Morphological changes of gonadotropin-releasing hormone neurons in the rat preoptic area across puberty
2014-06-01HaogangXueXiaodongGaiWeiqiSunChunLiQuanLiu
Haogang Xue, Xiaodong Gai, Weiqi Sun, Chun Li, Quan Liu
1 Department of Orthopedic Surgery, Af fi liated Hospital of Beihua University, Changchun, Jilin Province, China
2 Department of Pathology, Beihua University, Changchun, Jilin Province, China
3 College of Public Health, Beihua University, Changchun, Jilin Province, China
4 Department of Cardiovascular Disease, the First Hospital of Jilin University, Changchun, Jilin Province, China
Morphological changes of gonadotropin-releasing hormone neurons in the rat preoptic area across puberty
Haogang Xue1, Xiaodong Gai2, Weiqi Sun3, Chun Li2, Quan Liu4
1 Department of Orthopedic Surgery, Af fi liated Hospital of Beihua University, Changchun, Jilin Province, China
2 Department of Pathology, Beihua University, Changchun, Jilin Province, China
3 College of Public Health, Beihua University, Changchun, Jilin Province, China
4 Department of Cardiovascular Disease, the First Hospital of Jilin University, Changchun, Jilin Province, China
Gonadotropin-releasing hormone (GnRH) neurons in the preoptic area may undergo morphological changes during the pubertal period when their activities are upregulated. To clarify the regulatory mechanism of puberty onset, this study aimed to investigate the morphological changes of GnRH neurons in the preoptic area of GnRH-enhanced green fl uorescent protein transgenic rats. Under confocal laser microscopy, pubertal GnRH neurons exhibited an inverted Y distribution pattern. Prepubertal GnRH neurons were generally unipolar and bipolar, and were distinguished as smooth type cells with few small processes or irregular type cells with many spine-like processes in the proximal dendrites. The number of GnRH neurons in the preoptic area and spine-like processes were increased during the course of reproductive maturation. There was no signi fi cant difference between male and female rats. Immuno fl uorescence staining revealed synaptophysin punctae close to the distal end of GnRH neurons, indicating that some presynaptic terminals may form a synaptic linkage with these neurons.
nerve regeneration; preoptic area; gonadotropin-releasing hormone; neurons; pubertal period; luteinizing hormone; transgenesis; bipolar neurons; neural regeneration
Xue HG, Gai XD, Sun WQ, Li C, Liu Q. Morphological changes of gonadotropin-releasing hormone neurons in the rat preoptic area across puberty. Neural Regen Res. 2014;9(13):1303-1312.
Introduction
Gonadotropin-releasing hormone (GnRH) (also known as luteinizing hormone-releasing hormone or luliberin), is a trophic peptide hormone responsible for the release of follicle-stimulating hormone and luteinizing hormone from the anterior pituitary. GnRH is a decapeptide synthesized in GnRH neurons of the medial forebrain (Arroyo et al., 2011; von Wolff et al., 2011; Sun et al., 2013). Cell bodies of GnRH neurons in rodents are mainly scattered in the medial septum, diagonal band of Broca, and preoptic area, at the level of the organum vasculosum of the lamina terminalis (Arroyo et al., 2011). The preoptic area of the hypothalamus is a key region for the production of GnRH. This area contains most of the GnRH-secreting neurons. GnRH neurons originate in the nasal region and migrate to the brain. These neurons are scattered throughout the medial septum and hypothalamus and form a network through their very long dendrites (> 1 mm long). This interwoven network therefore receives a shared synaptic input. This process allows these neurons to synchronize the release of GnRH. GnRH neurons in the preoptic area are considered to directly regulate sexual maturation and reproductive behavior (Kinoshita et al., 2005; Clarkson and Herbison, 2006b; Adachi et al., 2007). Axons of preoptic GnRH neurons extend to the median eminence of hypothalamus and release GnRH into the hypophyseal portal blood (Yin et al., 2010). Once in the anterior pituitary, GnRH controls the synthesis and secretion of gonadotropins, luteinizing hormone, and follicle-stimulating hormone (Debruyne et al., 2010; Farkas et al., 2010; Gentil et al., 2012).
GnRH neurons originate from the olfactory placode, and during embryonic development, these neurons migrate from this region to the brain along olfactory neuronal axons (Wray, 2002; Yu et al., 2011). GnRH neurons synthesize the various hormones well before puberty. Indeed, endogenous secretion of GnRH has been shown to occur during late fetal development or early neonatal development (Ko et al., 2010; Pandit and Saxena, 2010). However, the activity of GnRH neurons before puberty remains at a low level, which may be due to a central inhibition of GnRH secretion (Monje et al., 2010) or a shift in sensitivity resulting from the feedback of gonadal steroids (Shaw et al., 2012). Either response increases the release of GnRH and triggers the pubertal process (Shaw et al., 2012; Tada et al., 2013). Previous studies in different animal species have suggested that the activation of GnRH neurons in late postnatal development initiates puberty (Monje et al., 2010; Shaw et al., 2012; Tada et al., 2013). However, the mechanism underlying this effect remains debatable across these different species.
The morphology of GnRH neurons is altered following the onset of puberty. In the mouse, prepubertal GnRH neurons have been reported to appear as a relatively complex network of dendritic branches compared with those at postpuberty(Cottrell et al., 2006). During postnatal development, the soma and dendritic spine density in GnRH neurons of mice are significantly increased, which reflects an abundance of excitatory inputs to GnRH neurons at the adult stage (Cottrell et al., 2006). Whether a similar morphological characterization of GnRH neurons occurs in the rat remains to be determined. Postnatal morphological changes of GnRH neurons in the rat have been reported (Sharif et al., 2013). However, their observations were only made by immunocytochemistry, and this method does not provide extensive labeling of dendritic arborizations and spine-like processes of neurons. Detailed morphological studies are important to better understand the regulatory mechanism of puberty onset.
Transgenic mice expressing green fluorescent protein (GFP) or enhanced GFP (EGFP) in GnRH neurons have recently been established to visualize living GnRH neurons (Teixeira et al., 2010; Wojniusz et al., 2011). The present study aimed to investigate the morphological changes of GnRH neurons in the preoptic area using GnRH-EGFP transgenic rats.
Materials and Methods
Animals
For the morphological investigations of the preoptic GnRH neurons, male and female Wistar rats (n = 16 for each sex) aged 3 weeks (prepuberty) and 49-84 days (postpuberty; adult) were used to express EGFP under the control of the GnRH promoter, according to Teixeira et al. (2010). All rats were provided by the Experimental Animal Center of College of Basic Medical Science, Jilin University, China (license No. SCXK (Ji) 2007-0003). Animals were maintained under a 14-hour light and 10-hour dark cycle with ad libitum access to a standard rodent chow diet and water. The stage of the estrous cycle in adult female rats was determined by vaginal smear histology, and diestrus female rats were used for the analysis of synaptic inputs to the GnRH neurons (Teixeira et al., 2010). All experiments were performed with the approval of the Beihua University Animal Care Committee, China.
Tissue preparation
Rats were anesthetized by intraperitoneal injection of 5 mg/kg pentobarbital sodium (Dainippon Sumitomo Pharma, Osaka, Japan) and then perfused through the left ventricle with 0.9% saline followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer (PB; pH 7.4). Rats were then quickly decapitated. The brains were removed from the cranium and post- fi xed in a fresh solution of the same fi xative at 4°C for 24 hours. The post- fi xed brains were immersed in 30% sucrose in PB at 4°C for 3 days and then frozen rapidly by immersing them in n-hexane at -60°C. Serial frontal sections were cut (100 μm and 30 μm in thickness) on a cryostat (Leica Microsystems, Wetzlar, Germany) from the diagonal band of Broca to the caudal extent of the mammillary bodies (Yu et al., 2011). Sections were collected in three sets, transferred to individual wells in a 6-well plate containing 0.1 mol/L PBS (pH 7.4), and stored at 4°C until further use. Sections at 100 μm and 30 μm thickness were used for GnRH neuronal morphology and immunocytochemistry, respectively.
Morphological analyses of GnRH neurons
Prepubertal (four males and four females) and postpubertal (four males and four females) GnRH-EGFP transgenic rats were used for morphological analyses. Floating sections 100 μm thick were soaked in PB, mounted on gelatin-coated glass slides, and then covered with coverslips with Gel/Mount Aqueous Mounting Medium (Biomeda Corporation, Hayward, CA, USA). Detailed morphological analysis of EGFP-expressing GnRH neurons in the preoptic area was observed by a confocal laser scanning microscope (Digital Eclipse C1 TE2000-E, 3D; Nikon Insteck, Tokyo, Japan) with a green argon laser (excitation: 488 nm, emission: 515/530 nm). Fluorescence images were acquired and analyzed using a Nikon Plan Fluor 10 × objective (numerical aperture, 0.3) and 100 × oil immersion objective (numerical aperture, 1.45) lens with the confocal microscope Digital Eclipse C1 control software EZ-C1 (Version 2.30; Nikon Insteck). The confocal image represented the maximal projection of the optical image stack, reconstructed from a series of images at 400-nm intervals. A three-dimensional image was obtained using the high-performance three-dimensional imaging software, Volocity Visualization (Version 3.6.1; Improvision, Lexington, MA, USA). The brightness and contrast of the images were adjusted in Adobe Photoshop CS. Morphological data obtained from each rat were counted and averaged as a single observation for statistical analyses.
Immuno fl uorescence staining of synaptophysin
GnRH-EGFP transgenic rats at prepuberty (four males and four females) and postpuberty (four males and four females) were used for synaptophysin staining. Twenty- fi ve prepubertal and 40 postpubertal GnRH-EGFP neurons in male and female rats, respectively, were used for the analysis of synaptic inputs to GnRH neurons. Floating sections (30 μm thick) were rinsed in PBS and incubated with 5% normal goat serum in 0.1 mol/L PBS containing 0.3% Triton X-100 (PBST; pH 7.4; Sigma, St. Louis, MO, USA) for 2 hours at room temperature to reduce nonspeci fi c binding. Sections were incubated with the primary antibody, mouse anti-rat synaptophysin monoclonal antibody (1:200 in PBST; Clone SVP-38, Sigma) for 48 hours. After washing (three times for 10 minutes) in PBST, sections were then incubated for 2 hours with the secondary antibody, Alexa Flour 568 goat anti-mouse IgG (1:1,000 in PBST; Molecular Probes, Eugene, OR, USA). Sections were then washed, mounted on gelatin-coated glass slides, and covered with a coverslip. All procedures were performed at room temperature and in darkness. Images were acquired and processed as described for morphological analyses of GnRH neurons. The Z-series stack of confocal images at 400-nm intervals was collected using the 100 × oil immersion objective (numerical aperture, 1.45) with a green argon laser exciting at 488 nm (for EGFP) and a red helium-neon laser exciting at 543 nm (for synaptophysin). The number ofspines in the cell bodies and initial 50-μm length of dendrites were measured in reconstructed three-dimensional images obtained using the software, Volocity Visualization (Version 3.6.1, Improvision). The number of synaptophysin-positive puncta directly opposite to GnRH-EGFP soma and dendrites were counted and combined to provide mean values for each neuron. The initial 50-μm length of dendrite from the soma was designated for analyzing the number of synaptophysin appositions in the dendrites.

Figure 1 The effect of prepuberty and postpuberty on the number of GnRH-EGFP neurons in the preoptic area.
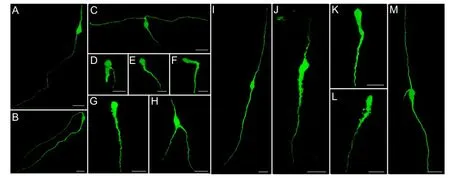
Figure 2 The effect of prepuberty and postpuberty on the morphology of GnRH-EGFP neurons in the preoptic area.
Statistical analysis
All data are expressed as mean ± SEM and were analyzed by paired or unpaired Student’s t-tests using SPSS 19.0 software (IBM Corporation, Armonk, NY, USA). Significance was reached at values of P < 0.05.
Results
The effect of prepuberty and postpuberty on the number of GnRH-EGFP neurons in the preoptic area
In the preoptic area of EGFP transgenic rats, EGFP was localized in all GnRH neurons. Furthermore, cell boundaries, including dendrites and spine-like processes, were clearly visible, which are observations that have not been adequately identified with immunocytochemistry (Kato et al., 2003; Farkas et al., 2010; Wojniusz et al., 2011; Sharif et al., 2013; Srivastava et al., 2014). GnRH-EGFP neurons in both the prepubertal and postpubertal preoptic area possessed a similar inverted Y distributional pattern (Figure 1A-D). The number of GnRH neurons in the preoptic area was significantly (P < 0.05) higher in postpubertal rats compared with prepubertal rats (Figure 1A-D). In the preoptic area of male rats, the number of postpubertal GnRH neurons increased 5.5-fold compared with prepubertal neurons (Figure 1A, C). In the preoptic area of female rats, the number of postpubertal GnRH neurons increased 7.1-fold compared with prepubertal neurons (Figure 1B, D). The number of GnRH-EGFP neurons in the preoptic area between male and female rats was not signi fi cantly different (Figure 1E).
The effect of prepuberty and postpuberty on the morphology of GnRH-EGFP neurons in the preoptic areaThree morphological phenotypes of GnRH-EGFP neurons with medium-sized somata (of 10-16 μm) were detected in both the prepubertal and postpubertal preoptic area. Although fewer (approximately 0.82%) multipolar neurons were detected, GnRH neurons were generally unipolar and bipolar (Figure 2). In the preoptic area frontal sections, the majority of unipolar GnRH neurons were oval-shaped with a long dendrite that emerged from the basal part of the soma and extended ventrally (Figure 2). In contrast, bipolar GnRH neurons were mostly fusiform-shaped with both dorsal and ventral dendritic extensions (Figure 2). Approximately one third of both unipolar and bipolar neurons exhibited oblique dendritic orientation (Figure 2). Some GnRH neurons displayed differing morphologies, such as a unidirectional extension of both dorsal and ventral dendrites (Figure 2B), bifurcation of dendrites (Figure 2C), and two dendrites of different thickness originating from the basal region of the soma(Figure 2D). These complex neuronal morphologies were mainly observed in preoptic area bipolar GnRH cells of prepubertal rats (Figure 2). In contrast, the postpubertal preoptic area principally exhibited typical bipolar and unipolar GnRH neuronal features (Figure 2I-L). The number of unipolar and bipolar GnRH neurons in the preoptic area at prepuberty was similar (Figure 3A). Postpubertal GnRH neurons were primarily bipolar compared (P <0.05;Figure 3B). No sex differences were found in the number of unipolar and bipolar neurons (Figure 3A, B).
Because spine-like processes were detected in nearly all the preoptic GnRH neurons, both unipolar and bipolar GnRH neurons were subdivided into the smooth type with few small processes (the number of processes ≤ 5) and the irregular type with many spine-like processes in the soma and proximal dendrite (the number of processes > 5). In prepubertal and postpubertal rats of both sexes, unipolar GnRH neurons were primarily an irregular type (P < 0.01;Figure 3C, D), whereas the bipolar neurons were mostly a smooth type (P < 0.05 or P < 0.01;Figure 3E, F). In prepubertal rats, irregular GnRH neurons were similar to the smooth type (Figure 3G). The number of smooth type neurons in postpubertal rats was not signi fi cantly higher than the irregular type (Figure 3H). Both the smooth and irregular GnRH-EGFP neurons were signi fi cantly (P < 0.05) increased following the development of sexual maturation (Figure 3A-H). No signi fi cant differences were observed in the GnRH neuronal sub-types between males and females at both maturation periods (Figure 3I, J).
The effect of prepuberty and postpuberty on the number of spine-like processes of GnRH-EGFP neurons in the preoptic area
Spine-like processes were observed in all examined GnRH-EGFP neurons, including the somata and dendrites (Figure 4A-D). Because the distal extremities of most dendrites were cut off during processing of the sections, the length of every dendrite appeared to be largely different. Furthermore, the majority of processes were present within the first 50 μm of the dendrites, and counts of processes revealed the number of processes according to their length from the soma. We found a gradual reduction in the number of processes with increasing distance from the soma (Figure 4A-C). Therefore, in the present study, soma and the initial 50-μm length of dendrite were used for counting the number of spine-like processes and synaptophysin appositions in GnRH-EGFP neurons. In prepubertal rats, the average number of spines in smooth GnRH neurons was 3.5 ± 1.02 and the mean irregular type in unipolar bipolar GnRH neurons consisted of 16.9 ± 4.15 spine-like processes. The number of spine-like processes increased in postpubertal neurons (19.7 ± 5.83), but no signi fi cant differences were detected between prepubertal and postpubertal rats (Figure 4E, F). Sex differences in the number of spine-like processeswere not detected in prepubertal or postpubertal GnRH neurons (Figure 4E).
The effect of prepuberty and postpuberty on the synaptic inputs to GnRH-EGFP neurons in the preoptic area
To evaluate synaptic inputs to GnRH neurons, we examined the apposition of synaptophysin to the somata and proximal dendrites of GnRH neurons by the confocal analysis. Plain images showed that numerous synaptophysin-immunoreactive punctae were in contact with GnRH neuronal somata and proximal dendrites (Figure 4A-C). However, few synaptophysin punctae was found adjacent to GnRH neurons. Three-dimensional rotation images showed that the apposition of synaptophysin punctae to GnRH neurons was approximately one third of the plan images. Some punctate staining of synaptophysin in the plan images was observed to be in contact with GnRH neuronal dendrites and/or somata, whereas three-dimensional image rotation along the dorsoventral axis indicated that they had exited the neuronal dendrites/somata (Figure 4A, B). This punctate staining of synaptophysin adjacent to the GnRH neurons in the plan images did not make contact with the dendrites and/or somata of GnRH neurons. The close apposition of synaptophysin with GnRH neurons occurred mainly on the proximal dendrites of GnRH neurons and with fewer observations on the somata and distal dendrites (Figure 4AD). In the irregular GnRH neurons of postpubertal rats, a signi fi cant (P < 0.05) increase of close appositions between synaptophysin and GnRH neurons was found in both sexes, and was not observed in the prepubertal rats (Figure 4F). However, no differences were detected between prepubertal and postpubertal male and female rats. No signi fi cant differences were found in the number of synaptophysin punctae in smooth GnRH neurons of both prepubertal and postpubertal rats (data not shown).
Discussion
Puberty is regarded as a process of maturation of reproductive function, representing the transition from childhood to adulthood in mammals (Ebling, 2005; Clarkson and Herbison, 2006a; Monje et al., 2010). GnRH neurons in the preoptic area may be altered morphologically during the pubertal period when their activities are upregulated (Cottrell et al., 2006; Sharif et al., 2013). Identifying the morphological changes of GnRH neurons following reproductive maturation is helpful in understanding the regulatory mechanisms underlying pubertal onset. GnRH-EGFP transgenic animals exhibit stronger EGFP expression in GnRH neurons (Spergel et al., 1999; Cariboni et al., 2007; Rosati et al., 2011; Shapiro et al., 2011; Vollaard et al., 2011) and clearly show dendritic arborizations and spine-like processes of these cells (Clarkson and Herbison, 2006b; Cottrell et al., 2006; Rosati et al., 2011). Therefore, in the present study, GnRH-EGFP transgenic rats were used for the morphological investigation of GnRH neurons (including synaptic inputs) in the preoptic area.
GnRH-EGFP neurons at both prepubertal and postpubertal stages in animals reveal a typical distribution pattern of GnRH neurons. The majority of GnRH cell bodies are present in the preoptic area where these neurons are involved in the generation of GnRH/luteinizing hormone, which initiates ovulation in mammals (Kinoshita et al., 2005; Clarkson and Herbison, 2006b; Adachi et al., 2007). However, the number of GnRH neurons varies greatly. Our results showed a predominance of GnRH-EGFP neurons in postpubertal rats over prepubertal animals. However, a previous study has shown a constant distribution of GnRH neurons throughout postnatal development in rats (Gao et al., 2011). The discrepancy between the two studies may lie in the different animals and methods used. The topographical distribution of GnRH neurons in the forebrain is fully established at the earliest neonatal stages, which may be involved in sexual dimorphism of the preoptic area (Gao et al., 2011). Results from our study indicate that a decline in GnRH synthetic capacity or transcriptional activity at prepuberty may result in lower EGFP expression in GnRH neurons of the transgenic rats. Furthermore, although the activity of GnRH neurons at prepuberty is lower, these neurons may have the capacity to synthesize GnRH, and the total cell number may remain unchanged during postnatal development. Therefore, low expression of GnRH peptide in wild-type rats may be labeled by immunocytochemistry. Sex differences throughout development in GnRH neurons of the preoptic area that were detected by Gao et al. (2011) were not observed in the present study. The reason for this is unknown and should be clari fi ed in future studies.
Bipolar and unipolar GnRH neurons have been previously classified as smooth type and irregular type with spine-like processes (Krisch, 1980; Sharova et al., 2013). Similarly to previously studies using immunocytochemistry, GnRH-EGFP neurons in the present model exhibited spinelike processes on bipolar neurons as well as on unipolar neurons (Saito et al., 2010; Gao et al., 2011; Sharif et al., 2013; Srivastava et al., 2014). These processes were observed on almost every GnRH-EGFP neuron. Because dendritic spines are typically involved in excitatory synapses (Clarkson and Herbison, 2006a; Cottrell et al., 2006), both unipolar and bipolar GnRH neurons in the present study were further subdivided into smooth type with few small processes and irregular type with numerous spine-like processes on somata and dendrites. Most unipolar neurons showed the irregular type whereas the majority of the bipolar neurons were the smooth type at all ages. Irregular GnRH neurons predominated at prepuberty, whereas no signi fi cant differences were detected between the two sub-types at postpuberty. Both sub-types of GnRH neurons significantly increased during the puberty period. However, a previous study using immunocytochemistry revealed a decrease in smooth neurons and an increase in irregular neurons with a constant total number of GnRH neurons during postnatal development (Sharif et al., 2013). The reason for the discrepancy between the two studies may be a technical variation and a different mode ofanimal selectivity.
Immunofluorescence for GnRH neurons in mice has revealed that spines are principally present on somata and the fi rst 30 μm of dendrites (Cottrell et al., 2006). However, the study did not show spines on dendrites beyond this length. GnRH-EGFP transgenic rats in the present study provided a clearer image than that of immuno fl uorescence. However, the majority of spine-like processes were detected on the proximal dendrites of irregular GnRH neurons. A trend was only observed for an increase in spine-like processes on GnRH neurons during puberty. In mice, spine numbers on GnRH-EGFP neurons increase two-fold between the end of the second week after birth and adulthood (Cottrell et al., 2006). Numerous spine-like processes were also seen on the cell bodies (Cottrell et al., 2006; Rosati et al., 2011). Biocytin filling of GnRH-EGFP neurons may provide a better identi fi cation of small processes on neurons compared with the present GnRH-EGFP neurons. In the present study, the somata exhibited fewer processes. The spines are known to represent the location of synaptic inputs to neurons (Krisch, 1980; Shepherd, 1996; Cariboni et al., 2007; Xu et al., 2011; Merriam et al., 2013; Sharova et al., 2013). Therefore, excitatory synaptic inputs to GnRH neurons may be predominantly transmitted via the spine-like structures of irregular GnRH neurons. An increase in spine density or number has been suggested to elevate direct excitatory inputs to GnRH neurons during puberty (Cottrell et al., 2006).
Ultrastructural studies in rats have revealed the presence of synapses on all GnRH neurons, including both smooth and irregular types (Xu et al., 2011; Sharova et al., 2013). However, irregular GnRH neurons possess more synaptic pro fi les compared with smooth neurons (Sharova et al., 2013). Nevertheless, the functional distinction of the two neuronal subtype is unknown. Smooth GnRH neurons are considered to be associated with sensory input emerging from the olfactory epithelium (Cheung et al., 2011; Sukhbaatar et al., 2014). Lesion of either the olfactory epithelium or olfactory bulb disturbs reproductive function and sexual behavior (Sotonyi et al., 2010; Navarro et al., 2011). Irregular GnRH neurons may receive multiple synaptic inputs or be integrated in acomplex formation (Sharova et al., 2013). Similarly to previous studies, the present immunofluorescence findings showed that most synaptic inputs to GnRH neurons consisted of spine-like processes (Clarkson and Herbison, 2006b; Cottrell et al., 2006). These neurons have been suggested to receive multiple synaptic inputs from GABAergic (Cottrell et al., 2006; McFarlane et al., 2011), glutamatergic (Lin et al., 2003; McFarlane et al., 2011), and kisspeptin fi bers (Kinoshita et al., 2005; Clarkson and Herbison, 2006b). Gamma-aminobutyric acid (GABA) and glutamate are the principal inhibitory and excitatory neurotransmitters, regulating the activity of GnRH neurons via their respective receptors expressing GnRH neurons in the preoptic area (Lin et al., 2003; Kroll et al., 2010; Papanikolaou et al., 2011; Quintanar et al., 2011). GABA induces a switch from depolarization to hyperpolarization in GnRH neurons of pubertal female mice (Ottem et al., 2002), and rapid GABA activation excites GnRH neurons in adult mice (Han et al., 2002). The excitatory action of GABA on GnRH neurons has also been reported in rats (Kim et al., 2011). Under current-clamp conditions in the rat, the activation of GABA receptor subunits depolarizes GnRH neurons (Kim et al., 2011).
The present study revealed fewer synaptophysin appositions to GnRH neurons, which is consistent with electron microscopic observations showing that GnRH neurons receive relatively sparse synaptic input (Yin et al., 2008; Sharova et al., 2013). Synaptophysin punctae were mostly present on the proximal dendrites of irregular GnRH neurons and rarely on somata and smooth neurons. Synaptophysin is a 38 kDa glycoprotein present on the membrane of neuronal presynaptic vesicles, which may be involved in the formation of synaptic vesicles and exocytosis (Jahn et al., 1985; Arauz et al., 2010). Terminals of the preoptic area containing GABAergic and glutamatergic vesicles that colocalize with synaptophysin provide abundant synaptic inputs to GnRH neurons (McFarlane et al., 2011). Activation of GnRH neurons is a key event in the onset of puberty (Ebling, 2005; Clarkson and Herbison, 2006a; Monje et al., 2010). Kisspeptin is regarded as the most potent activator of GnRH neurons (Clarkson and Herbison, 2006b; Abbasi et al., 2013) and its input and subsequent response are increased during postnatal development in the mouse (Yu et al., 2013). Findings from the present study revealed an increase of synaptophysin punctae in contact with the GnRH neuron during puberty. This result corroborates with Clarkson and Herbison (2006b) who have shown synaptic inputs to GnRH neurons during reproductive maturation. Images from previous studies have shown numerous immunofluorescent punctae adjacent to GnRH neurons (Clarkson and Herbison, 2006b; Cottrell et al., 2006; Rosati et al., 2011). In a similar manner, the present study also showed numerous synaptophysin-immunoreactive punctae closely adjacent to the GnRH neuron in the plain photograph. However, in three-dimensional rotation images, few synaptophysin punctae were in contact with this neuron. Thus, GnRH neurons may receive fewer synaptic inputs, which has been suggested by ultrastructural studies in rats (Han et al., 2005; Yin et al., 2008; Karamizadeh et al., 2013; Rosen fi eld et al., 2013; Sharova et al., 2013; Thornton et al., 2014). The differences in the number of synaptic inputs to GnRH neurons may re fl ect the differences between rats and mice or the method of observation (Lee et al., 2012; Lem et al., 2012; Rosen fi eld et al., 2012). Further morphological studies and the inclusion of electrophysiological studies in both species will be important to effectively clarify this discrepancy.
Acknowledgments:We are grateful to Dr. Park MK, Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Japan, for LRH13 antibody and to thank Dr. Ozawa H and Dr. Kato M, Nippon Medical School, Tokyo, Japan for the valuable discussion and constructive criticism on the manuscript.
Author contributions:Liu Q and Xue HG conceived and designed the experiments. Gai XD performed the experiments. Sun WQ analyzed the data. Li C provided experimental reagents/materials/analysis tools. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Abbasi NZ, Zahur Z, Sheikh AS, Khan AA, Ali F, Memon KH, Moizuddin SS, Loya A, Barkat N (2013) Solid neuroendocrine carcinoma of the breast. J Coll Physicians Surg Pak 23:820-822.
Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K (2007) Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367-378.
Arauz RF, Solomon BD, Pineda-Alvarez DE, Gropman AL, Parsons JA, Roessler E, Muenke M (2010) A hypomorphic allele in the FGF8 gene contributes to holoprosencephaly and is allelic to gonadotropin-releasing hormone de fi ciency in humans. Mol Syndromol 1:59-66.
Arroyo A, Kim BS, Biehl A, Yeh J, Bett GC (2011) Expression of kv4.3 voltage-gated potassium channels in rat gonadotrophin-releasing hormone (GnRH) neurons during the estrous cycle. Reprod Sci 18:136-144.
Cariboni A, Hickok J, Rakic S, Andrews W, Maggi R, Tischkau S, Parnavelas JG (2007) Neuropilins and their ligands are important in the migration of gonadotropin-releasing hormone neurons. J Neurosci 27:2387-2395.
Cheung LW, Mak AS, Cheung AN, Ngan HY, Leung PC, Wong AS (2011) P-cadherin cooperates with insulin-like growth factor-1 receptor to promote metastatic signaling of gonadotropin-releasing hormone in ovarian cancer via p120 catenin. Oncogene 30:2964-2974.
Clarkson J, Herbison AE (2006a) Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol Cell Endocrinol 254:32-38.
Clarkson J, Herbison AE (2006b) Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817-5825.
Cottrell EC, Campbell RE, Han SK, Herbison AE (2006) Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology 147:3652-3661.
Debruyne F, Tzvetkov M, Altarac S, Geavlete PA (2010) Dose-ranging study of the luteinizing hormone-releasing hormone receptor antagonist cetrorelix pamoate in the treatment of patients with symptomatic benign prostatic hyperplasia. Urology 76:927-933.
Ebling FJ (2005) The neuroendocrine timing of puberty. Reproduction 129:675-683.
Farkas I, Kalló I, Deli L, Vida B, Hrabovszky E, Fekete C, Moenter SM, Watanabe M, Liposits Z (2010) Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology 151:5818-5829.
Gao CQ, Fraeyman N, Eertmans F, Dhooge W, Kaufman JM (2011) Further evaluation of the biological activity of the unique gonadotropin-releasing hormone peptide in the guinea pig brain. Neurosci Lett 487:246-249.
Gentil M, Hoffmann B, Spang A, Failing K, Goericke-Pesch S (2012) Restart of steroidogenesis in dogs during recrudescence of testicular function following downregulation with a GnRH-agonist implant. Cell Tissue Res 350:513-523.
Han SK, Abraham IM, Herbison AE (2002) Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 143:1459-1466.
Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE (2005) Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349-11356.
Jahn R, Schiebler W, Ouimet C, Greengard P (1985) A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A 82:4137-4141.
Karamizadeh Z, Tabebordbar M, Saki F, Karamifar H, Amirhakimi G (2013) The side effects of gonadotropin releasing hormone analog (diphereline) in treatment of idiopathic central precocious puberty. Acta Med Iran 51:41-46.
Kato M, Ui-Tei K, Watanabe M, Sakuma Y (2003) Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fl uorescent protein in rats. Endocrinology 144:5118-5125.
Kim DK, Cho EB, Moon MJ, Park S, Hwang JI, Kah O, Sower SA, Vaudry H, Seong JY (2011) Revisiting the evolution of gonadotropin-releasing hormones and their receptors in vertebrates: secrets hidden in genomes. Gen Comp Endocrinol 170:68-78.
Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K (2005) Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431-4436.
Ko JH, Lee HS, Lim JS, Kim SM, Hwang JS (2010) Changes in bone mineral density and body composition in children with central precocious puberty and early puberty before and after one year of treatment with GnRH agonist. Horm Res Paediatr 75:174-179.
Krisch B (1980) Two types of luliberin-immunoreactive perikarya in the preoptic area of the rat. Cell Tissue Res 212:443-455.
Kroll H, Bolsover S, Hsu J, Kim SH, Bouloux PM (2010) Kisspeptin-evoked calcium signals in isolated primary rat gonadotropin-releasing hormone neurones. Neuroendocrinology 93:114-120.
Lee HS, Park HK, Ko JH, Kim YJ, Hwang JS (2012) Impact of body mass index on luteinizing hormone secretion in gonadotropin-releasing hormone stimulation tests of boys experiencing precocious puberty. Neuroendocrinology 97:225-231.
Lem AJ, van der Kaay DC, Hokken-Koelega AC (2012) Bone mineral density and body composition in short children born SGA during growth hormone and gonadotropin releasing hormone analog treatment. J Clin Endocrinol Metab 98:77-86.
Lin W, McKinney K, Liu L, Lakhlani S, Jennes L (2003) Distribution of vesicular glutamate transporter-2 messenger ribonucleic acid and protein in the septum-hypothalamus of the rat. Endocrinology 144: 662-670.
McFarlane HO, Joseph NT, Maddineni SR, Ramachandran R, Bédécarrats GY (2011) Development, validation, and utilization of a novel antibody speci fi c to the type III chicken gonadotropin-releasing hormone receptor. Domest Anim Endocrin 40:110-118.
Merriam EB, Millette M, Lumbard DC, Saengsawang W, Fothergill T, Hu X, Ferhat L, Dent EW (2013) Synaptic regulation of microtubule dynamics in dendritic spines by calcium, F-actin, and drebrin. J Neurosci 33:16471-16482.
Monje L, Varayoud J, Muñoz-de-Toro M, Luque EH, Ramos JG (2010) Exposure of neonatal female rats to bisphenol A disrupts hypothalamic LHRH pre-mRNA processing and estrogen receptor alpha expression in nuclei controlling estrous cyclicity. Reprod Toxicol 30: 625-634.
Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA (2011) Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. American journal of physiology Endocrinology and metabolism 300:E202-210.
Ottem EN, Godwin JG, Petersen SL (2002) Glutamatergic signaling through the N-methyl-D-aspartate receptor directly activates medial subpopulations of luteinizing hormone-releasing hormone (LHRH) neurons, but does not appear to mediate the effects of estradiol on LHRH gene expression. Endocrinology 143:4837-4845.
Pandit MA, Saxena RN (2010) Galanin regulation of LH release in male rats. Indian J Exp Biol 48:544-548.
Papanikolaou EG, Verpoest W, Fatemi H, Tarlatzis B, Devroey P, Tournaye H (2011) A novel method of luteal supplementation with recombinant luteinizing hormone when a gonadotropin-releasing hormone agonist is used instead of human chorionic gonadotropin for ovulation triggering: a randomized prospective proof of concept study. Fertil Steril 95:1174-1177.
Quintanar JL, Salinas E, Quintanar-Stephano A (2011) Gonadotropin-releasing hormone reduces the severity of experimental autoimmune encephalomyelitis, a model of multiple sclerosis. Neuropeptides 45:43-48.
Rosati F, Sturli N, Cungi MC, Morello M, Villanelli F, Bartolucci G, Finocchi C, Peri A, Serio M, Danza G (2011) Gonadotropin-releasing hormone modulates cholesterol synthesis and steroidogenesis in SHSY5Y cells. J Steroid Biochem Mol Biol 124:77-83.
Rosenfield RL, Bordini B, Yu C (2012) Comparison of detection of normal puberty in boys by a hormonal sleep test and a gonadotropin-releasing hormone agonist test. J Clin Endocrinol Metab 97: 4596-4604.
Rosenfield RL, Bordini B, Yu C (2013) Comparison of detection of normal puberty in girls by a hormonal sleep test and a gonadotropin-releasing hormone agonist test. J Clin Endocrinol Metab 98: 1591-1601.
Saito TH, Nakane R, Akazome Y, Abe H, Oka Y (2010) Electrophysiological analysis of the inhibitory effects of FMRFamide-like peptides on the pacemaker activity of gonadotropin-releasing hormone neurons. J Neurophysiol 104:3518-3529.
Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C (2011) Efficacy of induced luteinizing hormone surge after“trigger” with gonadotropin-releasing hormone agonist. Fertil Steril 95:826-828.
Sharif A, Baroncini M, Prevot V (2013) Role of glia in the regulation of gonadotropin-releasing hormone neuronal activity and secretion. Neuroendocrinology 98:1-15.
Sharova VS, Izvol’skaya MS, Zakharova LA (2013) Effect of prenatal infection of mice with bacterial endotoxin on the migration of neurons producing gonadotropin-releasing hormone. Dokl Biol Sci 452:273-276.
Shaw ND, Butler JP, McKinney SM, Nelson SA, Ellenbogen JM, Hall JE (2012) Insights into puberty: the relationship between sleep stages and pulsatile LH secretion. J Clin Endocrinol Metab 97:E2055-E2062.
Shepherd GM (1996) The dendritic spine: a multifunctional integrative unit. J Neurophysiol 75:2197-2210.
Sotonyi P, Mezei G, Racz B, Dallman MF, Abizaid A, Horvath TL (2010) Gonadotropin-releasing hormone fi bers contact POMC neurons in the hypothalamic arcuate nucleus. Reprod Sci 17:1024-1028.
Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH (1999) GABA-and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037-2050.
Srivastava VK, Hiney JK, Dees WL (2014) Actions and interactions of alcohol and transforming growth factor β1 on prepubertal hypothalamic gonadotropin-releasing hormone. Alcohol Clin Exp Res 38:1321-1329.
Sukhbaatar U, Kanasaki H, Mijiddorj T, Oride A, Miyazaki K (2014) Expression of gonadotropin-inhibitory hormone receptors in mouse pituitary gonadotroph LβT2 cells and hypothalamic gonadotropin-releasing hormone-producing GT1-7 cells. Endocr J 61:25-34.
Sun C, He M, Ko WK, Wong AO (2013) Gene expression of luteinizing hormone receptor in carp somatotrophs differentially regulated by local action of gonadotropin and dopamine D1 receptor activation. Mol Cell Endocrinol 374:22-34.
Tada H, Kuroki Y, Funabashi T, Kamiya Y, Goto T, Suyama K, Sano A, Mitsushima D, Etgen AM, Takahashi T (2013) Phasic synaptic incorporation of GluR2-lacking AMPA receptors at gonadotropin-releasing hormone neurons is involved in the generation of the luteinizing hormone surge in female rats. Neuroscience 248:664-669.
Teixeira L, Guimiot F, Dodé C, Fallet-Bianco C, Millar RP, Delezoide AL, Hardelin JP (2010) Defective migration of neuroendocrine GnRH cells in human arrhinencephalic conditions. J Clin Invest 120:3668-3672.
Thornton P, Silverman LA, Geffner ME, Neely EK, Gould E, Danoff TM (2014) Review of outcomes after cessation of gonadotropin-releasing hormone agonist treatment of girls with precocious puberty. Pediatr Endocrinol Rev 11:306-317.
Vollaard ES, van Beek AP, Verburg FA, Roos A, Land JA (2011) Gonadotropin-releasing hormone agonist treatment in postmenopausal women with hyperandrogenism of ovarian origin. J Clin Endocrinol Metab 96:1197-1201.
von Wolff M, Kämmerer U, Kollmann Z, Santi A, Dietl J, Frambach T (2011) Combination of gonadotropin-releasing hormone (GnRH) agonists with GnRH antagonists before chemotherapy reduce but does not completely prevent a follicle-stimulating hormone fl are-up. Fertil Steril 95:452-454.
Wojniusz S, Vögele C, Ropstad E, Evans N, Robinson J, Sütterlin S, Erhard HW, Solbakk AK, Endestad T, Olberg DE, Haraldsen IR (2011) Prepubertal gonadotropin-releasing hormone analog leads to exaggerated behavioral and emotional sex differences in sheep. Horm Behav 59:22-27.
Wray S (2002) Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol 23:292-316.
Xu Z, Kaga S, Mochiduki A, Tsubomizu J, Adachi S, Sakai T, Inoue K, Adachi AA (2011) Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr J 59:161-171.
Yin C, Ishii H, Tanaka N, Sakuma Y, Kato M (2008) Activation of A-type gamma-amino butyric acid receptors excites gonadotrophin-releasing hormone neurones isolated from adult rats. J Neuroendocrinol 20:566-575.
Yin W, Gore AC (2010) The hypothalamic median eminence and its role in reproductive aging. Ann N Y Acad Sci 1204:113-122.
Yu B, Ruman J, Christman G (2011) The role of peripheral gonadotropin-releasing hormone receptors in female reproduction. Fertil Steril 95:465-473.
Yu EP, Dengler CG, Frausto SF, Putt ME, Yue C, Takano H, Coulter DA (2013) Protracted postnatal development of sparse, speci fi c dentate granule cell activation in the mouse hippocampus. J Neurosci 33: 2947-2960.
Copyedited by Mark F, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.137578
Quan Liu, Ph.D., Department of Cardiovascular Disease, the First Hospital of Jilin University, Changchun, Jilin Province, China, quanliu888@163.com.
http://www.nrronline.org/
Accepted: 2014-05-12
杂志排行
中国神经再生研究(英文版)的其它文章
- Stem cell therapy for central nerve system injuries: glial cells hold the key
- Beyond taxol: microtubule-based strategies for promoting nerve regeneration after injury
- Neuroprotective effect of the traditional Chinese herbal formula Tongxinluo: a PET imaging study in rats
- Neuroprotective effects of Asiaticoside
- Treating Alzheimer’s disease with Yizhijiannao granules by regulating expression of multiple proteins in temporal lobe
- Autophagy activation aggravates neuronal injury in the hippocampus of vascular dementia rats
