石榴嗜蓝孢孔菌发酵液中一个新drimane型倍半萜
2014-05-17郭怀宇陆云德李正辉刘吉开
郭怀宇,陆云德,李正辉,王 刚,刘吉开*
1安徽中医药大学药学院现代中药安徽省重点实验室,合肥230031;2中国科学院昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室,昆明650204
石榴嗜蓝孢孔菌(Fomitiporia punicata Y.C.Dai,B.K.Cui& Decock)为担子菌门(Basidiomycota)、伞菌纲(Agaricomycetes)、锈革孔菌目(Hymenochaetales)、锈革孔菌科(Hymenochaetaceae)、嗜蓝孢孔菌属(Fomitiporia Murrill)高等真菌[1],子实体为多年生、木栓质、黄褐色,三角形或马蹄形[2]。该菌最初发现于陕西,生长在活的石榴(Punicagranatum L.)树上[3],是一种寄生菌[4]。迄今为止,从该属真菌中分离得到的化合物主要结构涉及甾类和萜类化合物。经文献查阅,同属的其他真菌具有活血痛经、祛瘀止痛、降压等作用,像椭圆嗜蓝孢孔菌据《本草纲目》记载就有利五脏,止血活血等作用[5]。因此,为了进一步了解石榴嗜蓝孢孔菌的药理活性,本实验对该菌的化学成分进行了系统研究,从石榴嗜蓝孢孔菌发酵液的乙酸乙酯提取物中分离鉴定了11个化合物,其中化合物10为新化合物。
1 仪器与材料
比旋光度值由Horbia SEPA-300旋光仪测定;UV由UV-210A型分光光度计测定;IR由Bruker Tensor27型红外光谱仪测定(KBr压片);FAB-MS用VG Auto spec-3000型质谱仪测定,ESI+-MS由VG AutoSpec-3000质谱仪测定,高分辨质谱HR-EIMS由Waters AutoSpec Premier P776质谱仪测定;1H和13C-NMR由Bruker AM-400和DRX 500测定,内标为TMS;柱层析硅胶(80~100目和200~300目)和薄层层析材料均为青岛海洋化工厂生产;分析型和制备型HPLC为Agilent1100 HPLC,色谱柱为Agilent Zorbax SB-C18和 YMC柱;Sephadex LH-20为Amersham Biosciences公司产品;RP-18(40~63 μm)为德国Merck公司产品。显色方法为254、365 nm荧光,10%硫酸乙醇溶液和硫酸香草醛处理后加热及碘蒸气显色。
石榴嗜蓝孢孔菌(Fomitiporia punicata)于2006年8月采自陕西华山,由北京林业大学戴玉成教授鉴定。
2 培养与发酵
由昆明植物研究所李正辉工程师采用斜面转摇瓶液体培养的方法对该菌种进行发酵培养。培养基:葡萄糖5%,猪肉蛋白胨0.15%,酵母粉0.5%,KH2PO4和 MgSO4各0.05%,150 rpm,24 ℃摇床发酵25 d,发酵液总量为20 L。
3 提取与分离
石榴嗜蓝孢孔菌发酵液为20 L,用乙酸乙酯萃取三次,合并萃取液浓缩得浸膏8 g。经正相硅胶柱层析(CHCl3/MeOH,100∶0 ~ 0∶100,V/V)梯度洗脱得10个组分(A-J)。组分A以石油醚-乙酸乙酯(10∶1→1∶1)梯度洗脱得化合物 1(8.0 mg)以及 2个亚组分A1和A2。对A1以石油醚-乙酸乙酯(50∶1→1∶1)梯度洗脱得化合物 2(6.8 mg)、3(6.1 mg)、4(5.2 mg)。对第 A2经反相 RP-18 以甲醇-水(85∶15→0∶100)梯度洗脱得 5(11 mg)。组分 B 以石油醚-丙酮(10∶1→1∶1)梯度洗脱得化合物 6(3.9 mg)和一亚组分B2。对B2经Sephadex LH-20柱色谱以丙酮体系洗脱柱色谱得化合物7(3.4 mg)和8(3.3 mg)。组分 D 以石油醚-丙酮(5∶1→1∶1)梯度洗脱得亚组分D1和D2,对D2组分经反相-高效液相色谱法(RP-HPLC)制备柱色谱以(10% ~30%,乙腈/水)得化合物9(2.1 mg)。组分E经Sephadex LH-20以三氯甲烷-甲醇(1∶1)洗脱柱色谱,得5个亚组分(E1~E5),对 E5经反相-高效液相色谱法(RP-HPLC)制备柱色谱以(10% ~50%,乙腈/水)得化合物10(0.8 mg)。组分F以石油醚-丙酮(4∶1→1∶1)梯度洗脱的两个亚组分F1和F2,对F2先后经Sephadex LH-20柱色谱以三氯甲烷-甲醇(1∶1)洗脱柱色谱后再经反相RP-18以甲醇-水(85∶15→0∶100)梯度洗脱得化合物11(20.1 mg)。
4 结构鉴定
化合物1 无色透明油状液体,FAB-MSm/z:411.3[M+H]+。1H NMR(CDCl3,400 MHz)δ:5.08 ~5.15(6H,m,H-3,H-7,H-11,H-14,H-18,H-22),1.96 ~ 2.11(20H,m,H-4,H-5,H-8,H-9,H-12,H-13,H-16,H-17,H-2,H-21),1.68(6H,s,H-1,H-24),1.60(18H,s,2-CH3,6-CH3,10-CH3,15-CH3,19-CH3,23-CH3);13C NMR(CDCl3,100 MHz)δ:135.0(s,C-6,C-19),134.8(s,C-10,C-15),131.1(s,C-2,C-23),124.4(d,C-3,C-22),124.3(d,C-7,C-11,C-14,C-18),39.7(t,C-5,C-9,C-16,C-20),28.3(t,C-12,C-13),26.8(t,C-4,C-8,C-17,H-21),25.6(q,C-1,C-24),17.6(q,2-CH3,23-CH3),16.0(q,6-CH3,10-CH3,15-CH3,19-CH3)。以上波谱数据与文献[6]数据报道一致,确定该化合物为角鲨烯。
化合物2 白色无定型粉末,EI-MS(%):256[M]+(89),239 [M-OH]+(5),227 [M-C2H5]+(19),213(54),199(20),185(30),171(26),157(29),143(15),129(68),115(23),97(27),85(38),73(100),57(75);1H NMR(400 MHz,CDCl3)δ:2.35(2 H,t,J=7.2 Hz,H-2),1.63(2H,m,H-3),1.24(br.s,多个-CH2-),0.88(3H,t,J=6.8 Hz,-CH3);13C NMR(CDCl3,100 MHz)δ:179.9(s,C-1),34.0(t,C-2),31.9(m,C-14),29.6(m,C-6,7,8,9,10,11),29.2(m,C-5,12),29.1(m,C-4,13),24.7(m,C-3),22.7(m,C-15),14.1(t,C-16)。以上波谱数据与文献[7]数据报道一致,确定该化合物为棕榈酸。
化合物3 无色油状物。1H NMR(400 MHz,CDCl3)δ:5.34(4H,m,H-9,H-10,H-12,H-13),2.77(2H,t,J=6.8 Hz,H-11),2.34(2H,t,J=7.5 Hz,H-2),2.04(4H,m,H-8,H-14),1.61(2H,m,H-3),1.20 ~1.35(m,H-4 ~ H-7,H-15 ~H-17),0.89(3H,t,J=6.8 Hz,H-18);13C NMR(CDCl3,100 MHz)δ:180.6(s,C-1),130.1(d,C-9),130.0(d,C-13),128.0(d,C-10),127.9(d,C-12),34.1(t,C-2),31.5(t,C-16),29.0 ~ 29.7(t,C-4 ~ C-7,C-15),27.2(t,C-8,C-14),25.6(t,C-11),24.6(t,C-3),22.6(t,C-17),14.0(q,C-18)。以上波谱数据与文献[8]数据报道一致,确定化合物为(9Z,12Z)-十八烷二烯酸。
化合物4 无色针晶,EI-MS m/z(%):398[M]+(34),383 [M-Me]+(15),300(20),271(100),255(76),213(34),147(43),107(63),69(67);1H NMR(400 MHz,CDCl3)δ:5.14 ~ 5.10(m,H-23),3.58(m,H-3),0.99(d,J=6.6 Hz,H-21),0.88(d,J=6.8 Hz,H-27),0.82(d,J=6.3 Hz,H-27),0.80(d,J=6.3 Hz,H-26),0.77(s,H-19),0.52(s,H-18);13CNMR(CDCl3,100 MHz)δ:139.6(s,C-8),135.1(d,C-22),131.9(d,C-23),117.4(d,C-7),71.0(d,C-3),56.0(d,C-17),55.1(d,C-14),49.4(d,C-9),43.3(s,C-13),42.8(d,C-24),40.5(d,C-20),40.2(d,C-5),39.4(t,C-12),38.0(t,C-4),37.1(t,C-1),34.2(s,C-10),33.1(d,C-25),31.4(t,C-6),29.6(t,C-2),28.1(t,C-16),22.9(t,C-15),21.5(t,C-11),21.1(q,C-21),19.9(q,C-28),19.6(q,C-27),17.7(q,C-26),13.0(q,C-19),12.1(q,C-18)。以上波谱数据与文献[9]报道一致,确定该化合物为(22E,24R)-ergosta-7,22-dien-3β-ol。
化合物5 淡黄色油状物,EI-MS m/z(%):294[M]+(45),263(34),220(2),149(18),109(40),95(77),81(100),74(31),67(88);1H NMR(CDCl3,400 MHz)δ:5.35(4H,m,H-9,H-10,H-12,H-13),3.66(3H,s,-OCH3),2.77(2H,t,J=6.8 Hz,H-11),2.30(2H,t,J=7.2 Hz,H-2),2.04(4H,m,H-8,H-14),1.60(2H,m,H-3),1.25~1.37(m,H-4 ~H-7,H-15 ~ H-17),0.88(3H,t,J=6.8 Hz,H-18);13C NMR(CDCl3,100 MHz)δ:174.3(s,C-1),130.1(d,C-9),130.0(d,C-10),128.0(d,C-12),127.8(d,C-13),51.4(q,-OCH3),34.0(t,C-2),31.9(t,C-8),31.5(t,C-14),29.7 ~ 22.5(t,C-3 ~ C-7 and C-15 ~ C-17),14.0(q,C-18)。以上数据与文献[10]数据报道一致,确定该化合物为亚油酸甲酯。
化合物6 浅黄色晶体,mp 112~114℃;EI-MS m/z(%):392 [M]+(15),377(3),349(4),268(100),253(30),214(26),173(23),69(47);1H NMR(CDCl3,400 MHz)δ:6.58(1H,d,J=9.4 Hz,H-7),6.00(1H,d,J=9.4 Hz,H-6),5.70(1H,s,H-4),5.24(1H,dd,J=15.2,7.2 Hz,H-23),5.18(1H,dd,J=15.2,7.2 Hz,H-22),1.21 ~2.53(18H,m,甾体母核),1.03(3H,d,J=6.8 Hz,H-21),0.97(3H,s,H-19),0.93(3H,s,H-18),0.90(3H,d,J=6.8 Hz,H-28),0.82(3H,d,J=6.8 Hz,H-27),0.78(3H,d,J=6.8 Hz,H-26);13C NMR(CDCl3,100 MHz)δ:199.3(s,C-3),164.2(s,C-5),156.0(s,C-14),135.0(d,C-22),133.9(d,C-7),132.6(d,C-23),124.5(d,C-6),124.3(s,C-8),123.0(d,C-4),55.8(d,C-17),44.0(d,C-9),44.0(s,C-13),42.9(d,C-24),39.2(d,C-20),36.8(s,C-10),35.7(t,C-15),34.2(t,C-12),34.1(t,C-1),33.1(d,C-25),27.7(t,C-16),25.4(t,C-11),21.2(q,C-21),20.0(q,C-27),19.7(q,C-26),19.0(t,C-2),18.9(q,C-19),17.6(q,C-28),16.7(q,C-18)。以上波谱数据与文献[11]数据报道一致,确定该化合物为麦角甾-4,6,8(14),22-四烯-3-酮。
化合物7 白色晶体,mp 152~154℃;EI-MS m/z(%):396[M]+(55),363(61),337(40),271(17),253(48),211(37),197(28),185(23),171(30),157(55),143(57),131(27),119(30),91(28),81(27),69(100),55(56);1H NMR(CDCl3,400 MHz)δ:5.58(1H,m,H-6),5.38(1H,m,H-7),5.14 ~ 5.26(2H,m,H-22,H-23),3.60(1H,m,H-3),1.03(3H,d,J=6.8 Hz,H-21),0.95(3H,s,H-19),0.92(3H,d,J=6.8 Hz,H-28),0.84(3H,d,J=6.8 Hz,H-27),0.82(3H,d,J=6.8 Hz,H-26),0.61(3H,s,H-18);13C NMR(CDCl3,100 MHz)δ:141.3(s,C-8),139.8(s,C-5),135.6(d,C-22),132.1(d,C-23),119.6(d,C-6),116.4(d,C-7),70.5(d,C-3),55.9(d,C-17),54.6(d,C-14),46.4(d,C-9),42.9(s,C-13),40.9(t,C-4),40.8(d,C-24),40.4(d,C-20),39.2(t,C-12),38.4(t,C-1),37.1(s,C-10),33.1(d,C-25),32.1(t,C-2),28.3(t,C-16),23.0(t,C-15),21.2(t,C-11),21.2(q,C-21),19.9(q,C-26),19.7(q,C-27),17.6(q,C-28),16.3(q,C-19),12.1(q,C-18)。以上波谱数据与文献[12]数据报道一致,确定该化合物为麦角甾-5,7,22-三烯-3β-醇。
化合物8 无色针晶,mp 177~178℃;EI-MS m/z(%):428[M]+(10),410(4),396(100),363(35),271(7),255(37),251(14),152(30),107(22),69(63);1H NMR(CDCl3,400 MHz)δ:6.50(1H,d,J=8.4 Hz,H-7),6.24(1H,d,J=8.4 Hz,H-6),5.22(1H,dd,J=15.2,7.4 Hz,H-22),5.13(1H,dd,J=15.2 Hz,7.4 Hz,H-23),3.97(1H,m,H-3),0.99(3H,d,J=6.8 Hz,H-21),0.90(3H,d,J=6.8 Hz,H-28),0.88(3H,s,H-19),0.83(3H,d,J=6.8 Hz,H-27),0.82(3H,s,H-18),0.80(3H,d,J=6.8 Hz,H-26);13C NMR(CDCl3,100 MHz)δ:135.4(d,C-6),135.2(d,C-22),132.3(d,C-23),130.7(d,C-7),82.1(s,C-5),79.4(s,C-8),66.4(d,C-3),56.2(d,C-17),51.6(d,C-14),51.0(d,C-9),44.5(s,C-13),42.7(d,C-24),39.7(d,C-20),39.3(t,C-12),36.9(t,C-4),36.9(s,C-10),34.7(t,C-1),33.0(d,C-25),30.1(t,C-2),28.6(t,C-15),23.4(t,C-11),20.8(q,C-21),20.6(t,C-16),19.9(q,C-27),19.6(q,C-26),18.1(q,C-19),17.5(q,C-28),12.8(q,C-18)。以上波谱数据与文献[13]数据报道一致,确定该化合物为过氧麦角甾醇。
化合物 9 黄色油状物,1H NMR(CDCl3,400 MHz)δ:5.40(1H,m,H-7),5.32(1H,dd,J=15.4,8.1 Hz,H-24),5.16(1H,dd,J=15.4,8.1 Hz,H-24),4.05(1H,m,H-3),3.39(3H,s,H-18),3.16(1H,d,J=4.7 Hz,H-3),1.51(3H,s,H-20),0.92(3H,d,J=6.8 Hz,H-29),0.84(3H,d,J=6.7 Hz,H-28),0.82(3H,d,J=6.7 Hz,H-28),0.59(3H,s,H-19);13C NMR(CDCl3,100 MHz)δ:143.6(s,C-8),135.4(d,C-22),132.0(d,C-23),114.9(d,C-7),82.4(d,C-2),76.3(s,C-9),67.8(d,C-3),55.9(d,C-17),54.9(d,C-14),43.8(s,C-13),42.7(d,C-5),42.7(d,C-24),40.4(d,C-20),39.3(t,C-12),37.2(s,C-10),33.0(t,C-1),32.7(d,C-25),32.4(t,C-4),30.86(t,C-6),27.9(t,C-16),26.9(t,C-11),22.8(t,C-15),21.0(q,C-21),19.9(q,C-26),19.6(q,C-27),18.3(q,C-19),17.6(q,C-28),12.3(q,C-18)。以上波谱数据与文献[14]数据报道一致,确定该化合物为2α-methoxyl-3β,9β-dihydroxyergosta-7,22-diene。
化合物10 无色油状物,[α]25D-21.8(c 0.08,MeOH);UVλmax(MeOH):201;IR显示有 OH(3423,3440 cm-1)、C=O(1740 cm-1)、C=C(1631 cm-1)等吸收信号的存在。ESI-MS显示准分子离子峰[M+H]+在 m/z 267,HR-EI-MSm/z 266.1511,分子式为C15H22O4(计算值266.1518)。
1H NMR谱显示了3个单峰甲基信号1.31(3H,s,H-14)、δH1.22(3H,s,H-13)和 1.06(3H,s,H-15),一个连氧的亚甲基 δH4.46(1H,dd,J=9.0,9.0 Hz,H-11β)和 4.12(1H,dd,J=9.0,9.0 Hz,H-11α),两个连氧的次甲基 δH4.76(1H,dd,J=4.2,7.1 Hz,H-6)和 3.26(1H,m,H-3)。13C NMR和DEPT数据显示该化合物包括15个碳原子信号峰,分别归属为3个甲基、3个亚甲基,5个次甲基和4个季碳信号。其中有2个烯碳(δC135.5,C-7;δC127.8,C-8),一个连氧的亚甲基(δC67.3,C-11),两个连氧的次甲基(δC79.3,C-3;δC66.1,C-6)。通过比较化合物10和化合物12(2α,3β-dihydroxycinnamolide)、13(ugandenial A)的1H 和13C波谱数据[15,16],说明化合物10是一个 drimane型倍半萜。对比化合物10与12、13的2位、9位和11位的碳谱数据以及1H-1H COSY、HSQC和HMBC谱,得出羟基不是在2位、9位和11位(图1)。

图1 化合物10、12和13的化学结构Fig.1 Chemical structures of compounds 10,12 and 13
根据1H-1H COSY和HSQC谱分析可知该化合物具有3个自旋耦合系统,即CH2(1)-CH2(2)-CH(3)、CH(5)-CH(6)-CH(7)、CH(9)-CH2(11)。在HMBC谱中H-7与C-5和C-9相关;H-11与C-12、C-10 和 C-8 相关,显示 C-9 分别与 C-8、C-10、C-11相连,且H-11与C-12相关暗示出二者通过氧原子的连接形成了一个五元环;H-3与C-13和C-14相关,显示C-3与C-4相连。通过对比化合物10和12的2位和6位的δH和δC数据,说明化合物12的2位次甲基和6位亚甲基在化合物10中分别变成了亚甲基和次甲基,通过以上分析确定了化合物10的平面结构(图2)。

图2 化合物10的主要1 H-1 H COSY和HMBC相关图Fig.2 Key 1 H-1 H COSY and HMBC correlations of compound 10
化合物10的相对构型通过ROESY确定。在ROESY谱中,H-3α与 H-13和 H-5,H-13与 H-6,H-5与H-9存在着NOE效应,说明H-5、H-6、H-9和H-13的构型是α-构型。H-15与H-14存在着NOE效应,说明H-15和H-14的构型是β-构型(图3)。综上所述,化合物 10的结构被鉴定为 3β,6β-dihydroxycinnamolide。化合物10的1H NMR、13C NMR数据如下。
1H NMR(CDCl3,400 MHz)δ:6.8(1H,dd,J=3.6,3.6 Hz,H-7),4.76(1H,dd,J=4.2,7.2 Hz,H-6),4.46(1H,dd,J=9.0,9.0 Hz,H-11α),4.12(1H,dd,J=9.0,9.0 Hz,H-11β),3.26(1H,m,H-3),2.68(1H,m,H-9),1.69(1H,m,H-1α),1.68(2H,m,H-2),1.38(1H,m,H-1β),1.31(3H,s,H-14),1.22(3H,s,H-13),1.15(1H,d,J=4.8 Hz,H-5),1.06(3H,s,H-15);13C NMR(CDCl3,100 MHz)δ:170.1(C-12),135.5(C-7),127.8(C-8),79.1(C-3),67.3(C-11),66.1(C-6),54.1(C-5),51.8(C-9),39.7(C-4),39.1(C-1),33.4(C-10),27.1(C-13),26.9(C-2),17.2(C-14),15.7(C-15)。
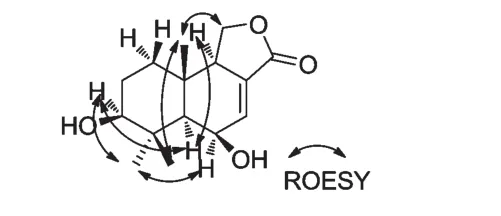
图3 化合物10的ROESY相关图Fig.3 The ROESY correlation of compound 10
化合物11 白色粉末;mp 224~226℃;EI-MS m/z(%):430[M]+(35),412(35),394(37),379(65),376(15),269(33),251(62),69(100);1H NMR(C5D5N,400 MHz)δ:5.74(1H,br.s,H-7),5.24(1H,dd,J=15.2,7.4 Hz,H-23),5.16(1H,dd,J=15.2,7.4 Hz,H-22),4.84(1H,m,H-3),4.32(1H,br.d,J=4.8 Hz,H-6),1.53(3H,s,H-19),1.07(3H,d,J=6.8 Hz,H-21),0.94(3H,d,J=6.8 Hz,H-28),0.85(3H,d,J=6.8 Hz,H-27),0.84(3H,d,J=6.8 Hz,H-26),0.67(3H,s,H-18);13C NMR(C5D5N,100 MHz)δ:141.6(s,C-8),136.2(d,C-22),132.5(d,C-23),120.4(d,C-7),76.5(s,C-5),74.3(d,C-6),67.6(d,C-3),56.5(d,C-17),55.2(d,C-14),43.9(s,C-13),43.8(d,C-9),43.0(d,C-24),42.0(t,C-4),40.7(d,C-20),40.1(t,C-12),38.1(s,C-10),33.8(t,C-1),33.1(d,C-25),32.6(t,C-2),28.2(t,C-16),23.5(t,C-15),22.4(t,C-11),21.3(q,C-21),20.1(q,C-27),20.67(q,C-26),18.8(q,C-19),17.6(q,C-28),12.3(q,C-18)。以上波谱数据与文献[17]数据报道一致,确定该化合物为麦角甾-7,22-二烯-3β,5α,6β-三醇。
1 Dai YC(戴玉成).Hymenochaetaceae(Basidiomycota)in China.Fungal Divers,2010,45:131-343.
2 Cui BK(崔宝凯),Du P(杜萍),Tao WQ(陶万强),et al.Two new pathogenic wood-rotting fungi from Beijing.Forest Res(林业科学研究),2009,22:274-278.
3 Dai YC(戴玉成).Pathogenic wood-decaying fungion woody plants in China.Mycosystema(菌物学报),2012,31:493-509.
4 Dai YC,Cui BK,Decock C.A new species of Fomitiporia(Hymenochaetaceae,Basidiomycota)from China based on morphological and molecular characters.Mycol Res,2008,112:375-380.
5 Zhu F,Yuan B,Huang TZ,etal.GC-MSanalysis of puny polar fractions from Fomitiporia punctata.Chin J Pharm Anal(药物分析杂志),2001,31:732.
6 Burger BV,Roux ML,Spies SC,etal.Mammalian pheromone studies-III.(e,e)-7,11,15-trimethyl-3-methylenehexa-deca-1,6,10,14-tetraene,anewditerpene analogue of β-farnesene from the dorsal gland of the springbok,Antidorcas marsupialis.Tetrahedron Lett,1978,19:5221.
7 Zan LF(昝立峰),Bau T(图立古尔),Bao HY(包海鹰),et al.Chemical constituents in fruiting body of Cortinarius rufo-olivaceus.Mycosystema(菌物学报),2008,27:284-288.
8 Liu R(刘荣).Investigation on chemical constituents of eight higher fungiand onemedicinal plant.Kunming:Kunming Institute of Botany,Chinese Academy of Sciences(中国科学院昆明植物研究所),PhD.2010.
9 Wang F(王飞),Liu JK(刘吉开).The chemical constituents of basidimycete Calodon Suaveolens.Nat Prod Res Dev(天然产物研究与开发),2004,16:204-206.
10 Chen SC,Hong LL,Chang CY,etal.Antiproliferative constituents from Gynura divaricata subsp.Formasana.Chin Pharm J,2003,55:109-119.
11 Deng HY(邓慧颖),Xing JG(邢建广),Luo DQ(罗都强).Metabolites of endophytic fungus Pestalotiopsis clavispora isolated from the stem of Bruguiera sexangula.Mycosystema(菌物学报),2011,30:263-267.
12 Shi Y(石瑛),Tian L(田黎),Wang J(王婧),et al.Studies on the chemical constituents of the fermentation liquid from marine actinomyces Micromonospora sp.and bacteria Oceanospirillum sp.Chin J Mar Drugs(中国海洋药物杂志),2006,25:6-10.
13 Liu C(刘超),Wang HQ(王洪庆),Li BM(李保明),et al.Studies on chemical constituents from the fruiting bodies of Ganoderma sinense Zhao,Xu et Zhang.China J Chin Mater Med(中国中药杂志),2007,32:235-237.
14 Lin CN,Kuo SH,Won SJ.Steroids of formosan Ganoderma amboinense.Phytochemistry,1993,32:1549-1551.
15 Zhang CF,Hu Y,Lin Y,et al.Anti-inflammatory activities of ethyl acetate of Polygonum jucundum and its phytochemical study.JMed Plant Res,2012,6:1505-1511.
16 Xu M,Marc L,Sabrina K,et al.Ugandenial A,a new drimane-type sesquiterpenoid from Warburgia ugandensis.Molecules,2009,14:3844-3850.
17 Gao JM,Hu L,Liu JK.A novel sterol from Chinese truffles Tuber indicum.Steroids,2006,66:771-775.
