
Longstaff C, Williams S, Thelwell C (2008) Fibrin binding and the regulation of plasminogen activators during thrombolytic therapy. Cardiovasc Hematol Agents Med Chem 6:212-223.
Lord ST (2007) Fibrinogen and fi brin: scaffold proteins in hemostasis. Curr Opin Hematol 14:236-241.
Luo R, Mutukumaraswamy S, Venkatraman SS, Neu B (2012) Engineering of erythrocyte-based drug carriers: control of protein release and bioactivity. J Mater Sci Mater Med 23:63-71.
Magnani M, Rossi L, Fraternale A, Bianchi M, Antonelli A, Crinelli R, Chiarantini L (2002) Erythrocyte-mediated delivery of drugs, peptides and modi fi ed oligonucleotides. Gene Ther 9:749-751.
Marsh JN, Senpan A, Hu G, Scott MJ, Gaffney PJ, Wickline SA, Lanza GM (2007) Fibrin-targeted per fl uorocarbon nanoparticles for targeted thrombolysis. Nanomedicine 2:533-543.
Marsh JN, Hu G, Scott MJ, Zhang H, Goette MJ, Gaffney PJ, Caruthers SD, Wickline SA, Abendschein D, Lanza GM (2011) A fi brin-specific thrombolytic nanomedicine approach to acute ischemic stroke. Nanomedicine (Lond) 6:605-615.
McCarthy JR, Sazonova IY, Erdem SS, Hara T, Thompson BD, Patel P, Botnaru I, Lin CP, Reed GL, Weissleder R, Jaffer FA (2012) Multifunctional nanoagent for thrombus-targeted fibrinolytic therapy. Nanomedicine (Lond) 7:1017-1028.
Meunier JM, Holland CK, Pancioli AM, Lindsell CJ, Shaw GJ (2009) Effect of low frequency ultrasound on combined rt-PA and epti fi batide thrombolysis in human clots. Thromb Res 123:528-536.
Meyer A, Auemheimer J, Modlinger A, Kessler H (2006) Targeting RGD recognizing integrins: Drug development, biomaterial research, tumor imaging and targeting. Curr Pharm Des 12:2723-2747.
Meyer JS, Gilroy J, Barnhart ME, Johnson JF (1965) Therapeutic thrombolysis in cerebral thromboembolism: randomized evaluation of intravenous streptokinase. In: Cerebral vascular diseases, Fourth Princeton Conference (Millikan CH, Siekert RG, Whisnant JP, eds). New York: Grune and Stratton.
Millan CG, Marinero ML, Castaneda AZ, Lanao JM (2004) Drug, enzyme and peptide delivery using erythrocytes as carriers. J Control Release 95:27-49.
Mosesson MW (2005) Fibrinogen and fi brin structure and functions. J Thromb Haemost 3:1894-1904.
Muni NI, Gross TP (2004) Problems with drug-eluting coronary stents - The FDA perspective. New Engl J Med 351:1593-1595.
Murciano JC, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR (2003) Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol 21:891-896.
Muzykantov VR (2010) Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin Drug Deliv 7:403-427.
Muzykantov VR (2013) Drug delivery carriers on the fringes: natural red blood cells versus synthetic multilayered capsules. Expert Opin Drug Del 10:1-4.
Okabayashi K, Tsujikawa M, Morita M, Einaga K, Tanaka K, Tanabe T, Yamanouchi K, Hirama M, Tait JF, Fujikawa K (1996) Secretory production of recombinant urokinase-type plasminogen activator-annexin V chimeras in Pichia pastoris. Gene 177:69-76.
Oyama E, Kitagawa Y, Takahashi H (2013) Primary structure and characterization of a non hemorrhagic metalloproteinase with fi brinolytic activity, from the snake venom of Protobothrops tokarensis (Tokara-habu). Toxicon 70:153-161.
Pierige F, Sera fi ni S, Rossi L, Magnani M (2008) Cell-based drug delivery. Adv Drug Deliv Rev 60:286-295.
Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM, Daginawala HF (2006) Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb J 4:14.
Rossi L, Sera fi ni S, Pierige F, Antonelli A, Cerasi A, Fraternale A, Chiarantini L, Magnani M (2005) Erythrocyte-based drug delivery. Expert Opin Drug Deliv 2:311-322.
Runge MS, Bode C, Matsueda GR, Haber E (1987) Antibody-enhanced thrombolysis: targeting of tissue plasminogen activator in vivo. Proc Natl Acad Sci U S A 84:7659-7662.
Runge MS, Harker LA, Bode C, Ruef J, Kelly AB, Marzec UM, Allen E, Caban R, Shaw SY, Haber E, Hanson SR (1996) Enhanced thrombolytic and antithrombotic potency of a fi brin-targeted plasminogen activator in baboons. Circulation 94:1412-1422.
Sera fi ni S, Rossi L, Antonelli A, Fraternale A, Cerasi A, Crinelli R, Chiarantini L, Schiavano GF, Magnani M (2004) Drug delivery through phagocytosis of red blood cells. Transfus Med Hemother 31:92-101.
Shaw GJ, Meunier JM, Huang SL, Lindsell CJ, McPherson DD, Holland CK (2009) Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thromb Res 124:306-310.
Siddiqui TI, Kumar KS, Dikshit DK (2013) Platelets and atherothrombosis: causes, targets and treatments for thrombosis. Curr Med Chem 20:2779-2797.
Simone EA, Dziubla TD, Muzykantov VR (2008) Polymeric carriers: role of geometry in drug delivery. Expert Opin Drug Deliv 5:1283-1300.
Sirol M, Aguinaldo JGS, Graham PB, Weisskoff R, Lauffer R, Mizsei G, Chereshnev I, Fallon JT, Reis E, Fuster V, Toussaint JF, Fayad ZA (2005) Fibrin-targeted contrast agent for improvement of in vivo acute thrombus detection with magnetic resonance imaging. Atherosclerosis 182:79-85.
Starmans LW, van Duijnhoven SM, Rossin R, Aime S, Daemen MJ, Nicolay K, Grüll H (2013) SPECT imaging of fibrin using fibrin-binding peptides. Contrast Media Mol Imaging 8:229-237.
Talens S, Leebeek FW, Demmers JA, Rijken DC (2012a) Identi fi cation of fi brin clot-bound plasma proteins. PLoS One 7:e41966.
Talens S, Lebbink JH, Malfliet JJ, Demmers JA, Uitte de Willige S, Leebeek FW, Rijken DC (2012b) Binding of carboxypeptidase N to fi brinogen and fi brin. Biochemical and biophysical research communications 427:421-425.
ten Berg JM, Plokker HT, Verheugt FW (2001) Antiplatelet and anticoagulant therapy in elective percutaneous coronary intervention. Curr Control Trials Cardiovasc Med 2:129-140.
Toombs CF (2001) New directions in thrombolytic therapy. Curr Opin Pharmacol 1:164-168.
Tsuruta W, Tsurushima H, Yamamoto T, Suzuki K, Yamazaki N, Matsumura A (2009) Application of liposomes incorporating doxorubicin with sialyl Lewis X to prevent stenosis after rat carotid artery injury. Biomaterials 30:118-125.
Uesugi Y, Kawata H, Jo J, Saito Y, Tabata Y (2010) An ultrasound-responsive nano delivery system of tissue-type plasminogen activator for thrombolytic therapy. J Control Release 147:269-277.
Vaidya B, Agrawal GP, Vyas SP (2011) Platelets directed liposomes for the delivery of streptokinase: Development and characterization. Eur J Pharm Sci 44:589-594.
Vaidya B, Agrawal GP, Vyas SP (2012) Functionalized carriers for the improved delivery of plasminogen activators. Int J Pharm 424:1-11.
van der Worp HB, van Gijn J (2007) Clinical practice: Acute ischemic stroke. N Engl J Med 357:572-579.
Varin R, Mirshahi S, Mirshahi P, Klein C, Jamshedov J, Chidiac J, Perzborn E, Mirshahi M, Soria C, Soria J (2013) Whole blood clots are more resistant to lysis than plasma clots--greater ef fi cacy of rivaroxaban. Thromb Res 131:e100-109.
Verstraete M (2000) Third-generation thrombolytic drugs. Am J Med 109:52-58.
Wadajkar AS, Santimano S, Rahimi M, Yuan BH, Banerjee S, Nguyen KT (2013) Deep vein thrombosis: Current status and nanotechnology advances. Biotechnol Adv 31:504-513.
Walker JB, Binette TM, Mackova M, Lambkin GR, Mitchell L, Bajzar L (2008) Proteolytic cleavage of carboxypeptidase N markedly increases its anti fi brinolytic activity. J Thromb Haemost 6:848-855.
Wang B, Zang WJ, Wang M, Ai H, Wang YW, Li YP, He GS, Wang L, Yu XJ (2006) Prolonging the ultrasound signal enhancement from thrombi using targeted microbubbles based on sulfur-hexafluoride- fi lled gas. Acad Radiol 13:428-433.
White HD, Chew DP (2008) Acute myocardial infarction. Lancet 372:570-584.
WRITING GROUP MEMBERS, Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, et al. (2010) Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 121:e46-e215.
Yurko Y, Maximov V, Andreozzi E, Thompson GL, Vertegel AA (2009) Design of biomedical nanodevices for dissolution of blood clots. Mater Sci Eng C 29:737-741.
Zaitsev S, Danielyan K, Murciano JC, Ganguly K, Krasik T, Taylor RP, Pincus S, Jones S, Cines DB, Muzykantov VR (2006) Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fi brinolysis. Blood 108:1895-1902.
Zaitzev S, Spitzer D, Murciano JC, Ding BS, Tliba S, Kowalska MA, Bdeir K, Kuo A, Stepanova V, Atkinson JP, Poncz M, Cines DB, Muzykantov VR (2010) Targeting of a mutant plasminogen activator to circulating red blood cells for prophylactic fi brinolysis. J Pharmacol Exp Ther 332:1022-1031.
Zhou XB, Qin H, Li J, Wang B, Wang CB, Liu YM, Jia XD, Shi N (2011) Platelet-targeted microbubbles inhibit re-occlusion after thrombolysis with transcutaneous ultrasound and microbubbles. Ultrasonics 51:270-274.
Copyedited by Elgin M, Raye W, Li CH, Song LP, Zhao M
10.4103/1673-5374.137580
Ruian Xu, Ph.D., School of Biomedical Sciences, Huaqiao University & Engineering Research Center of Molcelaur Medicine, Ministry of Education, No. 902, Fanhua Science Building, 668 Jimei Avenue, Jimei District, Xiamen 362021, Fujian Province, China, hmmedu@hqu.edu.cn.
http://www.nrronline.org/
Accepted: 2014-05-12
杂志排行
中国神经再生研究(英文版)的其它文章
- Stem cell therapy for central nerve system injuries: glial cells hold the key
- Beyond taxol: microtubule-based strategies for promoting nerve regeneration after injury
- Neuroprotective effect of the traditional Chinese herbal formula Tongxinluo: a PET imaging study in rats
- Neuroprotective effects of Asiaticoside
- Treating Alzheimer’s disease with Yizhijiannao granules by regulating expression of multiple proteins in temporal lobe
- Autophagy activation aggravates neuronal injury in the hippocampus of vascular dementia rats
