Pharmacognostical studies on the leaves of Canavalia virosa (Roxb.) Wight & Arn.
2014-05-15LvnyAlgusoliynShkilRmhndrnSsiklEthirjuluAyysmySengnDeprtmentofPhrmologyGunpdmGovernmentSiddhMedilCollegeAnn
Lvny Algusoliyn, Shkil Rmhndrn, Ssikl Ethirjulu, Ayysmy SengnDeprtment of Phrmology (Gunpdm), Government Siddh Medil College, Ann
Hospital Campus, Arumbakkam, Chennai 600106, India;
bDepartment of Chemistry, Siddha Central Research Institute (Central Council for Research in
Siddha), Anna Hospital Campus, Arumbakkam, Chennai 600106, India;
cDepartment of Pharmacognosy, Siddha Central Research Institute (Central Council for
Research in Siddha), Anna Hospital Campus, Arumbakkam, Chennai 600106, India
Regular articles
Pharmacognostical studies on the leaves of Canavalia virosa (Roxb.) Wight & Arn.
Lavanya Alagusolaiyana, Shakila Ramachandranb*, Sasikala Ethirajuluc, Ayyasamy SenganaaDepartment of Pharmacology (Gunapadam), Government Siddha Medical College, Anna
Hospital Campus, Arumbakkam, Chennai 600106, India;
bDepartment of Chemistry, Siddha Central Research Institute (Central Council for Research in
Siddha), Anna Hospital Campus, Arumbakkam, Chennai 600106, India;
cDepartment of Pharmacognosy, Siddha Central Research Institute (Central Council for
Research in Siddha), Anna Hospital Campus, Arumbakkam, Chennai 600106, India
Canavalia virosa (Roxb.) Wight & Arn. syn. C. ensiformis (Linn.) DC. var. virosa (Roxb.) Baker. belongs to the family Papilionaceae. It is known as Kozhi Avarai in Tamil and used in the Siddha System of Medicine. In the present study, the morphology of the plant, exo and endomorphic characteristics, powder analysis, preliminary phytochemical analysis, physicochemical analysis, TLC photo documentation and HPTLC finger print of leaf are investigated. The findings revealed that the occurrence of prism of calcium oxalate crystals, paired foliar epidermal cells with solitary styloid crystals, non-glandular uniseriate and glandular club shaped trichomes and paracytic stomata are diagnostic characters which would help in the identifi cation of the drug. TLC photo documentation showed ten spots in UV 254 and 366 nm, HPTLC fi nger print profi les of the chloroform extract showed thirteen peaks out of which 4 are major peaks.
Canavalia virosa; Canavalia ensiformis; Kozhi Avarai; phamacognosy; HPTLC fi nger print profi le
1 Introduction
Folk medical lore is a source of new drugs or new applications of a known drug. The present contribution is one such where the plant Kozhi avarai - Canavalia virosa (Roxb.) Wight & Arn. leaf is used in the treatment of infective hepatitis in the folk medical practice in Tanjore District of Tamil Nadu.
Patharthaguna cintamani [1], a Siddha text records that the leaves are specifi c in the treatment of Spleenomegaly, wind colic and other stomach ailments.
It is a climbing perennial, common throughout India and found wild in scrub jungles. Seeds are used in scorpion sting [2]. Root is used in malaria [3]. Root and bark are used to eradicate intestinal worms [4].
Chemical analysis of seeds showed the presence of crude protein (26%), pentosan (11.6%), water-soluble mucilage (4.1%). Protein and pentosan were absent in the mucilage [5, 6]. The aqueous extract produced CNS effects including potentiation of pentobarbitone hypnosis in mice at 1 mg/100 gand potentiation of morphine catalepsy in albino rats (1 mg/100 g) [7]. The application of seeds, after removal of seed coat, was effective in giving complete relief of the symptoms of scorpion and centipede poisoning within 5-7 h [8]. Leaves are reported to possess antiulcer activity [9]. As the leaves are predominately employed as medicine, the Pharmacognosy of this part was studied.
2 Materials and methods
Fresh plants were collected from Pudukkottai, Tamil Nadu. Botanical identifi cation was carried out using Flora [10] and confi rmed at Botanical Survey of India, Coimbatore. The voucher specimen of the samples was deposited in the department.
Leaf, petiole and petiolule were fixed in FAA solution (70% ethyl alcohol, formalin and acetic acid in the ratio of 90 ml : 5 ml : 5 ml) for histological studies. The materials were left in the fl uid for three days, after which they were washed in water and dehydrated with tertiary butyl alcohol. Paraffi n wax was infiltered and the specimens were embedded in wax for sectioning. Wax embedded microtome sections were prepared [11] and stained with safranin and fast green [12].
Clearing of leaves for studying stomatal number and stomatal index was done by using 5% sodium hydroxide along with chlorinated soda solution supplemented with gentle heat. Powder microscopy and quantitative values such as stomatal number, stomatal index, vein islet number, vein termination number and palisade ratio were determined [13, 14]. Observations were photomicrographed at different magnifi cations with the help of Nikon Eclipse E200 Microscope.
Preliminary phytochemical tests, physicochemical analysis and chromatographic studies were carried out using the shade dried powder by following prescribed methods [15-17]. Linomat IV applicator, TLC twin trough chamber, visualizer, TLC scanner 030618 attached with WINCATS 4 software (CAMAG, Muttenz, Switzerland) were the instruments used for TLC photo documentation and HPTLC fi nger printing. Aluminium plate precoated with silica gel 60F254(E Merck) were used for the chromatography and the plate was developed in a solvent system of toluene: ethyl acetate (7:1.5, v/v) for the chloroform extract. The developed plate was photo-documented in white light and under UV at wavelengths of 254 nm and 366 nm. Then the plate was dipped in vanillin-sulphuric acid reagent, heated at 105°C till the development of colour of the separated compounds.
3 Results
3.1 Plant morphology
A large perennial climber; leaves pinnately 3-foliolate; leaflets large obovate, acute or obtuse. Racemes with many pink fl owers; pods short, 4 to 6 inch long containing 4 to 6 mottled brown seeds [18].
3.2 Macroscopical characters
Leaf trifoliolate, 12 to 15 cm long, petiolate. Petiole 5 to 7 cm long; stipules 0.2 cm long; oblong, obtuse, deciduous. Leaflets, membraneous to subcoriaceous, 5 to 7 cm by 4 or 5 cm; broadly ovate, obovate or orbicular, emarginated nearly glabrous (inner photo), shortly acuminate, base cuneate; reticulately veined; the basal nerves opposite, conspicuous; petiolules 0.5 cm long (Fig. 1A).
3.3 Microscopical characters
Transverse section of petiolule is oval in outline (Fig. 1B). Epidermis is single layered and covered by a cuticle. Hairs are with short basal cell and a larger terminal cell (Fig. 1I, J). The ground tissue is madeup of small spherical closely arranged thick walled parenchyma cells. In the centre, an‘U’ shaped vascular bundle is seen (Fig. 1E). Smallgroups of pericyclic fi bres are noticed.
The transverse section of petiole shows planoconvex outline with two protruberances on the adaxial side. It measures 1650 μm in vertically and 1550 μm in horizontally (Fig. 1C). It shows a single layered epidermis, a well developed collenchyma and parenchymatous tissue, cylindrical stele and a broad zone of parenchymatous pith in the centre. The two accessory vascular bundles which are small and circular in outline are situated on either side of the median vascular cylinder towards the upper side of the petiole (Fig. 1F, G). On the dorsal side 2 or 3 layered sclerenchyma fibres are seen as a cap (Fig. 1G). The epidermis is externally covered with a thin cuticle and is composed of cubical cells with thick anticlinal walls. The epidermal cells measure 14-16-18 × 8-10-12 μm. Epidermis is followed by 2 or 3 layers of collenchyma cells and 2 to 4 layers of thin walled parenchymatous cells. Some of the innermost cortical layer i.e. outer boundary of the pericylic fibers contain prisms of calcium oxalate crystals (Fig. 1D). Pericyclic fibres are lignified, 2 or 3 layered and occur as a continuous ring. The cylindrical vascular bundle shows well developed phloem followed by a thin ring of cambium then xylary elements facing the pith. The xylem is endarch in nature and consists of vessels and tracheids.
Transverse section of midrib shows a convexity on the abaxial side (Fig. 2K). The midrib measures 475-500 μm in vertical plane & 420-440 μm in horizontal plane. The epidermis is externally covered by a cuticle. The palisade tissue is not continuous at major veins. Presence of patches of collenchyma are seen below the upper epidermis. The hypodermal region of the abaxial side is composed of 1 or 2 layers of collenchyma cells. Abaxial epidermal cells bear glandular club shaped trichomes (Fig. 1H). The ground tissue is large extensive madeup of thin walled parenchymatous cells. In the centre of the ground tissue an arc shaped vascular bundle is seen (Fig. 2L). It consists of xylem and well developed phloem.
A mature lamina measure 180-200 μm thickness and is dorsiventral in nature. The epidermis is single layered, mucilaginous and covered externally with thick cuticle. Upper epidermal cells measure 38-42 μm × 16-18 μm, while the lower epidermal cells measure 14-16 μm × 8-10 μm. Below the upper epidermis a double layered closely packed columnar palisade. Central layers of mesophyll occupied by cells containing little chlorophyll is frequently filled with tanniniferous contents which are coloured brown in the dried leaf. The spongy mesophyll is represented by 4 or 5 layers of loosely arranged chlorenchymatous cells of varying size and shape (Fig. 2M).
The stomatal index is 18-21/mm2for adaxial epidermis and 22-24/mm2for abaxial epidermis. Palisade ratio 5-8 and vein-islet number 8-12, vein termination number 8 to 10 (Fig. 2Q).
In surface view, the adaxial and abaxial epidermal cells possess grossly wavy outlines. The adaxial epidermal cells are slightly larger in size (Fig. 2N). Both the epidermii are perforated by paracytic stomata but the frequency is less in adaxial epidermis (Fig. 2N, P). The occurrence of paired epidermal cells with solitary styloid crystals (Fig. 2O) in both the epidermii of the leaf is a note worthy feature.
3.3.1 Powder analysis
The powder is greenish in colour, without any characteristic odour or taste. When the powder was treated with chloral hydrate and stained with phloroglucinol and HCl, the following structures were observed under the microscope. The powder showed epidermal cells with paracytic stomata, paired epidermal cells with solitary styloid crystals, parenchyma cells, fibres, vessels with spiral thickenings, uniseriate trichomes and rarely glandular trichomes.
3.3.2 Histochemical tests
The sections of the leaf when treated with Phloroglucinol and dilute hydrochloric acid (HCl) gave red colour indicating the presence of lignin. Treatment with Sudan III revealed the absence of oils. With ferric chloride bluish black colour developed showing the presence of tannins. Ruthenium red imparted red colour indicating the presence of mucilage. Dragendorff’s test showed no brown colour indicating the absence of alkaloids.
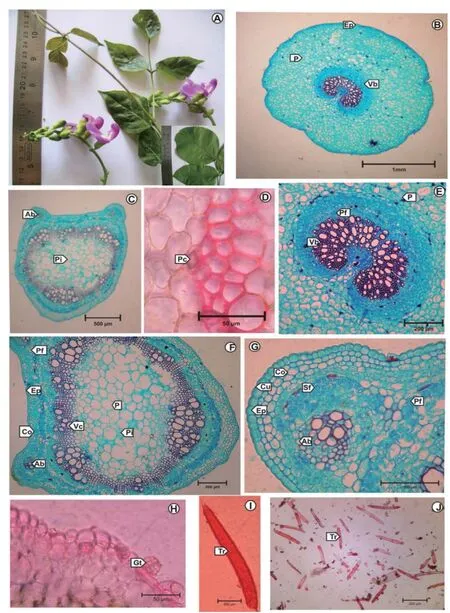
Fig. 1. A: Plant; B: T.S. of Petiolule; C: T.S. of Petiole; D: Prism of calcium oxalate crystal; E: T.S. of Petiolule(Enlarged); F: T.S. of Petiole (Enlarged); G:T.S. of Petiole - Accessory bundle enlarged; H: Glandular trichome; I: Non-glandular uniseriate trichomes; J: Uniseriate trichome – Enlarged. Ab: Accessory bundle; Co: Collenchyma; Cu: Cuticle; Ep: Epidermis; Gt: Glandular trichome; P: Parenchyma; Pc: Prism of calcium oxalate crystal; Pf: Pericyclic fi bre; Pi: Pith; Sf: Sclerenchyma fi bre; Tr: Trichome; Vb: Vascular bundle; Vc: Vascular cylinder.
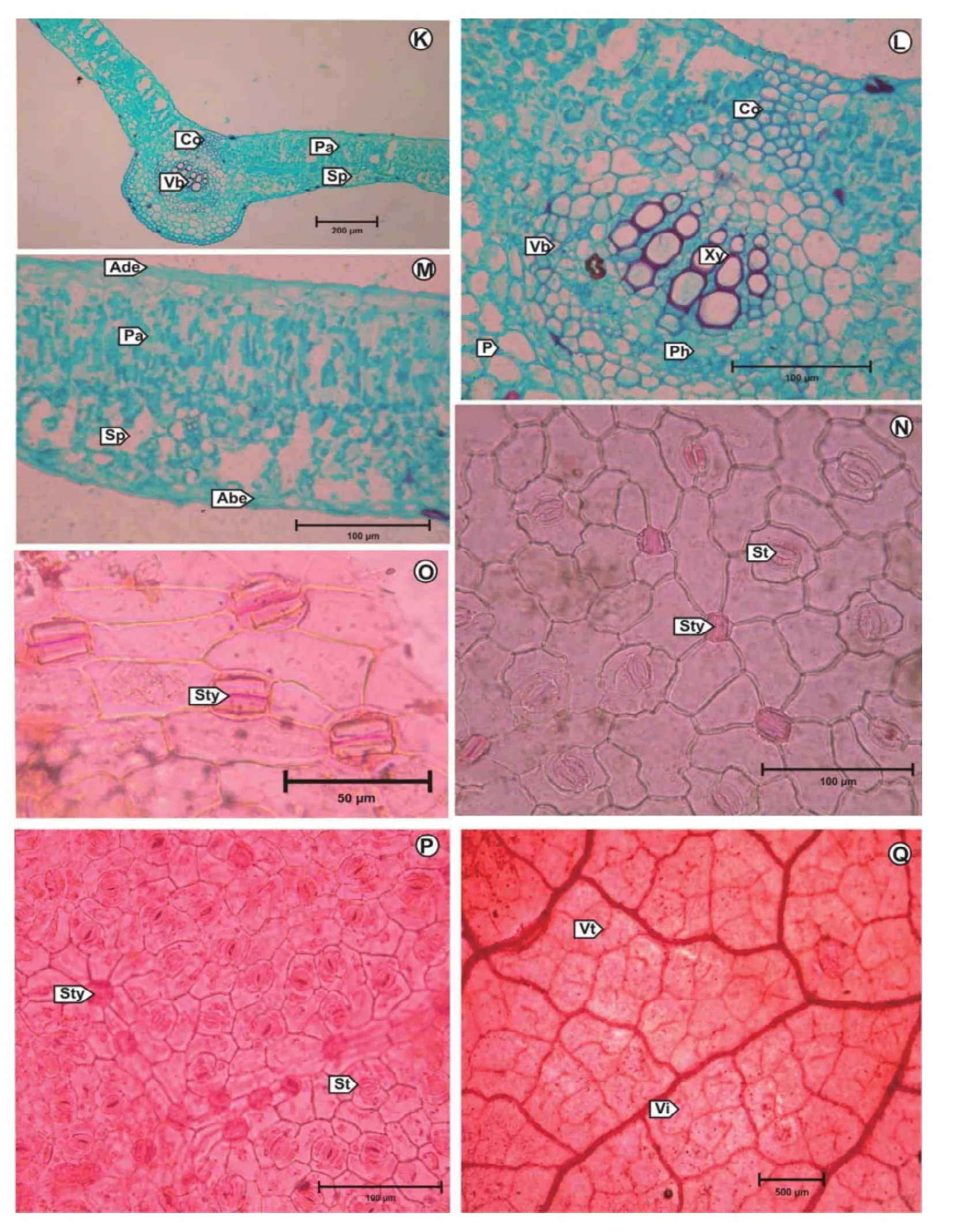
Fig. 2. K: T.S. of Leafl et; L: T.S. of Midrib (Enlarged); M: T.S. of Lamina (Enlarged); N: Adaxial foliar epidermis; O: Paired foliar epidermal cells with solitary styloid crystals; P: Abaxial foliar epidermis; Q: Vein islets and vein terminations.Abe: Abaxial epidermis; Ade: Adaxial epidermis; Co: Collenchyma; P: Parenchyma; Pa: Palisade tissue; Ph: Phloem; Sp: Spongy tissue; St: Stoma; Sty: Styloid crystals; Vb: Vascular bundle; Vi: Vein islet; Vt: Vein termination; Xy: Xylem.
4 Preliminary phytochemical tests
Preliminary phytochemical tests for various extracts were carried out and the results are tabulated in Table 1.
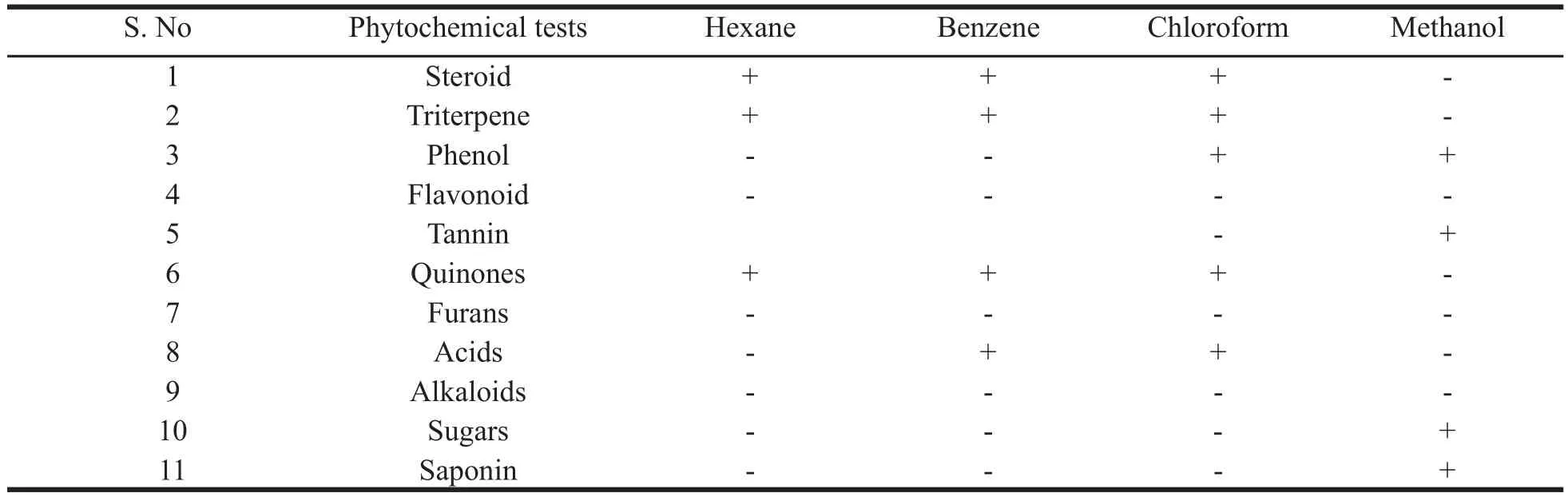
Table 1. The results of preliminary phytochemical tests.
5 Physico-chemical studies
The bulk sample was found to be free from foreign organic matter. All other parameters studies were done with powdered samples. The moisture content as 6.17%. The total ash was 10.69%. The water soluble ash was 1.821%. The acid insoluble ash was 1.28%. The ethanol and water soluble extractives were 11.49% and 30.94% respectively. The physico-chemical values and other qualitative inorganic test results are presented in the Table 2.
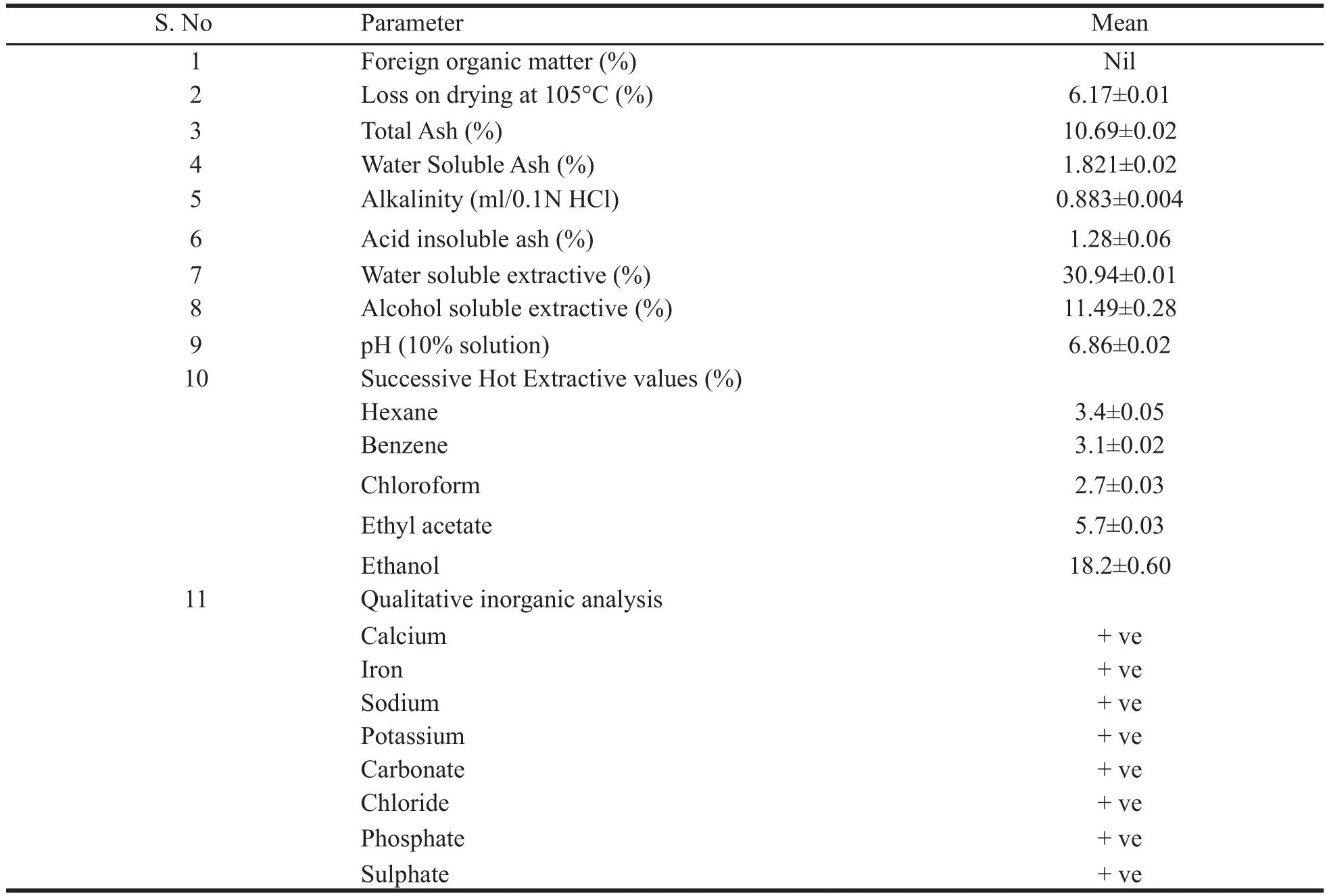
Table 2. Physico-chemical and qualitative analytical values.
6 Chromatographic studies
HPTLC profi le of the chloroform extract of the leaf was carried out. The chloroform extract revealed the presence of 13 spots under white light; 10 spots under a wavelength of 254 & 366 nm and 11 spots when it was dipped in vanillin-sulphuric acid reagent and heated at 105°C till the development of colour of the spot. The chromatograms are presented in Fig. 3 a-d and HPTLC fi nger print profi le in Fig. 4. The Rfvalues and colours are presented in Table 3 & 4.
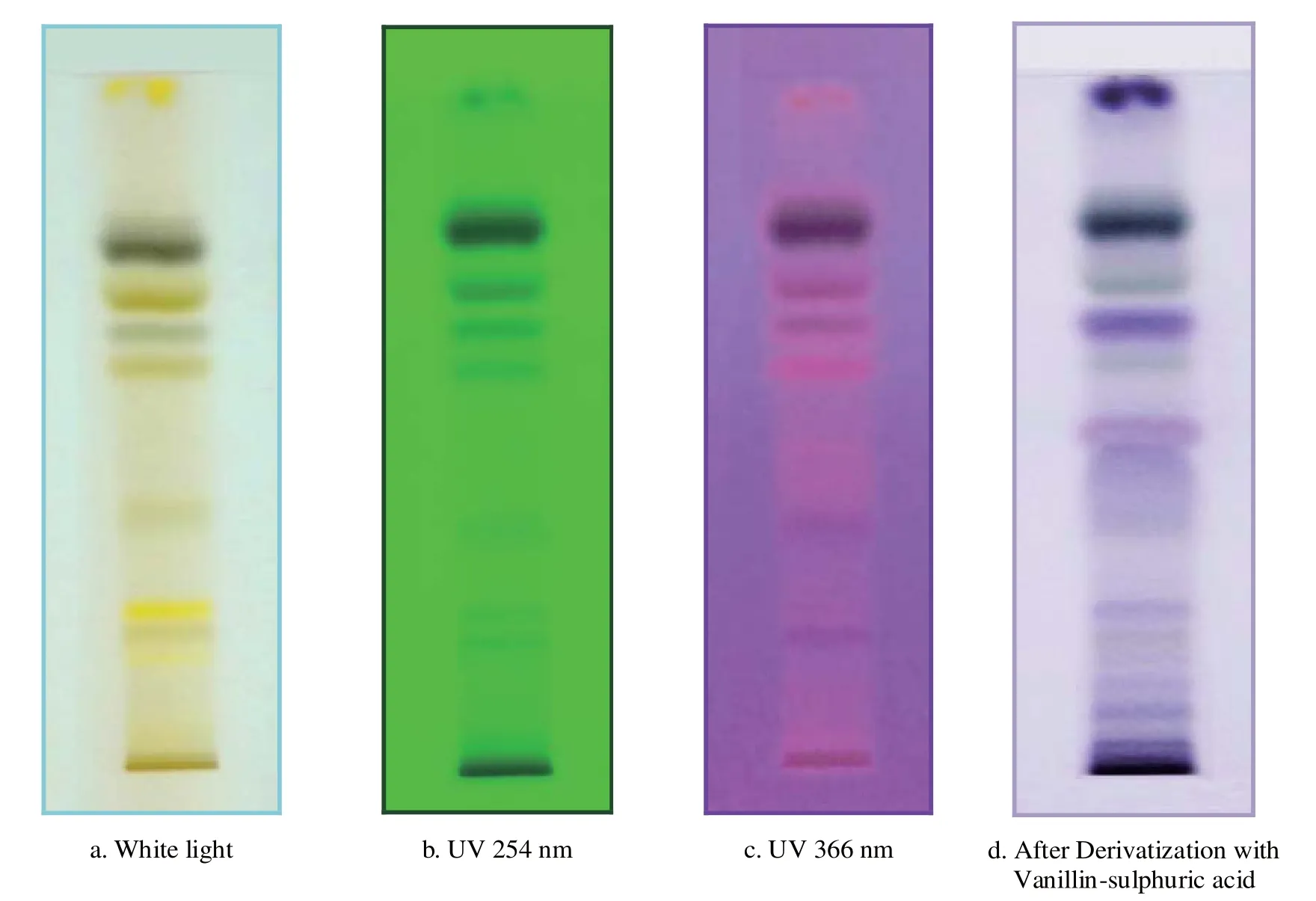
Fig. 3. Thin Layer Chromatograms of chloroform extract of Canavalia virosa leaf.
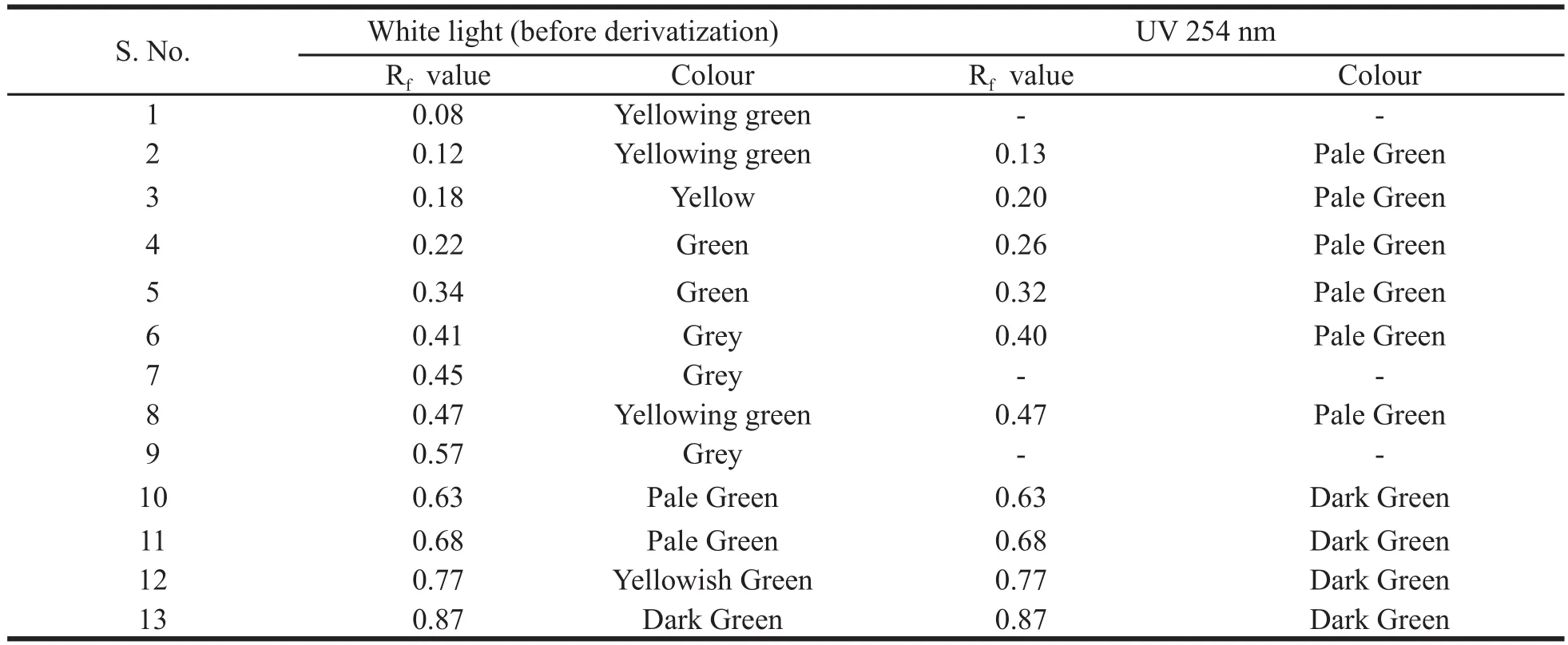
Table 3. Rfand colour details in white light and under UV 254 nm.
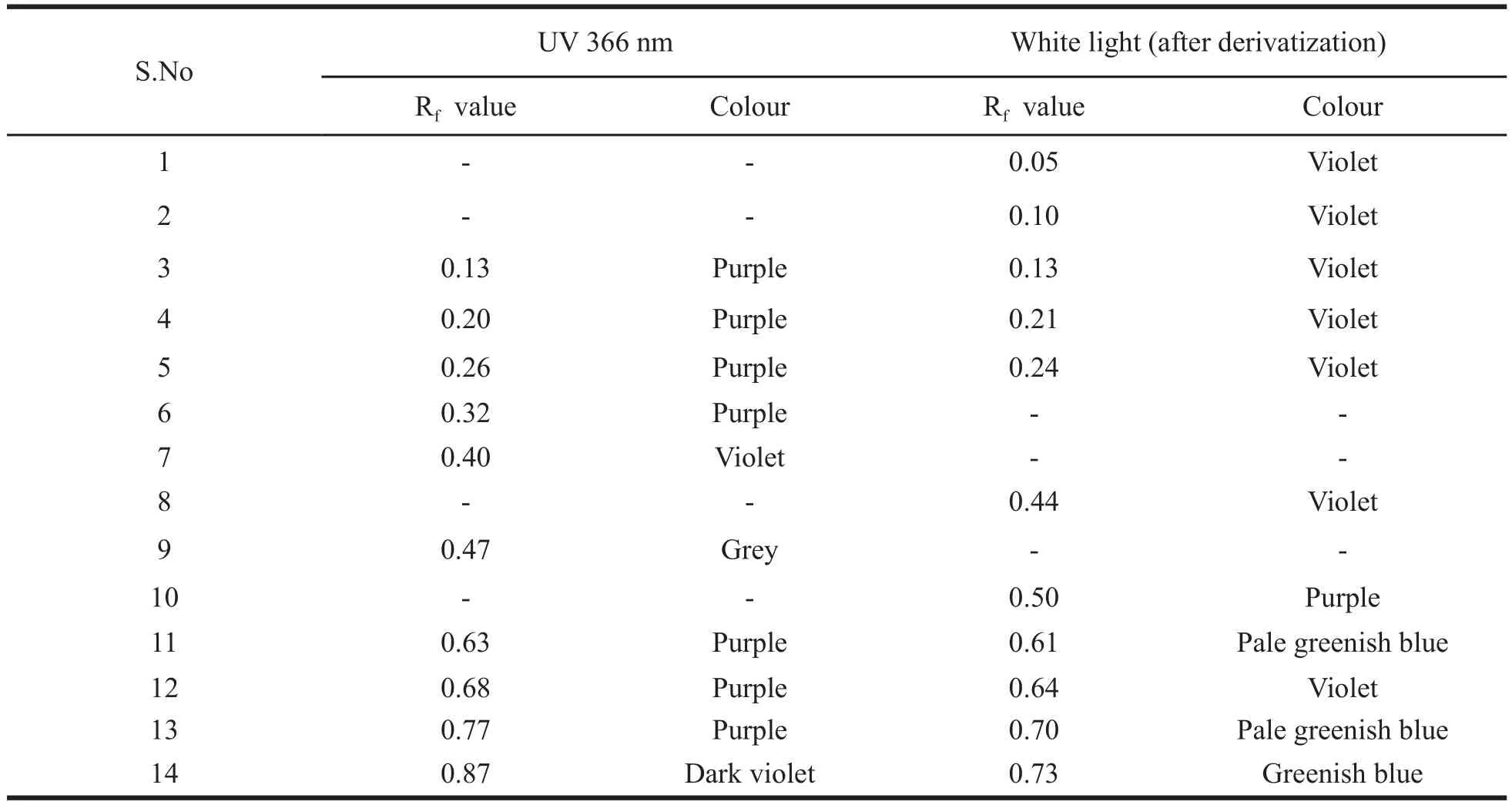
Table 4. Rfand colour details under UV 366 nm and in white light after derivatization.
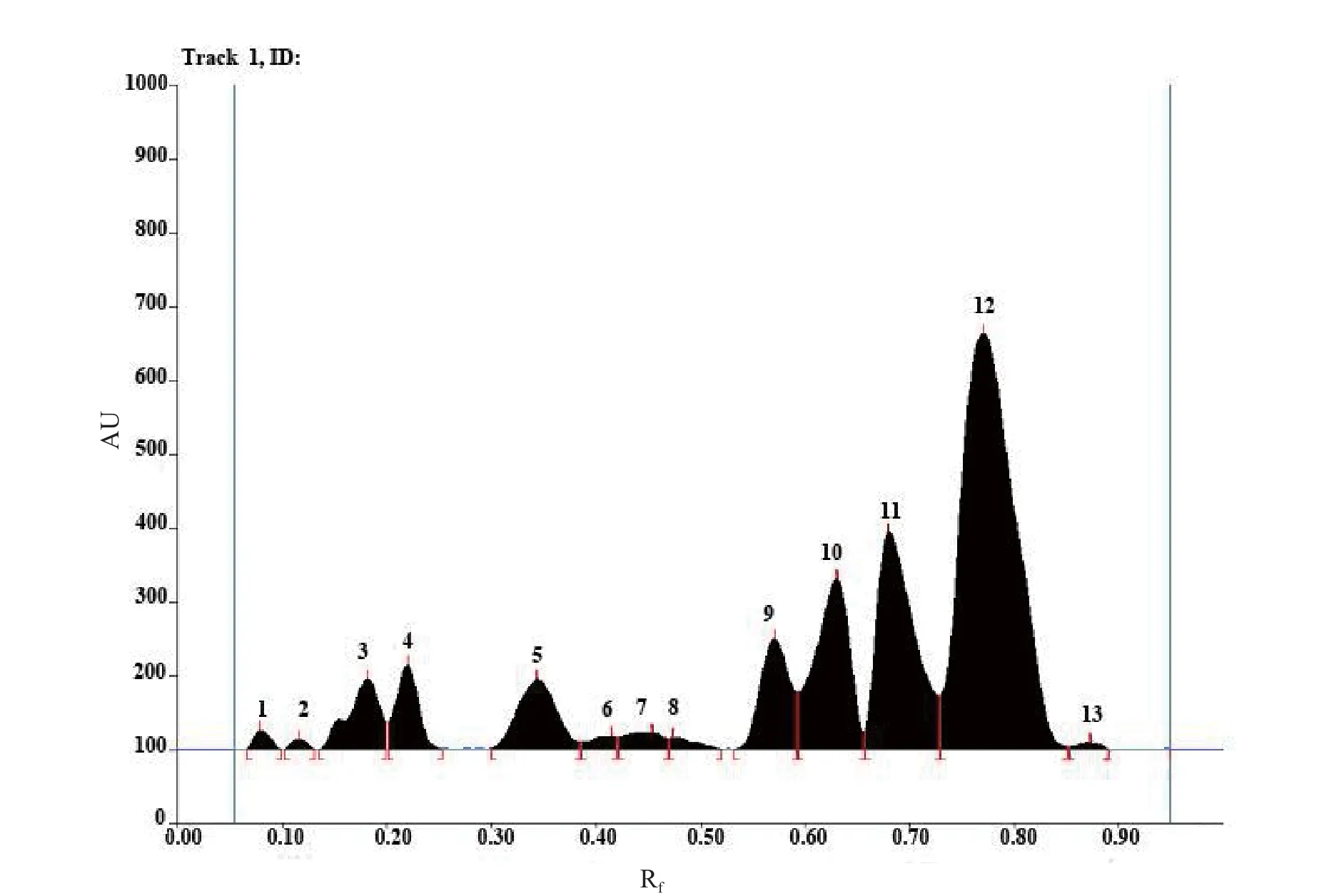
Fig. 4. HPTLC fi nger print profi le and Rftable of chloroform extract under UV 254 nm.
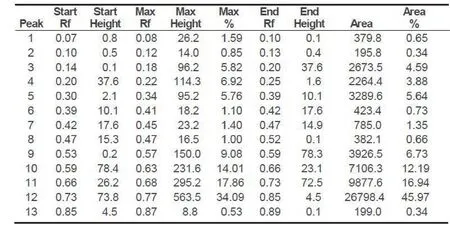
Fig. 4. HPTLC fi nger print profi le and Rftable of chloroform extract under UV 254 nm.
7 Discussion
The leaf which constitutes the drug is identifi ed by its diagnostic anatomical characters such as presence of paracytic (rubiaceous) stomata, paired foliar epidermal cells with styloid crystals, prism of calcium oxalate crystals, two layered palisade, uniseriate trichomes and club shaped glandular trichomes.
The preliminary phytochemical tests revealed the presence of steroid, triterpene, phenol, tannin, acid, quinone, sugar and saponins and absence of flavonoids, furans and alkaloids. The obtained ash value implies reveals the presence of considerable amount of inorganic content; the presence of calcium oxalate crystals also contribute to the higher ash value; water soluble ash implies that the plant is having signifi cant amount of water soluble inorganic salts. The alcohol and the water soluble extractive values show that the higher content of polar compounds. The extractive values in different organic solvents such as hexane, benzene, chloroform, ethyl acetate and ethanol showed 3.3, 3.1, 2.7, 5.7 and 18.2% respectively which also confirms the higher content of polar compounds rather than non-polar compounds. The pH of 10% solution was found to be 6.86 which is slightly acidic. Inorganic cations like sodium, potassium, iron and calcium and the anions carbonate, sulphate, chloride, oxalate and phosphate are also present which count for the higher ash content. In the TLC photodocumentation studies, it is observed that the compounds which are visualized under UV 254 nm are also visualized under UV 366 nm and the derivatized plate also shows similar number of compounds only. In the HPTLC finger print at UV nm, the peak at Rf0.77 contributes to 45.97% of the total separated compounds in the solvent system used; the peaks at Rf0.66 and 0.59 contribute to 16.94 and 12.19% respectively.
8 Conclusion
By this investigation, the identification of Canavalia virosa leaf can be achieved through the characters like calcium oxalate crystals, paired foliar epidermal cells with solitary styloid crystals, non-glandular uniseriate and glandular club shaped trichomes and paracytic stomata. Preliminary phytochemical study revealed the presence different phytochemicals devoid of fl avonoids and alkaloids. TLC and HPTLC study showed the presence ofmany compounds in the chloroform extract and the high alcohol soluble extractive value proves the presence of many more high polar compounds.
Acknowledgements
The authors are thankful to the Director General, CCRS for facilities and Assistant Director I/c, Siddha Central Research Institute for support.
[1] Anonymous. Patharthaguna Cintamani. Chennai: B. Rathina Nayakar Sons, 1932, 184.
[2] Raju MS. Native plants used in snake bite and poisonous animals among the tribals of East Godavari district, Andhra Pradesh. Aryavaidyan, 1996, 9: 251-255.
[3] Karuppusamy S, Kumuthakalavalli R. Some folklore medicinal claims on rare plants of Sirumalai hills, Dindigul district, Tamilnadu, India. Bull Med Ethnobot Res, 1998, 19: 145-150.
[4] Sudhakar S, Rao RS. Medicinal plants of upper East Godavari district (Andhra Pradesh) and need for establishment of medicinal farm. J Econ Tax Bot, 1985, 7: 399-406.
[5] Kapoor VP, Khan PSH, Farooqu MIH. Chemical analyses of seeds. Part IV. 50 Leguminous species. Sci Cult, 1978, 44: 191-192.
[6] Farooqi MIH, Kapoor VP, Khan PSH. Phytochemical observations on the seed galactomannan contents in some Indian Leguminosae. Res Ind, 1985, 30: 144-149.
[7] Mukhopadhyay M, Sarkar MK, Biswas M, et al. Some pharmacological studies on Canavalia virosa. Indian J Pharmacol, 1986, 18: 84-88.
[8] Jayavardhanan KK, Panikkar KR, Kesava M, et al. Antipoisonous property of Canavalia virosa. Ancient Sci Life, 1988, 8: 103-105.
[9] Lavanya A, Pitchiah Kumar M, Anbu J, et al. Antiulcer activity of Canavalia virosa (Roxb) W&A leaves in animal model. Int J Life Sci & Pharma Res, 2012, 2: 39-43.
[10] Gamble JS. The Flora of Presidency of Madras. Vol I. Calcutta: Botanical Survey of India, 1967, 253-254.
[11] Sass JE. Elements of Botanical Microtechnique. Newyork & London: Mc Graw Hill Book Co. Inc, 1940, 222.
[12] Johansen DA. Plant Microtechnique. New York: McGraw Hill,1940, 182-203.
[13] Wallis TE. Text book of Pharmacognosy. New Delhi: CBS Publishers & Distributors, 1985, 104-109.
[14] Trease GE, Evans WC. Pharmacognosy, 13th ed. London: Bailliere Tindall, 1989, 799-803.
[15] Harborne JB. Phytochemical Methods. London: Chapman and Hall, 1973, 70.
[16] Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas. 2nd ed. Berlin: Springer-Verlag, 1996, 21-45.
[17] Anonymous. Quality Control Methods of Medicinal Plant Materials. Geneva: World Health Organization (WHO), 1998, 25-28.
[18] Anonymous. The Wealth of India. Vol.II. New Delhi: CSIR, 1950, 57.
* Author to whom correspondence should be addressed. Address: Department of Chemistry, Siddha Central Research Institute (Central Council for Research in Siddha), Anna Hospital Campus, Arumbakkam, Chennai 600 106, India; Tel.: +88-44-26214925; Email: shakilasiva@gmail.com
Received: 2013-05-20 Accepted: 2013-08-30
