Research on the Critical Conditions for Clay Particle Release During Saline Aquifer Freshening Process
2014-05-05ZHENGXilaiandCHENRan
ZHENG Xilai, and CHEN Ran
1) Key Laboratory of Marine Environment and Ecology, Ministry of Education, Ocean University of China, Qingdao 266100, P. R. China
2)College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, P. R. China
Research on the Critical Conditions for Clay Particle Release During Saline Aquifer Freshening Process
ZHENG Xilai1),2),*, and CHEN Ran1)
1) Key Laboratory of Marine Environment and Ecology, Ministry of Education, Ocean University of China, Qingdao 266100, P. R. China
2)College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, P. R. China
Water sensitivity phenomenon occurs during saline aquifer freshening process in seawater intrusion area, and clay particles released in the phenomenon can damage the infiltration capacity of the aquifer. In order to find out the factors and mechanisms for clay particle release, laboratory column infiltration experiments simulating saline aquifer freshening process were designed to measure the critical conditions (critical flow velocity, critical salt concentration and critical ionic strength) and force analysis for clay particle according to DLVO electric double layer theory was employed to illustrate the mechanisms for particle release. The research results showed that critical flow velocity for clay particle release is influenced by salt concentration of injecting solution. When salt concentration of injecting solution is very high, clay particles are not released, indicating that there does not exist a critical flow velocity in this situation. As salt concentration of injecting solution decreases, particles start to be released. The critical salt concentration for clay particle release is 0.052 mol L-1in our work, which was determined by a constant-flux experiment for stepwise displacement of high concentration NaCl solution. The critical ionic strength for clay particle release decreases as Ca2+molar content percentage of the mixed solution of NaCl and CaCl2increases following the first-order exponential decay equationy= 0.0391e-0.266x+ 0.0015.
seawater intrusion area; clay particle release; critical flow velocity; critical salt concentration; critical ionic strength
1 Introduction
The water sensitivity is a colloidal phenomenon whereby the permeability of the porous media is decreased rapidly and significantly after the porous media is contacted with incompatible solution (Khilar and Fogler, 1983). The phenomena of water sensitivity have been found in many fields. For instance, in the field of oil and gas exploitation, it is possible to enhance the exploitation amount of oil and gas by controlling the water quality of injecting liquids to reduce or avoid the occurrence of water sensitivity in oil-containing rocks or sandstones (Ochi and Vernoux, 1998; Moghadasiet al., 2004; Pyatakhin, 2006; Gravelleet al., 2011). In the field of soil science, it is possible to control the irrigation water quality by studying water sensitivity of soil, so as to improve infiltration rate and save irrigation volume (Blumeet al., 2002; Choudharyet al., 2006; Mandalet al., 2008; Patilet al., 2011). In the field of groundwater contamination remediation, it has been found that the released clays with high surface area could facilitate the transport of many inorganic and organic contaminants that strongly adheres to the porous wall when water sensitivity happens (Kimet al., 2003; Grolimund and Borkovec, 2005; Sen and Khilar, 2006; Simuneket al., 2006; Gaoet al., 2011). Due to the critical roles of water sensitivity mentioned above, a lot of relevant studies have been conducted to acquire deep insight into the phenomena, mainly focusing on the mechanisms, causes, and factors for the occurrence of water sensitivity.
Clay particle release is assumed to be controlled by hydrodynamic and chemical perturbations. Some studies demonstrate that whether the clay particle release phenomenon can occur or not mainly depends on the critical conditions such as flow velocity, salt concentration and ionic strength of the injecting solution. Critical flow velocity refers to the flow velocity at which the clay particles adhering to the porous wall can be released by shear force of the fluid. It is found that increasing the velocity of water flow through porous media can enhance colloid release due to an increase of the hydrodynamic forces and torques that act on retained colloids (Bergendahl and Grasso, 2000; Elimelechet al., 2001; Bradfordet al., 2011). Some attempts have been made to study the effect of different parameters like flow velocity, ionic strength, and particle size on the release of colloidal particle from the porous media through experiments and simulations (Tripathy, 2010; Yuet al., 2013). Critical salt concentra-tion refers to the concentration of the single NaCl solution at which the clay particles start to release. The well head injection pressure increased from 4 MPa to 5 MPa during the injection project in Melleray doublet, in which the increase of the injection pressure was quasi-immediate as a consequence of an instantaneous and rapid movement of particles to cause blockage due to the difference of the salinity between injection water and groundwater (Boisdetet al., 1989). It is proposed that the release of clay particles possibly starts from a certain salt concentration at which the resultant energy and force of the particle are zero (Vaidya, 1991; Torkzabanet al., 2007). And it is found that at the critical salt concentration, neither clay particles adhered to the porous wall nor any energy barriers exist to prevent their release (Mohan, 1996). Critical ionic strength refers to the molar concentration of the mixed solution of NaCl and CaCl2at which clay particles start to release. Divalent ions (e.g., Ca2+) in solution will increase the solution ionic strength and decrease the double layer thickness compared with similar concentration of monovalent ions (e.g., Na+) (Bradford and Kim, 2010). Enhanced colloid release is known to occur with a decrease in the ionic strength due to an expansion of the double layer thickness and an increase in the magnitude of the surface potential (Bradford and Torkzaban, 2012;Sefriouiet al., 2013). Migration of interstitial clay particles clogs pores, reduces permeability, and decreases recovery efficiency, but a calcium preflush was found to reduce clay dispersion and lead to a higher recovery efficiency during the field experiment of freshwater injected into brackish aquifers in Norfolk, Virginia (Konikowet al., 2001).
For the aquifer suffering from seawater intrusion, injecting fresh water to replace saline water is an effective way to prevent seawater intrusion. However, water sensitivity phenomenon can occur during the saline aquifer freshening process. And it results in clay particles release and deposition. The occurrence of the phenomenon would clog the aquifer and further lower fresh water injection rate and saline water restoration efficiency. It deserves our serious attention, nevertheless, that the studies of water sensitivity occurring in the saline aquifer freshening process have rarely been reported before (Konikowet al., 2001; Zhouet al., 2009), especially under the critical conditions for clay particle release from water sensitivity phenomenon (Blumeet al., 2005). Therefore, the paper employed the aquifer material collected from Dagu River seawater intrusion aquifer as test material and designed laboratory column infiltration experiments simulating saline aquifer freshening process to investigate water sensitivity phenomenon. The objectives of this research are as follows: 1) to determine the critical conditions (critical flow velocity, critical salt concentration and critical ionic strength) which cause clay particle release from the porous media; 2) to find out the mechanisms of clay particle release caused by different factors based on forces analysis for clay particle according to DLVO electric double layer theory. The research could provide the foundation for further study on the water sensitivity in saline aquifer freshening process in seawater intrusion area.
2 Materials and Methods
2.1 Aquifer Materials
Aquifer materials collected from the lower reach of the Dagu River near the coastal city of Qingdao (Shandong Province, P. R. China) were employed in this study. The location of the sampling site is shown in Fig.1. The aquifer consisted of unconsolidated fluvial deposits. The material was obtained from the 5 m depth by drilling into a section of the aquifer that was affected by seawater intrusion. The aquifer material was air-dried, sieved through a 2 mm screen, thoroughly mixed, and stored in a closed 10 L plastic bucket.

Fig.1 Location of the investigation site.

Fig.2 Granulometric curve of the aquifer material.
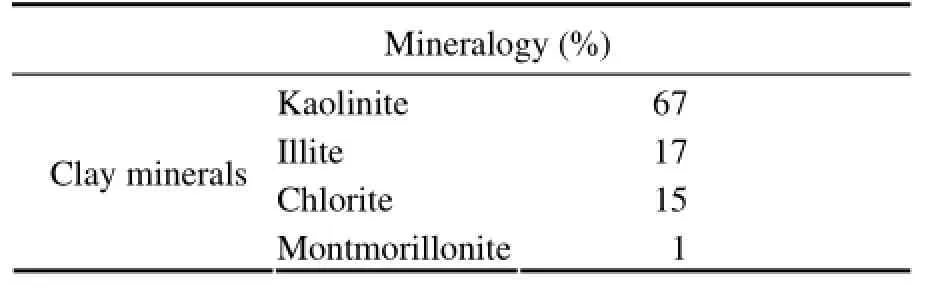
Table 1 Mineralogical analysis of the aquifer material
Basic characterizations of the aquifer material included particle size (Fig.2) and mineralogical analysis (X-ray diffraction) (Table 1). The material mainly consisted ofsand. The mean particle size was 0.38 mm, and the median particle diameter was 0.33 mm. The clay mineralogy mainly consisted of kaolinite, illite, chlorite and montmorillonite clays. Scanning electron microscopy (SEM) images showed that larger grains were covered with smaller clay particles (Fig.3a). Smaller particles showed morphologies of clay particles with different magnification. (Figs.3b, 3c, and 3d). As is seen from Fig.3, clay particles in the outflow had different shapes like fibrous shape, hairline shape, cyclic shape,etc. The shapes and structures of particles made them occur interception and bridging effects in the pores very likely.
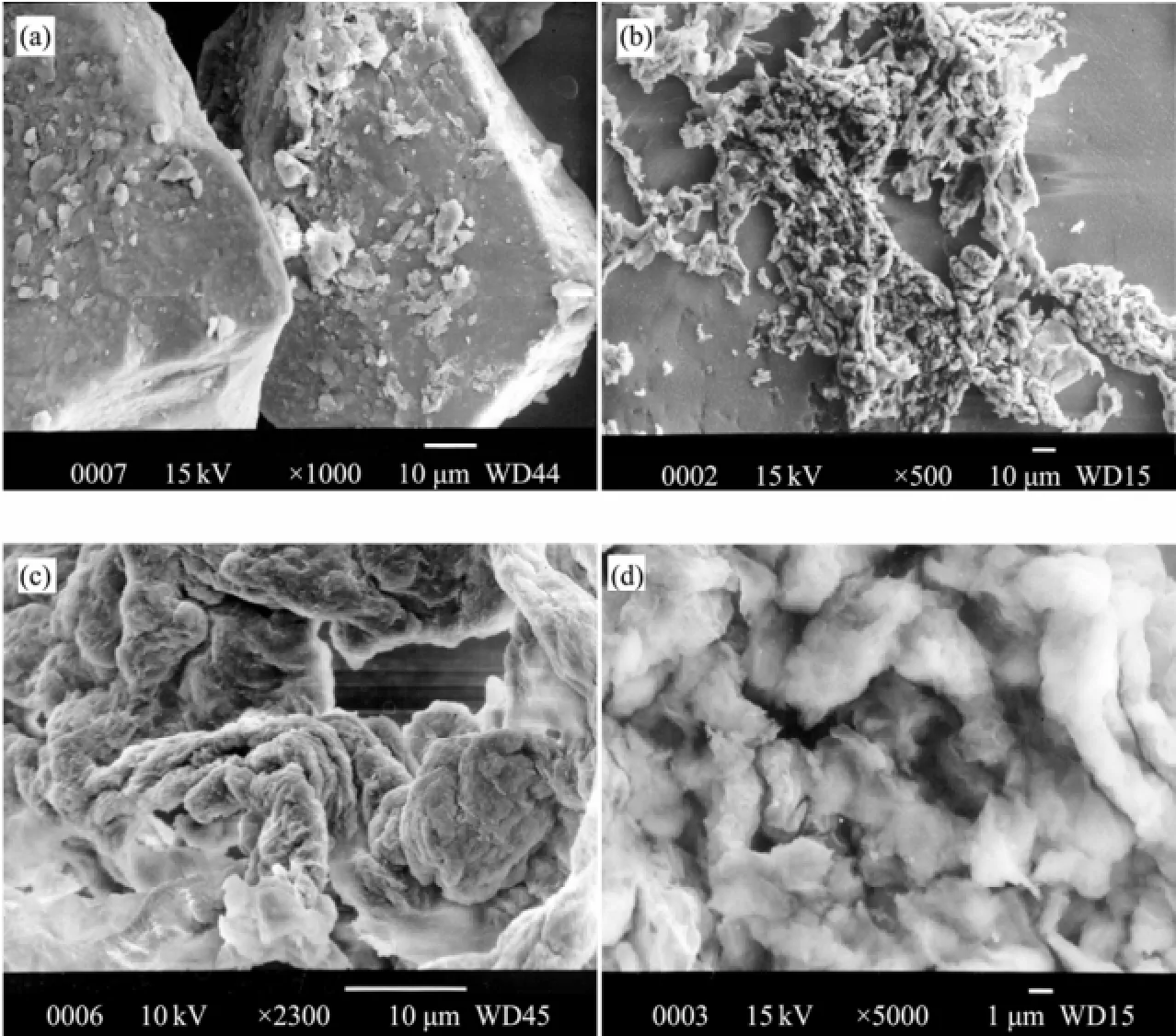
Fig.3 SEM images: (a) sand grains covered with smaller particles; (b), (c), (d) clay particles morphology of different magnification in column outflow (500 times, 2300 times, 5000 times).
2.2 Aqueous Solutions
The aqueous solutions used in the experiment were NaCl solutions with different concentrations formulated by deionized water and NaCl reagents, as well as mixed solutions of NaCl and CaCl2with different concentrations formulated by deionized water, NaCl reagents and CaCl2reagents. All chemicals used in the study were of analytical grade.
2.3 Experimental Setup
Dry aquifer material, thoroughly mixed, was packed into vertically oriented cylindrical glass columns (2.8cm i.d and 5cm length). The bulk density of the packed columns was 1.6±0.05g cm-3,and the porosity was 0.38± 0.2cm3cm-3. To achieve reproducible packings in terms of bulk density and porosity, a given amount of aquifer material was weighed out and packed into the columns. After packed, the columns were oriented horizontally, and saturated with 0.4 mol L-1NaCl solution (the same conductivity as seawater) under a partial vacuum to minimize the presence of air bubbles (Fig.4).

Fig.4 Schematic experimental setup.
2.4 The Determination of Critical Flow Velocity
To evaluate the impact of flow velocity on clay particle release and identify the critical flow velocity, released clay particle concentration was measured as a function of flow velocity.
After the columns were saturated, NaCl solutions with different concentrations were injected into the columns separately at different flow velocities (the flow velocities increased gradually from 0.20 to 15.60 cm min-1) by using the peristaltic pump. The flow velocity at which clay particles started to be detected in the outflow was identified as the critical flow velocity under the condition of specific NaCl concentration. The flow velocity was determined by measuring the volume of column outflow in the fraction collector. Column effluent was collected through a fraction collector, and was used for measuring conductivity (using a conductivity meter) and absorbance (using a spectrophotometer at the wavelength of 600 nm). Conductivity can be converted to ionic strength (expressed inmolar concentration) by a regression equation (Eq.(1)) (Marion and Babcock, 1976). The absorbance can be converted to clay particle concentration by a specific calibration curve (Fig.5). All experiments were conducted at an ambient laboratory temperature of 23℃ and repeated twice. The ionic strength can be calculated based on conductivity following Eq.(1).
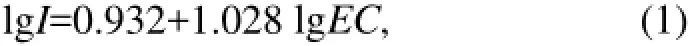
whereIis the ionic strength;ECis the conductivity of the solution.
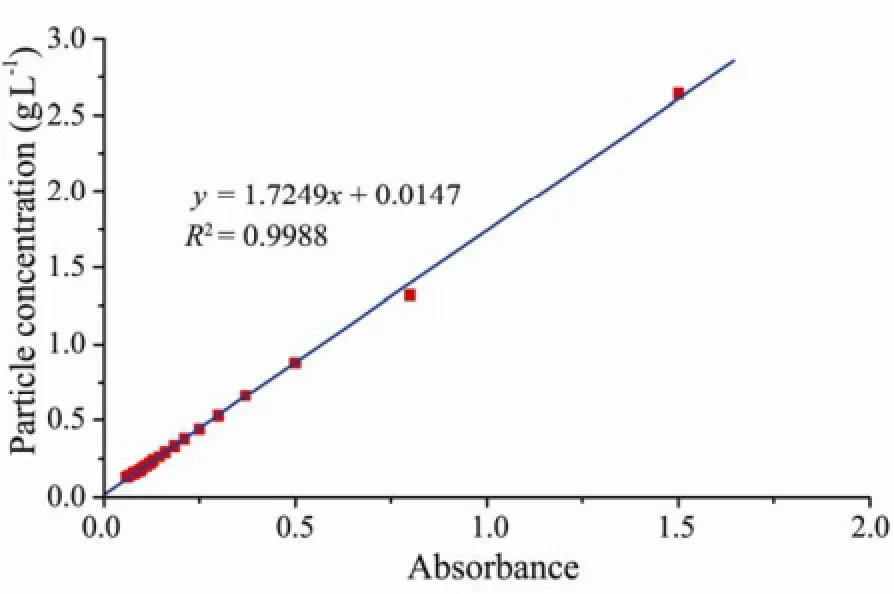
Fig.5 The calibration curve for absorbance against particle concentrations.
2.5 The Determination of Critical Salt Concentration
After the columns were saturated, NaCl solution with different concentrations (0.40, 0.23, 0.17, 0.11, 0.06 and 0.003 mol L-1) were injected continuously in turn by using the peristaltic pump at the specific flow velocity much lower than the critical flow velocity determined in Section 2.4 to determine the critical salt concentration. The salt concentration at which clay particles started to be detected in the outflow was the critical salt concentration. Then tests were performed in the same way as the experiments described in Section 2.4.
2.6 The Determination of Critical Ionic Strength
After the columns were saturated, the mixed solutions of NaCl and CaCl2with different Ca2+molar content percentages (0%, 1%, 5%, 10%, 15%, 20%, seperately) were injected into the columns at the same flow velocity of critical salt concentration experiment. The ionic strength at which clay particles started to be detected in the outflow was the critical ionic strength. Then tests were performed in the same way as the experiments described in Section 2.4.
3 Results and Discussion
3.1 Critical Flow Velocity
Fig.6 shows the relation curves between flow velocities and particle concentrations in the outflow when injecting different NaCl solutions.

Fig.6 Correlations between flow velocities and particle concentrations in the outflow when injecting different NaCl solutions. (a) 0.40 mol L-1; (b) 0.17 mol L-1; (c) 0.08 mol L-1.
When 0.40 mol L-1NaCl solution was injected into the column, no particle was detected in the outflow even if the flow velocity reached the maximum value of the peristaltic pump. It indicated that the fluid shear force almost exerted no effect on particle release when high con-centration NaCl solution was injected. However, when 0.17 mol L-1NaCl solution was injected into the column, particles started to be observed in the outflow when the flow velocity was higher than 3 cm min-1. So 3 cm min-1was identified to be the critical flow velocity for particle release. As the flow velocities further increased, the particle concentrations in the outflow increased rapidly and the maximum concentration finally attained 0.034 g L-1. When 0.08 mol L-1NaCl solution was injected, a lower critical flow velocity of 2.5 cm min-1was found. As flow velocities increased, the maximum particle concentration attained 0.560 g L-1. According to the experimental results above, in order to isolate hydrodynamic effect on particle release, a flow velocity lower than 2.5 cm min-1should be adopted in the subsequent experiments with critical salt concentration and critical ionic strength.
The above experimental results could be illustrated by force analysis of clay particle which adhered to the pore wall (Bradfordet al., 2011). Clay particle in the porous media is affected by the combined effects of its own gravity, Van der Waals attractive force, electric double layer repulsive force and fluid shear force. The schematic force analysis of the mechanical system is shown in Fig.7. In the system, the gravity (G) and Van der Waals attractive force (FA,F’A) keep the clay particle still, while the electric double layer repulsive force (FD) and the fluid shear force (FH) make particles produce migration trend. The combined effects of the forces determine whether the particle can be released from the grain skeleton. As the flow velocity increases, the shear force increases, and the force balance is disrupted. Therefore the particles are stripped from the pore wall and migrate with the fluid in the pore.
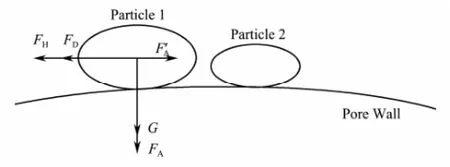
Fig.7 Schematic force analysis of clay particle.
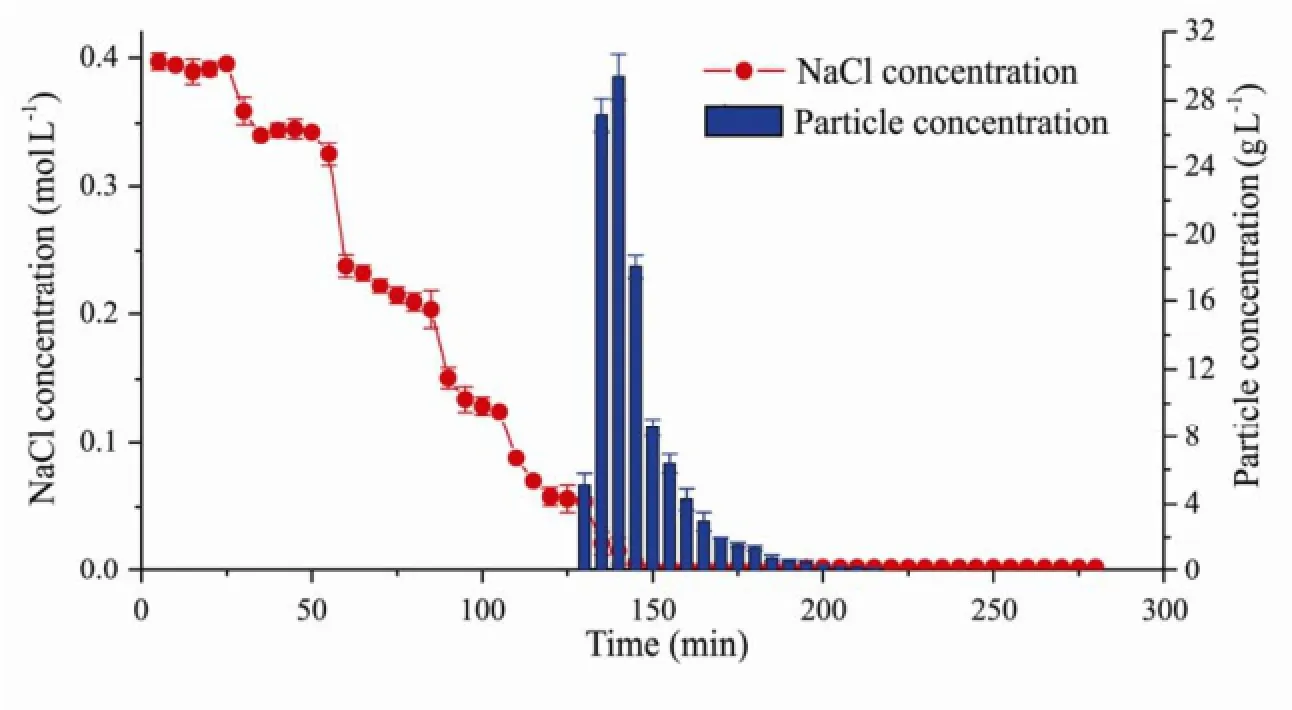
Fig.8 Released particle concentrations vs. NaCl solution concentrations changing with time.
3.2 Critical salt concentration
Fig.8 shows the curve of the released particle concentrations in the outflow and the injecting NaCl solution concentrations changing with time. The injecting NaCl solution concentrations decreased progressively as: 0.40, 0.23, 0.17, 0.11, 0.06 and 0.003 mol L-1. No clay particle was found in the outflow when the injecting NaCl solution concentrations decreased from 0.4 mol L-1to 0.06 mol L-1gradually. However, when the NaCl solution concentration turned to 0.003 mol L-1instantaneously, the outflow became turbid quickly, indicating that large amounts of particles had been released. And the salt concentration of the outflow was 0.052 mol L-1at this moment. Therefore, the concentration of 0.052 mol L-1was considered as the critical salt concentration for clay particle release.
Because the hydrodynamic effect on particle release was excluded, the particle release in the column was caused mainly by salt concentration alteration. The mechanism of particle release due to salt concentration alteration could also be illustrated from the perspective of the force analysis of clay particle. According to DLVO theory, the force balance between clay particle and sand grain skeleton is maintained by Van der Waals attractive force and electric double layer repulsive force. And the occurrence of particle release is the combined effect of the force changing with the distance. Under different salt concentration conditions in neutral environment, kaolinite (main composition of clay mineral) and quartz (main composition of sand grain skeleton) have different Zeta potential values (Fenget al., 2005) (Table 2). Van der Waals attractive force, electric double layer repulsive force, and the resultant force of attractive force and repulsive force can be calculated by Eqs. (2), (3), (4).

Table 2 Zeta potentials of kaolinite and quartz under different salt concentrations in neutral environment
Van der Waals attractive force can be calculated by:

whereAFis Van der Waals attractive force,Ais Hamaker constant,λis London wavelength (usually 100 nm) (Fenget al., 2005);his the distance between clay particle and sand grain skeleton.
Electric double layer repulsive force can be calculated by:

whereFDis electric double layer repulsive force,yiis the decreased surface potential (yi=zeψikT),kis Boltzman constant,Tis the absolute temperature,

εis dielectric constant of the media;n0is the ion number of the electric double layer in the infinity (iψ= 0);zis the absolute value of positive and negative valence;eis Coulomb constant; the meanings of other symbols are as defined above.
The resultant force can be calculated by:

whereTFis the resultant force, the meanings of other symbols are as defined above.
Because the volume of sand grain was much larger than that of clay particle, so sand grain was simplied as flat approximately in the calculation. On this basis, the Van der Waals attractive force, electric double layer repulsive force and the resultant force between kaolinite and quartz under different salt concentrations in neutral enrironment were calculated according to Eqs.(2)-(4) respectively. The results are shown by the curve of the resultant force changing with the distance under different salt concentration conditions (Fig.9).
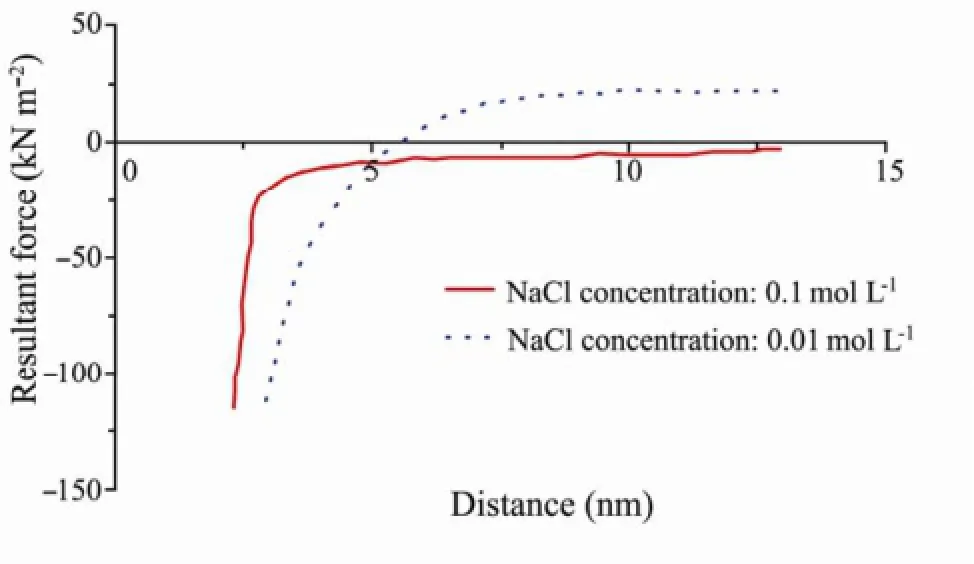
Fig.9 The resultant force vs. distance under different salt concentration conditions.
When the injecting NaCl solutiuon concentration equalled to 0.1 mol L-1, the resultant force between kaolinite and quartz changing with the distance was negative all the time, which meant that Van der Waals attractive force was dominant compared to electric double layer force. And the clay particle release did not occur under such situation. When the injecting NaCl solution concentration decreased to 0.01mol L-1, the resultant force reached zero at the distance about 5 nm, meaning that attractive force equalled to repulsive force at this distance. As the distance exeeded 5nm, the resultant force turned to be repulsive force, which made particle release possible. According to DLVO theory, high concentration electrolyte solution can compress the electric double layer greatly (Torkzabanet al., 2007). Therefore, 0.1 mol L-1NaCl solution made the double layer thickness become shorter, and resulted in shorter distance between clay particle and grain skeleton. Consequently the resultant force behaved as attractive force. However, 0.01 mol L-1NaCl solution exerted no obvious impact on compressing the electric double layer compared with 0.1 mol L-1NaCl solution, which meant that it only exerted a weak effect in shortening the distance between particle and grain skeleton. So the resultant force behaved as repulsive force gradually with the increase of the distance.
It could be seen from the foregoing force analysis that there must exist a NaCl concentration value higher than 0.01 mol L-1but lower than 0.1 mol L-1, at which the resultant force equalled to zero with the distance increase and clay particle started to release. It was in good agreement with the above experimental result that the critical salt concentration for clay particle release was 0.052 mol L-1.
3.3 Critical Ionic Strength
When the mixed NaCl and CaCl2solution with different Ca2+molar content percentages (0%, 1%, 5%, 10%, 15%) were seperately injected into the columns, the obtained critical ionic strengths were 0.041, 0.031, 0.011, 0.0063 and 0.0027 mol L-1respectively (Fig.10). However, for the injecting mixed solution with Ca2+molar content percentage further increased to 20%, even if the ionic strength of injecting solution decreased to zero, still no clay particle release was exhibited. Therefore, the critical ionic strength for particle release was negatively correlated with Ca2+molar content percentage of the mixed solution, and their relationship was quantified with the first-order exponential decay equationy= 0.0391e-0.266x+ 0.0015 (Fig.11). As Ca2+molar content percen- tage of the mixed solution increased, the critical ionic strength for particle release declined. When Ca2+molar content percentage of the mixed solution was high enough (up to 20%), the clay particle adhered to the porous media would no longer be released into the solution regardless of any low ionic strength (even zero).
The results of critical ionic strength experiments showed that critical ionic strength for clay particle release was determined by Ca2+molar content percentage of the mixed solution of NaCl and CaCl2, which could be explained from the following aspects. Firstly, when Ca2+of the mixed solution goes into the electric double layer, it can become counter-ion resulting in the decrease of Zeta potential on the particle surface (Bradford and Kim, 2010). Zeta potential decrease can directly trigger the weakening of double layer repulsive force, and increasethe difficulty of particle release. Secondly, Ca2+in the solution can replace Si4+and Al3+of the particle crystal structure, and increase the negative charges of the particle to decrease Zeta potential and weaken the repulsive force. Therefore, it can limit clay particles release from the grain skeleton. Furthermore, according to Schulze-Hardy rule of the colloid coagulation theory (Fenget al., 2005), the coagulation value ratio of different valence electrolytes with the same colloid is:
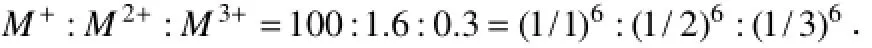
Thus, the coagulation capacity of 1.6 units Ca2+is equivalent to the coagulation capability of 100 units Na+. The coagulation effect of Ca2+promotes the combination of clay particle and sand skeleton, and so increases the difficulty of clay particle release.

Fig.10 Ionic strength and particle concentration of the outflow changing with time under the conditions of different Ca2+molar content percentages: (a) 0%, (b) 1%, (c) 5%, (d) 10%, (e) 15%, (f) 20%.
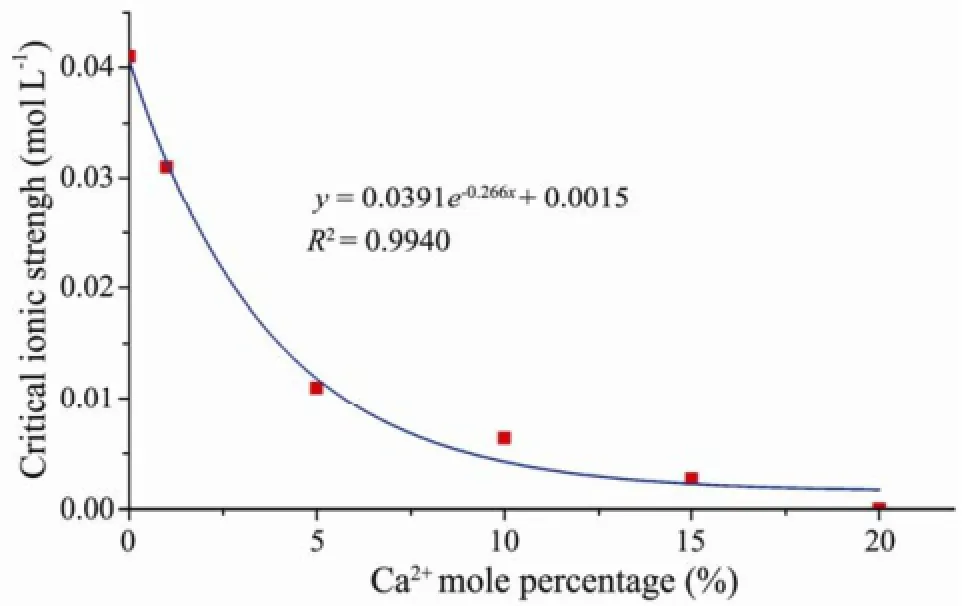
Fig.11 The fitting curve for Ca2+molar content percentage and ionic strength.
4 Conclusions
In the research, laboratory column infiltration experiments simulating saline aquifer freshening process were designed to find out the factors and mechanisms for clay particle release.
1) When 0.17 mol L-1and 0.08 mol L-1NaCl solution were injected into the column saturated with high salt concentration solution, the critical flow velocities of clay release were 3 cm min-1and 2.5 cm min-1respectively. However, when 0.4 mol L-1NaCl solution was injected, no clay particle release was observed even at a high flow velocity (15.60 cm min-1). When high salt concentration solution was injected into the column, fluid shear forcealmost displayed no effect on particle release comparing to chemical interaction force.
2) By injecting NaCl solutions in the way of decreasing the concentration gradually , it was found that as the injecting NaCl solution concentration decreased from 0.06 mol L-1to 0.003 mol L-1, large amounts of clay particles were detected in the outflow quickly. And the outflow salt concentration at this moment was the critical salt concentration for particle release, which was 0.052 mol L-1. The force analysis between clay particle and grain skeleton according to DLVO theory could explain and validate the experiment results.
3) The critical ionic strength for particle release was negatively correlated with Ca2+molar content percentage of the mixed NaCl and CaCl2solution following the correlation equation y = 0.0391e-0.266x+ 0.0015. A high Ca2+molar content percentage (up to 20%) of the mixed solution would curb the the clay particle adhered to the media from being released into the solution in spite of any low ionic strength (even zero).
Acknowledgements
This paper is supported by the National Natural Science Foundation of China (Grant No. 41172209) and National Public Welfare Scientific Research Project (Grant No. 201301090). The authors are grateful to Post Dr. Xin Jia (OUC) for her helpful comments.
Bergendahl, J., and Grasso, D., 2000. Prediction of colloid detachment in a model porous media: Hydrodynamics. Chemical Engineering Science, 55 (9): 1523-1532.
Blume, T., Weisbrod, N., and Selker, J. S., 2002. Permeability changes in layered sediments: impact of particle release. Ground Water, 40 (5): 466-474.
Blume, T., Weisbrod, N., and Selker, J. S., 2005. On the critical salt concentrations for particle detachment in homogeneous sand and heterogeneous Hanford sediments. Geoderma, 124: 121-132.
Boisdet, A., Cautru, J. P., and Czernichowske, I., 1989. Experiments on reinjection of geothermal brines in deep Triassic sandstones. In: Proceedings of the Fourth International Seminar on the Results of EC Geothermal Energy. Italy, 419-428.
Bradford, S. A., and Kim, H., 2010. Implications of cation exchange on clay release and colloid-facilitated transport in porous media. Journal of Environmental Quality, 39: 2040-2046.
Bradford, S., A., and Torkzaban, S., and Wiegmann, A., 2011. Pore-scale simulations to determine the applied hydrodynamic torque and colloid immobilization. Vadose Zone Journal, 10 (1): 252-261.
Bradford, S., A., and Torkzaban, S., 2012. Colloid adhesive parameters for chemically heterogeneous porous media. Langmuir, 28: 13643-13651.
Choudhary, O. P., Ghuman, B. S., Josan, A. S., and Bajwa, M. S., 2006. Effect of alternating irrigation with sodic and non-sodic waters on soil properties and sunflower yield. Agricultural Water Management, 85: 151-156.
Feng, X. S., Liu, H. G., and Hao, J. C., 2005. Colloid Chemistry. Chemical Industry Press of Beijing, Beijing, 30-55.
Gao, B., Cao, X., Dong, Y., Luo, Y., and Ma, L., 2011. Colloid deposition and release in soils and their association with heavy metals. Critical Reviews in Environmental Science and Technology, 41: 336-372.
Gravelle, A., Peysson, Y., Tabary, R., and Egermann, P., 2011. Experimental investigation and modeling of colloidal release in porous media. Transport in Porous Media, 88: 441-459.
Grolimund, D., and Borkovec, M., 2005. Colloid facilitated transport of strongly sorbing contaminants in natural porous media: mathematical modeling and laboratory column experiments. Environmental Science and Technology, 39 (17): 6378-6386.
Huang, P. M., Bollag, J. M., and Senesi, N., 2001. Interactions Between Soil Particles and Microorganisms: Impact on the Terrestrial Ecosystem. John Wiley & Sons Ltd, UK, 15-35.
Khilar, K. C., and Fogler, H. S., 1983. Water sensitivity of sandstones. SPE Journal, 23 (1): 55-64.
Kim, S. B., Corapcioglu, M. Y., and Kim D. J., 2003. Effect of dissolved organic matter and bacteria on contaminant transport in riverbank filtration. Journal of Contaminant Hydrology, 66: 1-23.
Konikow, L. F., August, L. L., and Voss, C. I., 2001. Effects of clay dispersion on aquifer storage and recovery in coastal aquifers. Transport in Porous Media, 43: 45-64.
Mandal, U. K., Bhardwaj, A. K., Warrington, D. N., Goldstein, D., and Levy, G. J., 2008. Changes in soil hydraulic conductivity, runoff,and soil loss due to irrigation with different types of saline-sodicwater. Geoderma, 144: 509-516.
Marion, G. M., and Babcock, K. L., 1976. Predicting specific conductance and salt concentration in dilute aqueous solutions. Soil Science, 122: 181-187.
Moghadasi, J., Steinhagen, H., and Jamialahmadi, M., 2004. Theoretical and experimental studay of particle movement and deposition in porous media during water injection. Journal of Petroleum Science & Engineering, 43: 163-181.
Mohan, K. K., 1996. Water sensitivity of porous media containing swelling clays. PhD thesis, The University of Michigan, 1-44.
Ochi, J., and Vernoux, J. F., 1998. Permeability decrease in sandstone reservoirs by fluid injection hydrodynamic and chemical effects. Journal of Hydrology, 208 (3-4): 237-248.
Patil, S., Tawfiq, K., and Chen, G., 2011. Colloid release and transport in agricultural soil as impacted by solution chemistry. Journal of Urban and Environmental Engineering, 5 (2): 84-90.
Pyatakhin, M. V., 2006. Stabilization of porous reservoir by stressed filter. Journal of Petroleum Science and Engineering, 50: 227-240.
Roy, S. B., and Dzombak, D, A., 1996. Na+-Ca2+exchange effects in the detachment of latex colloids deposited in glass bead porous media. Colloids and Surfaces. A: Physicochemical and Engineering Aspects, 119 (2): 133-139
Ryan, J. N., and Gschwend, P. M., 1994. Effects of ionic strength and flow rate on colloid release relating kinetics to intersurface potential energy. Journal of Colloid and Interface Science, 165 (2): 536.
Sefrioui, N., Ahmadi, A., Omari, A., and Bertin, H., 2013. Numerical simulation of retention and release of colloids in porous media at the pore scale. Colloids and Surfaces, A: Physicochemical and Engineering Aspects, 427: 33-40.
Sen, T. K., and Khilar, K. C., 2006. Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Advances in Colloids and Interface Sci-ence, 119 (2-3): 1-95.
Simunek, J., He, C., Pang, L., and Bradford, S. A., 2006. Colloid-facilitated transport in variably-saturated porous media: Numerical model and experimental verification. Vadose Zone Journal, 5: 1035-1047.
Torkzaban, S., Bradford, S. A., and Walker, S. L., 2007. Resolving the coupled effects of hydrodynamics and DLVO forces on colloid attachment in porous media. Langmuir, 23: 9652-9660.
Tripathy, A., 2010. Hydrodynamically and chemically induced in situ kaolin particle release from porous median experimental study. Advanced Powder Technology, 21: 564-572. Vaidya, R. N., 1991. Fines migration and formalation damage. PhD thesis, The University of Michigan, 1-61.
Yu, C., Carpena, R. M., Gao, B., and Ovilla, O. P., 2013. Effects of ionic strength, particle size, flow velocity, and vegetation type on colloid transport through a dense vegetation soil system: Experiments and modeling. Journal of Hydrology, 499: 316-323.
Zhou, J., Zheng, X., Flury, M., and Lin, G., 2009. Permeability changes during remediation of an aquifer affected by seawater intrusion: A laboratory column study. Journal of Hydrology, 376: 557-566.
(Edited by Ji Dechun)
(Received September 26, 2013; revised February 20, 2014; accepted March 17, 2014)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-532-66781759
E-mail: nwln2011@163.com
杂志排行
Journal of Ocean University of China的其它文章
- Numerieal Prediction of Storm Surge in the Qingdao Area Under the Impact of Climate Change
- Warmer-Get-Wetter or Wet-Get-Wetter? A Criterion to Classify Oceanic Precipitation
- Modeling Seasonal Variations of Subsurface Chlorophyll Maximum in South China Sea
- Assimilation of High Frequency Radar Data into a Shelf Sea Circulation Model
- A Physics-Based Dual-Frequency Approach for Altimeter Wind Speed Retrieval
- CFD-Based Numerical Analysis of a Variable Cross-Section Cylinder
