Improved anterior hepatic transection for isolated hepatocellular carcinoma in the caudate
2014-05-04TanToCheungWaiKeyYuenRonnieTPPoonSeeChingChanSheungTatFanandChungMauLo
Tan To Cheung, Wai Key Yuen, Ronnie TP Poon, See Ching Chan, Sheung Tat Fan and Chung Mau Lo
Hong Kong, China
Improved anterior hepatic transection for isolated hepatocellular carcinoma in the caudate
Tan To Cheung, Wai Key Yuen, Ronnie TP Poon, See Ching Chan, Sheung Tat Fan and Chung Mau Lo
Hong Kong, China
BACKGROUND:One of the best treatments for isolated hepatocellular carcinoma in the caudate lobe is major hepatectomy with caudate lobectomy, but it is not suitable for patients with poor liver function reserve. Isolated caudate lobectomy, which is a very difficult operation, is thus an alternative option.
METHODS:Here we report an isolated caudate lobectomy with an anterior approach in the treatment of a large hepatocellular carcinoma with underlying cirrhosis, with focus on the technical aspects.
RESULTS:In the operation, both the left and right lobes of the liver were mobilized. Hepatotomy was done along the round ligament where parenchymal transection was minimal. After exposure of the left and middle hepatic veins and the hilar plate, the caudate lobe and the tumor were resecteden blocwith a 5-mm margin.
CONCLUSION:Isolated caudate lobectomy can be performed safely with this anterior approach on patients with poor liver function reserve.
(Hepatobiliary Pancreat Dis Int 2014;13:219-222)
hepatocellular carcinoma;
hepatectomy;
liver cirrhosis
Introduction
The major challenge of surgical treatment for patients with isolated hepatocellular carcinoma and underlying cirrhosis is to preserve adequate postoperative liver function while achieving complete tumor clearance.[1]Tumor size, tumor location, and the severity of cirrhosis all determine the outcome. The challenge is even stronger if the tumor is located in the caudate lobe as resection of hepatocellular carcinomas in the caudate lobe demands the finest and most meticulous techniques.
The caudate lobe is the posterior part of the liver and can be subdivided into the left Spiegel lobe, the right caudate process, and the paracaval portion, but no definite landmarks for the boundaries can be observed from the liver surface.[2,3]To remove isolated hepatocellular carcinomas in the caudate lobe, an easier way is to perform a left or right hepatectomy together with caudate lobectomy. However, this can only be safely performed in patients with good liver function reserve. Isolated caudate lobectomy is thus a more sensible option for patients with advanced cirrhosis.
Isolated caudate lobectomy is not widely practiced because it is technically very demanding. As a result, there are only a few reports on it.[4,5]Here we report a simplified version of the anterior approach to isolated caudate lobectomy in the treatment of a large hepatocellular carcinoma with underlying cirrhosis.
Case report
The patient was a 68-year-old female carrier of hepatitis B virus with cirrhosis who joined a half-yearly ultrasound screening program. She had no jaundice or epigastric discomfort. She was found to have an 8-cm mass deep inside the liver, just anterior to the inferior vena cava. Blood test revealed that her serum totalbilirubin level was 40 µmol/L, serum albumin level was 32 g/L, serum α-fetoprotein level was 480 ng/mL, international normalized ratio was 1.3, platelet count was 80×109/L, and indocyanine green retention rate was 25% at 15 minutes. Contrast computed tomography was done, showing a mass of 8×6 cm in the caudate lobe of the liver which was compatible with hepatocellular carcinoma (Fig. 1). Since the patient did not want liver transplantation, isolated caudate lobectomy was conducted.
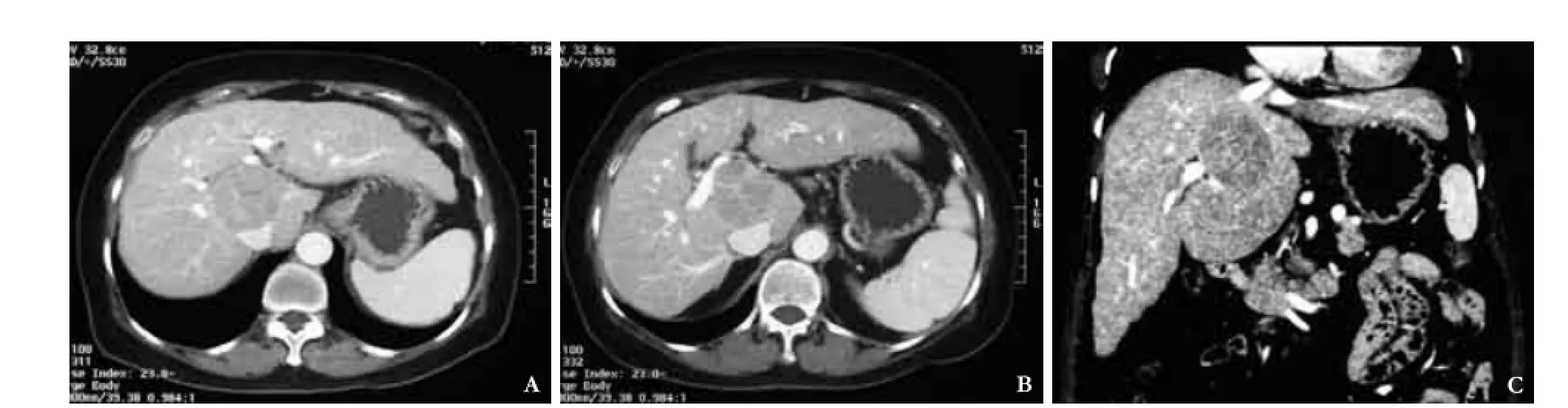
Fig. 1.A: Contrast computed tomography scan showing a caudate lobe tumor abutting on the hepatic veins before they enter the inferior vena cava;B: Contrast computed tomography scan showing the caudate lobe tumor compressing the hilar plate;C: Coronal reconstruction of computed tomography scan showing the caudate lobe tumor.
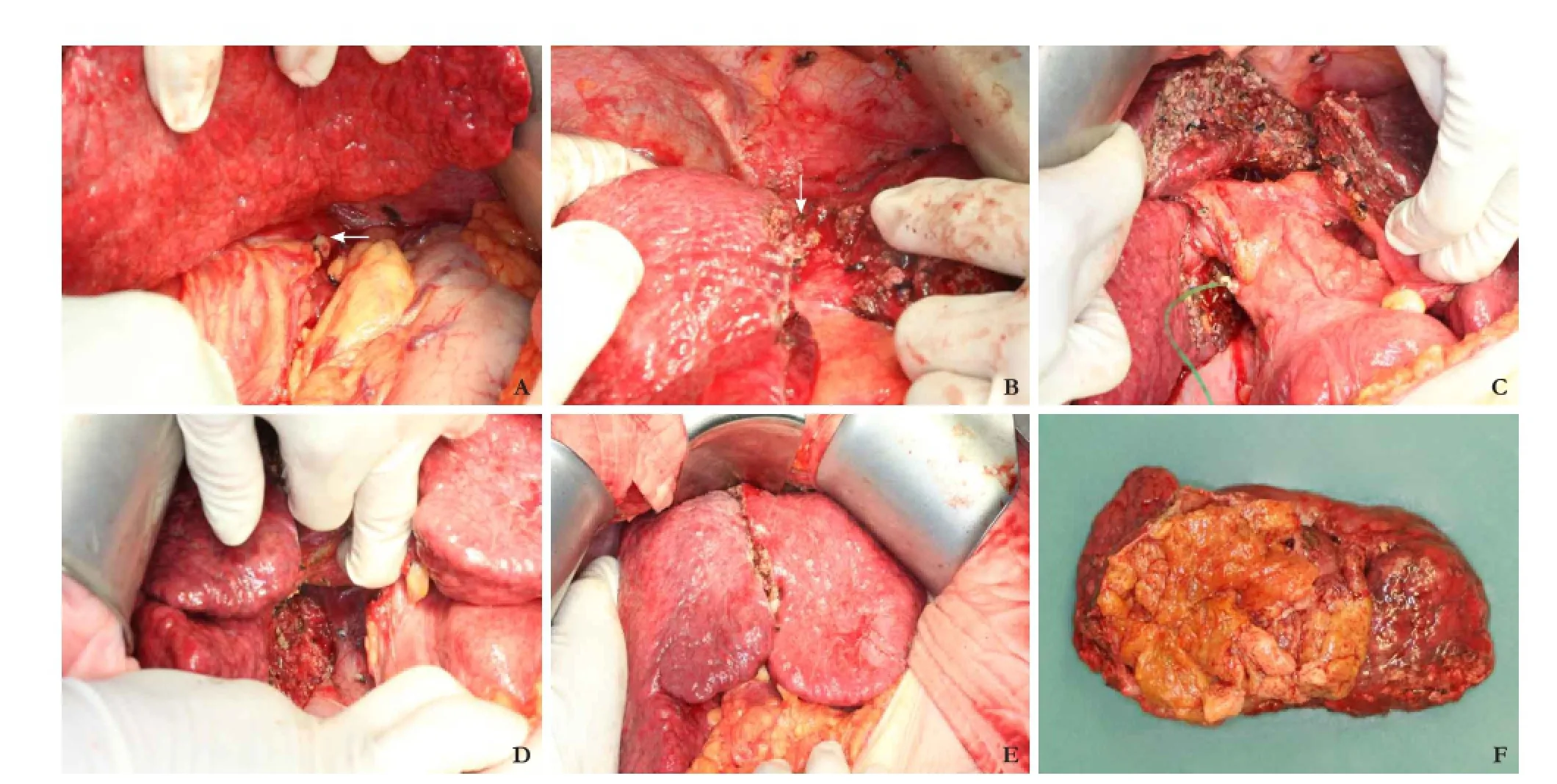
Fig. 2.A: A large caudate lobe lesion (arrow) revealed after mobilization of the left lateral section;B: Exposure of the middle hepatic vein (arrow) after transection along the round ligament with an anterior approach;C: Completion of caudate lobectomy with the middle hepatic vein and the hilar plate exposed;D: Completion of caudate lobectomy by division of the short hepatic veins from the inferior vena cava;E: Reposition of the liver after completion of caudate lobectomy;F: The caudate lobe and the encapsulated hepatocellular carcinoma resecteden bloc.
The laparotomy started with a right subcostal incision with midline extension. Intraoperative ultrasonography showed an isolated tumor in the caudate lobe, close to the middle and left hepatic veins. The posterior border of the tumor was compressing the inferior vena cava without infiltration to the lumen. The anterior border of the lesion was close to the hilar plate. Cholecystectomy was performed and the cystic duct was cannulated with an Fr-3.5 catheter and prepared for a methylene blue leakage test. The left lateral border of the encapsulated tumor was noted to be in the Spiegel lobe after mobilization of the left lateral section of the liver (Fig. 2A). To reduce liver transection time, a hepatotomy with an anterior approach along the round ligament was performed. Liver transection was performed with the use of a Cavitron ultrasonic aspirator. The anteriortransection was soon completed after exposing the left side of the middle hepatic vein and the junction of the middle and left hepatic veins (Fig. 2B). The hilar plate was identified. The left and right portal triads were isolated by exposing Glisson's capsule. Blood vessels to the caudate lobe were ligated and divided without dividing the major branches. The outline of the large tumor together with the caudate lobe was fully exposed. Parenchymal transection was completed by exposing the junction of the middle and left hepatic veins. Segment 4 was then "lifted up" and separated from the caudate lobe (Fig. 2C). The short hepatic veins were divided between the isolated caudate lobe and the inferior vena cava (Fig. 2D). The liver was repositioned (Fig. 2E). The encapsulated tumor remained intact and was delivered with the caudate lobe en bloc (Fig. 2F). The operation time was 520 minutes and the blood loss was 820 mL. Methylene blue leakage test was negative and the cystic duct was ligated. A drain was placed on the transection surface.
There was no output from the drain on the first 5 days. After then, 20 mL of bile-stained output was collected daily, signifying mild biliary leakage from the transection surface. The amount of output reduced gradually and the drain was removed 15 days after the surgery. Pathological examination showed a welldifferentiated hepatocellular carcinoma. The capsule was intact and the resection margin was 5 mm.
Contrast computed tomography of the abdomen was performed 12 months after the surgery, showing no recurrence of the disease (Fig. 3). Liver function remained static and there was no postoperative ascites.
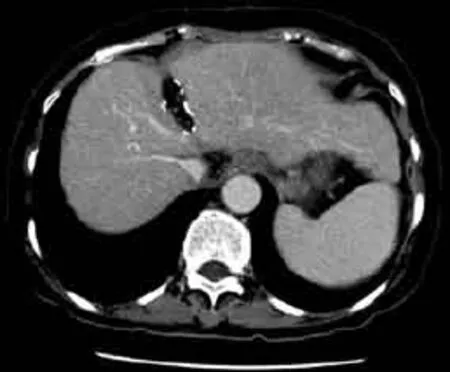
Fig. 3. Contrast computed tomography performed 12 months after the surgery showing no recurrence of disease.
Discussion
Caudate lobectomy combined with hepatectomy is generally better than isolated caudate lobectomy in terms of survival outcome, but patients with advanced cirrhosis cannot tolerate a major hepatectomy.[6,7]Liver transplantation is the best modality but its application is limited due to the shortage of liver grafts. In many transplantation centers, patients with larger tumors are not entitled to deceased donor liver transplantation.[8]On the other hand, live liver donors are not always available. Transarterial chemoembolization is another treatment option, but it may be less effective for tumors in the caudate lobe because such tumors are usually supplied by different arteries.[9,10]For the patient in this report, whose liver function was poor as indicated by an indocyanine green retention test, isolated caudate lobectomy seemed to be the only option.[11]
Different approaches to isolated caudate lobectomy have been reported, including the posterior approach with or without total hepatic vascular exclusion described by Yanaga et al,[12]the left lateral approach described by Colonna et al,[13]and the anterior approach described by Yamamoto et al.[4]The anterior approach is technically challenging but it provides best exposure of the liver vasculature. However, a prolonged transection along the Cantlie line would be anticipated on most occasions. The distorted and compressed middle hepatic vein may also be injured during transection. In addition, the larger transection surface for tumor exposure may cause more blood loss, especially in patients with cirrhosis, portal hypertension, thrombocytopenia, or a high international normalized ratio. The transection plane along the junction of the left medial and lateral sections is usually thinner, therefore the anterior approach could simplify the resection. In addition, the approach also provides a quick exposure of the middle and left hepatic veins before their entry into the inferior vena cava. Some measures can be taken to reduce blood loss. At our center, a central venous catheter is inserted in anticipation of a difficult major hepatectomy for closer monitoring of the central venous pressure, which is kept below 5 cmH2O under vigilant monitoring of fluid input by the anesthesiologist. The central venous pressure can also be lowered by placing the patient in the reverse Tredelunberg position. Intermittent inflow control by the Pringle maneuver would also provide a relatively bloodless operation field during parenchymal transection.[14]
In conclusion, the anterior approach along the left lateral section is a safe and effective method for complete caudate lobectomy. It is a simple and less timeconsuming alternative approach for large caudate lobe tumors in patients with cirrhosis.
Contributors:CTT drafted the manuscript; CSC, YWK and FSTrevised the manuscript; FST, PRTP and LCM revised and approved the manuscript. CTT is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from any commercial party related directly or indirectly to the subject of this article.
1 Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl 2004;10:S39-45.
2 Filipponi F, Romagnoli P, Mosca F, Couinaud C. The dorsal sector of human liver: embryological, anatomical and clinical relevance. Hepatogastroenterology 2000;47:1726-1731.
3 Couinaud C. Surgical approach to the dorsal section of the liver. Chirurgie 1993-1994;119:485-488.
4 Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T, Makuuchi M. Anterior transhepatic approach for isolated resection of the caudate lobe of the liver. World J Surg 1999;23:97-101.
5 Kosuge T, Yamamoto J, Takayama T, Shimada K, Yamasaki S, Makuuchi M, et al. An isolated, complete resection of the caudate lobe, including the paracaval portion, for hepatocellular carcinoma. Arch Surg 1994;129:280-284.
6 Liu P, Qiu BA, Bai G, Bai HW, Xia NX, Yang YX, et al. Choice of approach for hepatectomy for hepatocellular carcinoma located in the caudate lobe: isolated or combined lobectomy? World J Gastroenterol 2012;18:3904-3909.
7 Wang Y, Zhang LY, Yuan L, Sun FY, Wei TG. Isolated caudate lobe resection for hepatic tumor: surgical approaches and perioperative outcomes. Am J Surg 2010;200:346-351.
8 Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg 2007;94:78-86.
9 Terayama N, Miyayama S, Tatsu H, Yamamoto T, Toya D, Tanaka N, et al. Subsegmental transcatheter arterial embolization for hepatocellular carcinoma in the caudate lobe. J Vasc Interv Radiol 1998;9:501-508.
10 Yoon CJ, Chung JW, Cho BH, Jae HJ, Kang SG, Kim HC, et al. Hepatocellular carcinoma in the caudate lobe of the liver: angiographic analysis of tumor-feeding arteries according to subsegmental location. J Vasc Interv Radiol 2008;19:1543-1550.
11 Cheung TT, Chan SC, Chok KS, Chan AC, Yu WC, Poon RT, et al. Rapid measurement of indocyanine green retention by pulse spectrophotometry: a validation study in 70 patients with Child-Pugh A cirrhosis before hepatectomy for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2012;11:267-271.
12 Yanaga K, Matsumata T, Hayashi H, Shimada M, Urata K, Sugimachi K. Isolated hepatic caudate lobectomy. Surgery 1994;115:757-761.
13 Colonna JO 2nd, Shaked A, Gelabert HA, Busuttil RW. Resection of the caudate lobe through "bloody gultch". Surg Gynecol Obstet 1993;176:401-402.
14 Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg 2011;253:745-758.
Received March 28, 2013
Accepted after revision May 17, 2013
AuthorAffiliations:Department of Surgery (Cheung TT, Yuen WK, Poon RTP, Chan SC, Fan ST and Lo CM) and State Key Laboratory for Liver Research (Poon RTP, Chan SC, Fan ST and Lo CM), The University of Hong Kong, 102 Pok Fu Lam Road, Hong Kong, China
Tan To Cheung, MD, Department of Surgery, The University of Hong Kong, 102 Pok Fu Lam Road, Hong Kong, China (Tel: 852-22553025; Fax: 852-28165284; Email: tantocheung@hotmail.com)
© 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60035-7
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Multi-visceral resection of locally advanced extra-pancreatic carcinoma
- Effects of plasma exchange combined with continuous renal replacement therapy on acute fatty liver of pregnancy
- FBW7 increases chemosensitivity in hepatocellular carcinoma cells through suppression of epithelialmesenchymal transition
- Pancreatic head cancer in patients with chronic pancreatitis
- Familial chylomicronemia syndrome related chronic pancreatitis: a single-center study
- Instrumental detection of cystic duct stones during laparoscopic cholecystectomy
