Effects of melatonin on the oxidative damage and pancreatic antioxidant defenses in ceruleininduced acute pancreatitis in rats
2014-05-04CristinaCarrascoAnaBeatrizRodrguezandJosPariente
Cristina Carrasco, Ana Beatriz Rodríguez and José A Pariente
Badajoz, Spain
Effects of melatonin on the oxidative damage and pancreatic antioxidant defenses in ceruleininduced acute pancreatitis in rats
Cristina Carrasco, Ana Beatriz Rodríguez and José A Pariente
Badajoz, Spain
BACKGROUND:Oxidative stress is recognized as a pivotal effector of several pathogenic processes, including acute pancreatitis. Reactive oxygen species not just cause damage on the main cellular components, but also influence the expression of antioxidant system genes. Antioxidant molecules, such as melatonin, could be good candidates for the treatment of this multidimensional disease. The present study was to evaluate the chemopreventive effect of melatonin in a rat model of ceruleininduced acute pancreatitis.
METHODS:Four subcutaneous injections of cerulein (20 µg/kg body weight) were given to Wistar rats at two hours intervals; melatonin was injected intraperitoneally (25 mg/kg body weight) 30 minutes before each injection of cerulein. Lipid peroxidation, protein oxidation (carbonyl groups), total antioxidant status, and glutathione peroxidase activity were determined in pancreatic tissue using commercial kits.
RESULTS:The chemopreventive administration of melatonin caused a reduction in lipid peroxidation and protein oxidation due to injections of cerulein. Additionally, melatonin treatment was also able to revert glutathione peroxidase activity and total antioxidant status near to control levels, suggesting that melatonin could prevent from oxidative phenomena in the pancreas, such as lipid peroxidation and protein oxidation, and could stimulate, directly or indirectly, the expression of antioxidant enzymes.
CONCLUSION:Melatonin, a polyvalent antioxidant, protected the pancreatic damage via the decrease of oxidative stress andincrease of the activities of antioxidant enzymes in ceruleininduced acute pancreatitis.
(Hepatobiliary Pancreat Dis Int 2014;13:442-446)
acute pancreatitis;
antioxidants;
cerulein;
melatonin;
oxidative stress
Introduction
Oxidative stress is recognized as a pivotal effector of several pathogenic processes, including acute pancreatitis.[1,2]Imbalance between the antioxidant system of organisms, production of reactive oxygen species (ROS), and other reactive species, could predispose or even directly cause this disease.[3]Previous studies[4,5]showed that some of the main acinar components (lipids, proteins, cytoskeleton and DNA) and organelles (mitochondrion, zymogen granules, etc.) are highly susceptible to oxidative damage; additionally, it has been observed that nuclear factor-kappa B (NF-κB) and nuclear factor erythroid 2-related factor 2 (Nrf2) activities, transcription factors related to the expression of several genes (e.g. inflammatory mediators, antioxidant enzymes, etc.), are affected by ROS in the pancreas.[6-8]The acinar cell malfunction and the spread of the local inflammatory status could culminate in a multisystem organ failure and/or septic complications for the patient.[9]
Many antioxidant families (vitamins, catechins, phytoalexins, carotenoids, etc.) seem to counteract the excessive exposure and/or production of these harmful molecules, acting as direct scavengers and/or indirect regulators of levels of antioxidant enzymes, transcription factors and immunitary mediators. For this reason, antioxidant supplementation is gathering force in clinical practice as a possible option in the treatment ofseveral diseases.[10,11]Melatonin,[12,13]resveratrol[14-16]and lycopene[17,18]between other antioxidant components found in food, have demonstrated their usefulness in the management of inflammatory illness such as acute pancreatitis, at least in animal models. Effectiveness and lack of secondary effects at low doses are some of the strong points of this kind of natural molecules.[19]
Taking in account the observed implication of ROS in the pathophysiology of acute pancreatitis and the effectiveness of melatonin to counteract its deleterious effects, the purpose of this study was to evaluate the effect of exogenous administration of melatonin on the pancreatic oxidative status and structural phenomena, during the induction process of the disease caused by the cholecystokinin-analogue cerulein in rats.
Methods
Reagents
Lipid peroxidation and antioxidant total status commercial assay kits were supplied by Oxford Biomedical Research (Barcelona, Spain). Protein carbonyl and glutathione peroxidase assay kits were from Cayman Chemical Co. (Madrid, Spain). Cerulein was from Sigma Co. (Madrid, Spain), melatonin from Fagron Ibérica (Barcelona, Spain) and ketamine (Imalgen 500®) from Merial Laboratories SA (Barcelona, Spain). All other reagents were of analytical grade.
Animal and experimental model
Six-week-old male and female Wistar rats (150-200 g of body weight) were obtained from the Animal House of the Faculty of Medicine of University of Extremadura. The rats were housed individually in cages under standard conditions at room temperature on a 12-hour light/12-hour dark cycle with commercial pellet chow and drinking waterad libitum. All experiments were performed according to the protocols approved by the Bioethical Committee for Animal Experimentation of the University of Extremadura.
The rats were divided into a control group, a pancreatitis group and a treatment group. After 18-hour fasting, the rats were subcutaneously injected with cerulein (20 µg/kg body weight) or saline solution for four times at 2-hour intervals, as previously described.[12]In the treatment group, melatonin was injected intraperitoneally (25 mg/kg body weight, dissolved in saline solution) 30 minutes before each cerulein injection. The rats were killed by decapitation 12 hours after the final cerulein injection, under sedation by ketamine (150 mg/kg body weight). Pancreases of the rats were quickly removed, frozen in liquid nitrogen, and stored at -80 ℃ for later oxidative stress studies.
Measurement of lipid peroxidation, protein oxidation, glutathione peroxidase activity and total antioxidant status in pancreatic tissue
After pancreatic extractions, 0.1 g of tissue specimens were homogenized in 1 mL of a particular buffer (according to the protocols of used kits) and placed on ice until their use. Determinations were corrected for the protein content of the tissue by the Bradford method.[20]
To determine pancreatic lipid peroxidation, malondialdehyde (MDA) concentration was measured in the homogenates; as a product of fatty acid decomposition, MDA reacts with N-methyl-2-phenylindole at 45 ℃, resulting in a stable chromophore with a maximal absorbance of 586 nm. The data were expressed in terms of µmol/mg total protein of MDA.
Protein oxidation was studied by measuring carbonyl groups in the homogenates; these groups were formed by metal-catalyzed protein oxidation and quantified via their reaction with 2, 4-dinitrophenylhydrazine (DNPH). The resulting hydrazone was analyzed spectrophotometrically at 385 nm. The data were expressed in nmol/mL of protein carbonyl groups.
Glutathione peroxidase (GPx) activity was indirectly measured in the homogenates, via a coupled reaction with glutathione reductase and oxidized glutathione; in this reaction, the oxidation of NADPH causes a reduction in absorbance at 340 nm which is proportional to GPx activity in the sample. The data were expressed in terms of nmol/min/mL of GPx activity.
Finally, total antioxidant status was determined as the power of the sample to reduce copper ion. Reduced copper (Cu+) formed a stable complex with a chromogen, which has a maximal absorbance at 450 nm. The data were expressed as µmol/L of Trolox equivalents.
Statistical analysis
The data were expressed as mean±SEM. Statistical significance was calculated by using the one-way ANOVA, followed by Tukey's procedure for multiple comparison. AP<0.05 was considered statistically significant.
Results
MDA concentration was significantly increased in the cerulein-treated rats (pancreatitis group) compared to the non-treated group (control group) (Fig. 1,P<0.05); melatonin administration significantly reduced the levels of MDA (P<0.05) (Fig. 1). Protein oxidation(expressed by protein carbonyl content) was significantly increased in the pancreatitis group compared with the control group, and melatonin injection significantly prevented protein oxidation (Fig. 2,P<0.05). GPx activity was significantly lower in the pancreatitis group than in the control group (Fig. 3,P<0.05). Melatonin administration significantly increased the enzymatic activity (Fig. 3,P<0.05). Compared to the control group, cerulein administration significantly decreased pancreatic total antioxidant status (Fig. 4,P<0.05). Moreover, exogenous administration of melatonin increased significantly pancreatic total antioxidant status in cerulein-treated rats (Fig. 4,P<0.05).

Fig. 1.Lipid peroxidation in pancreatic samples. Rats were injected subcutaneously with four injections of cerulein (Cer) (20 µg/kg body weight) (pancreatitis group) or vehicle (saline solution) (control group) at 2-hour intervals, and melatonin (Cer+Mel) (25 mg/kg body weight) (treatment group) was administered intraperitoneally 30 minutes before each cerulein injection, as indicated in the Methods section. Data are represented as mean± SEM (n=6-8). *:P<0.05, versus control group; #:P<0.05, versus pancreatitis group.
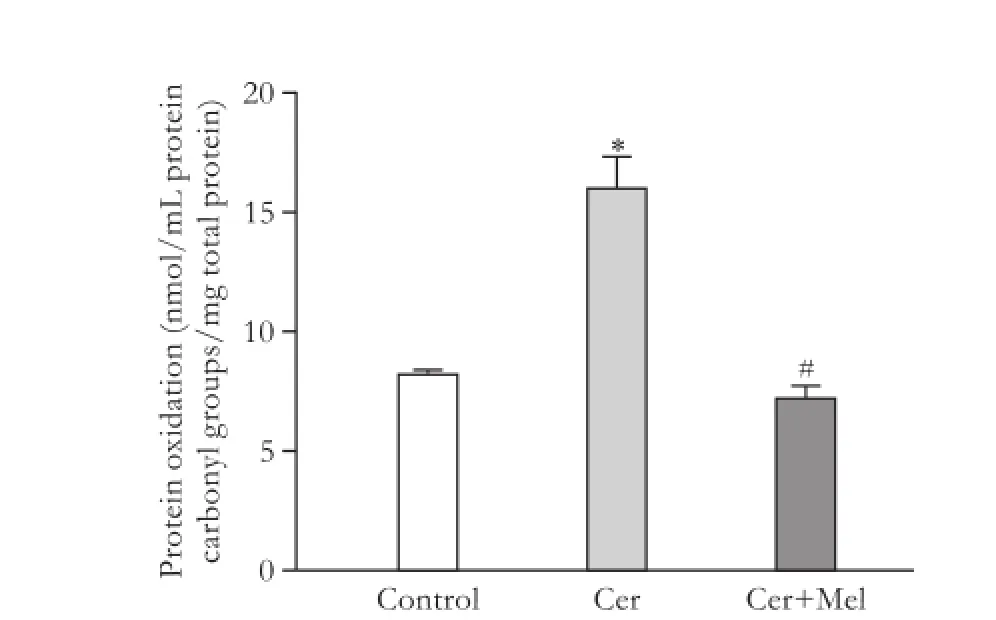
Fig. 2.Protein oxidation in pancreatic samples. Rats were injected subcutaneously with four injections of cerulein (Cer) (20 µg/kg body weight) (pancreatitis group) or vehicle (saline solution) (control group) at 2-hour intervals, and melatonin (Cer+Mel) (25 mg/kg body weight) (treatment group) was administered intraperitoneally 30 minutes before each cerulein injection, as indicated in the Methods section. Data are represented as mean± SEM (n=6-8). *:P<0.05, versus control group; #:P<0.05, versus pancreatitis group.
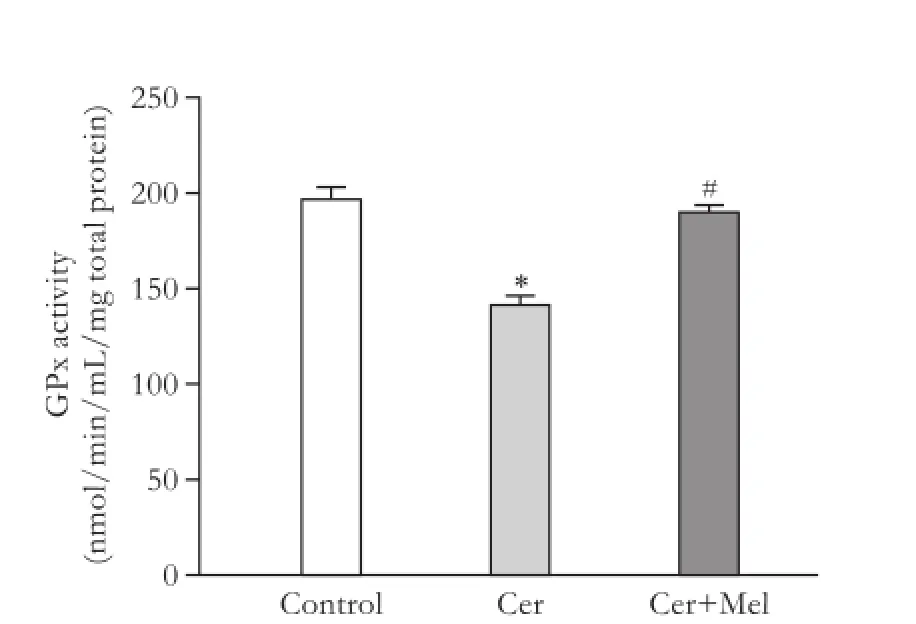
Fig. 3.GPx activity in pancreatic samples. Rats were injected subcutaneously with four injections of cerulein (Cer) (20 µg/kg body weight) (pancreatitis group) or vehicle (saline solution) (control group) at 2-hour intervals, and melatonin (Cer+Mel) (25 mg/kg body weight) (treatment group) was administered intraperitoneally 30 minutes before each cerulein injection, as indicated in the Methods section. Data are represented as mean± SEM (n=6-8). *:P<0.05, versus control group; #:P<0.05, versus pancreatitis group.
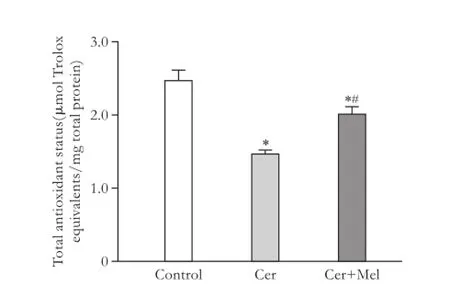
Fig. 4.Total antioxidant status in pancreatic samples. Rats were injected subcutaneously with four injections of cerulein (Cer) (20 µg/kg body weight) (pancreatitis group) or vehicle (saline solution) (control group) at 2-hour intervals, and melatonin (Cer+Mel) (25 mg/kg body weight) (treatment group) was administered intraperitoneally 30 minutes before each cerulein injection, as indicated in the Methods section. Data are represented as mean± SEM (n=6-8). *:P<0.05, versus control group; #:P<0.05, versus pancreatitis group.
Discussion
Since Sanfey et al[21]pointed out the implication of ROS in the pathophysiology of acute pancreatitis, manyin vitroandin vivostudies have confirmed the participation of these highly reactive molecules in the development of the disease. Nevertheless, it is not well understood if free radicals act as initiators or amplifiers of the inflammatory event.[5]
ROS levels as well as the levels of other reactive species resulting from normal cell metabolism are usually balanced by the action of the antioxidant system. Thus, the first line of defense is composed by detoxifying enzymes, such as superoxide dismutase,catalase and GPx. These enzymes metabolize potentially harmful molecules to innocuous byproducts. Loss of equilibrium between oxidants/antioxidants levels in the organism is a situation known as oxidative stress. It has been observed that multiple factors are responsible for the expression and activity of the enzymatic antioxidant system; oxidative status is considered to be the main regulator factor.[22]Without enough defenses, ROS causes structural damages (lipid peroxidation, protein oxidation, etc.) and, consequently, cell dysfunction which leads to some diseases such as acute pancreatitis.[23]
This study confirmed the implication of reactive species at early stage of cerulein-induced acute pancreatitis in rats. In general, it is thought that NADPH oxidase is the major source of ROS in human cells and, in particular, in this experimental model.[8,24]Free radicals could attack some cell components, mainly lipids and proteins. In the present study, MDA levels as well as modified proteins levels were increased in the pancreatitis group. However, concentrations of these markers were diminished in the treatment group, showing the protective effect of the exogenous administration of melatonin on these harmful structural phenomena. Other studies[6,25-27]demonstrated the capacity of melatonin to counteract lipid peroxidation in pancreatic tissue as well as in other antioxidant molecules.[14,17,28-30]Hence, antioxidant treatment protects not just fatty acids in acinar cell membrane, but also zymogen granules integrity and other intracellular lipid structures. As a result, lipid peroxidation-induced toxicity such as increased membrane fluidity, cytosolic efflux, loss of membrane protein activity and even cell death,[4]are minimized. This fact is of importance in the management of inflammatory response, considering that fatty acids of cell membranes are precursors of inflammatory mediators (e.g. prostaglandins).[31]Additionally, lipid peroxidation in the endothelial layer of capillaries and venules is also reduced.[32]At the same time, the dysfunction of membrane proteins, derived from the lipid peroxidation phenomenon, is aggravated for the direct attack of ROS to these essential cellular components. The present study is the first to show that melatonin pre-treatment diminished elevated levels of carbonyl groups in a cerulein-induced acute pancreatitis. This protective effect was also demonstrated in different animal models.[33,34]Thus, protein oxidation is another early event that could be prevented by antioxidant before treatment.
Obviously, the antioxidant defense system is also affected during the development of acute pancreatitis, aggravating the progression of the disease. In agreement with the previous studies,[6,27]our results showed that GPx levels are depressed during acute pancreatitis. Moreover, total antioxidant status seems to be reduced in rats treated with cerulein; thus, in our study the pancreatitis group had a compromised antioxidant capacity compared with the other two groups. Nevertheless, this work confirmed that pre-treatment with melatonin was able to make the concentration of these biomarkers in pancreatic tissue return to the control levels. It has been documented that melatonin regulates antioxidant defense expression[23]through influence on the function of important transcription factors such as NF-κB.[6]Franco et al[22]pointed out that the response of antioxidant enzyme genes to oxidative stress differs in a tissue specific manner. The balance between positive and negative regulatory factors determines different patterns of gene induction according to oxidative stimuli and tissues. On the other hand, stimulationin vitroof antioxidant enzyme genes occurs at nanomolar concentration of melatonin in cell culture, being far from the non-physiological doses administered to rats in experimental models of acute pancreatitis.[23]Thus, antioxidant effect of melatonin in acute pancreatitis is related not just to its scavenger abilities, but also to a direct stimulation of antioxidant defense expression.
In conclusion, the imbalance of oxidative stress and antioxidants plays an important role in cerulein-induced acute pancreatitis. Melatonin, a polyvalent antioxidant, significantly decreases oxidative stress and increases the activities of antioxidant enzymes in the pancreatitis model of rats while protecting from pancreatic damage.
Contributors:PJA proposed the study. CC and RAB performed research and wrote the first draft. CC collected and analyzed data. All authors contributed to the design and interpretation of the study and to further drafts. PJA is the guarantor.
Funding:This study was supported by grants from MICINNFEDER (BFU2010-15049), Gobierno de Extremadura (Re: GRU10003) and Plan of Recruitment and Training of Human Resources on Research of University of Extremadura (1076).
Ethical approval:The study was approved by Bioethical Committee for Animal Experimentation of the University of Extremadura.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Dabrowski A, Konturek SJ, Konturek JW, Gabryelewicz A. Role of oxidative stress in the pathogenesis of caeruleininduced acute pancreatitis. Eur J Pharmacol 1999;377:1-11.
2 Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal 2009;11:135-165.
3 Park BK, Chung JB, Lee JH, Suh JH, Park SW, Song SY,et al. Role of oxygen free radicals in patients with acute pancreatitis. World J Gastroenterol 2003;9:2266-2269.
4 Armstrong JA, Cash N, Soares PM, Souza MH, Sutton R, Criddle DN. Oxidative stress in acute pancreatitis: lost in translation? Free Radic Res 2013;47:917-933.
5 Closa D. Free radicals and acute pancreatitis: much ado about… something. Free Radic Res 2013;47:934-940.
6 Jung KH, Hong SW, Zheng HM, Lee HS, Lee H, Lee DH, et al. Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J Pineal Res 2010;48:239-250.
7 Kim H, Seo JY, Roh KH, Lim JW, Kim KH. Suppression of NF-kappaB activation and cytokine production by N-acetylcysteine in pancreatic acinar cells. Free Radic Biol Med 2000;29:674-683.
8 Yu JH, Kim H. Role of janus kinase/signal transducers and activators of transcription in the pathogenesis of pancreatitis and pancreatic cancer. Gut Liver 2012;6:417-422.
9 Ramudo L, Manso MA, Vicente S, De Dios I. Pro- and anti-inflammatory response of acinar cells during acute pancreatitis. Effect of N-acetyl cysteine. Cytokine 2005;32: 125-131.
10 Fantini L, Tomassetti P, Pezzilli R. Management of acute pancreatitis: current knowledge and future perspectives. World J Emerg Surg 2006;1:16.
11 Esrefoglu M. Experimental and clinical evidence of antioxidant therapy in acute pancreatitis. World J Gastroenterol 2012;18: 5533-5541.
12 Carrasco C, Marchena AM, Holguín-Arévalo MS, Martín-Partido G, Rodríguez AB, Paredes SD, et al. Anti-inflammatory effects of melatonin in a rat model of caerulein-induced acute pancreatitis. Cell Biochem Funct 2013;31:585-590.
13 Jaworek J, Szklarczyk J, Jaworek AK, Nawrot-Porąbka K, Leja-Szpak A, Bonior J, et al. Protective effect of melatonin on acute pancreatitis. Int J Inflam 2012;2012:173675.
14 Carrasco C, Holguín-Arévalo MS, Martín-Partido G, Rodríguez AB, Pariente JA. Chemopreventive effects of resveratrol in a rat model of cerulein-induced acute pancreatitis. Mol Cell Biochem 2014;387:217-225.
15 Li ZD, Ma QY, Wang CA. Effect of resveratrol on pancreatic oxygen free radicals in rats with severe acute pancreatitis. World J Gastroenterol 2006;12:137-140.
16 Szabolcs A, Varga IS, Varga C, Berkó A, Kaszaki J, Letoha T, et al. Beneficial effect of resveratrol on cholecystokinin-induced experimental pancreatitis. Eur J Pharmacol 2006;532:187-193.
17 Ozkan E, Akyüz C, Dulundu E, Topaloğlu U, Sehirli AÖ, Ercan F, et al. Protective effects of lycopene on ceruleininduced experimental acute pancreatitis in rats. J Surg Res 2012;176:232-238.
18 Kang M, Park KS, Seo JY, Kim H. Lycopene inhibits IL-6 expression in cerulein-stimulated pancreatic acinar cells. Genes Nutr 2011;6:117-123.
19 Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol Sci 2004;82: 614-619.
20 Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-254.
21 Sanfey H, Bulkley GB, Cameron JL. The role of oxygenderived free radicals in the pathogenesis of acute pancreatitis. Ann Surg 1984;200:405-413.
22 Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med 1999;27:1122-1132.
23 Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 2004;36:1-9.
24 Brieger K, Schiavone S, Miller FJ Jr, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly 2012;142:w13659.
25 Col C, Dinler K, Hasdemir O, Buyukasik O, Bugdayci G. Oxidative stress and lipid peroxidation products: effect of pinealectomy or exogenous melatonin injections on biomarkers of tissue damage during acute pancreatitis. Hepatobiliary Pancreat Dis Int 2010;9:78-82.
26 Jaworek J, Leja-Szpak A, Bonior J, Nawrot K, Tomaszewska R, Stachura J, et al. Protective effect of melatonin and its precursor L-tryptophan on acute pancreatitis induced by caerulein overstimulation or ischemia/reperfusion. J Pineal Res 2003;34:40-52.
27 Jaworek J, Zwirska-Korczala K, Szklarczyk J, Nawrot-Porąbka K, Leja-Szpak A, Jaworek AK, et al. Pinealectomy aggravates acute pancreatitis in the rat. Pharmacol Rep 2010;62:864-873.
28 Zhang DQ, Feng H, Chen WC. Effects of hydrogen-rich saline on taurocholate-induced acute pancreatitis in rat. Evid Based Complement Alternat Med 2013;2013:731932.
29 Carvalho KM, Morais TC, de Melo TS, de Castro Brito GA, de Andrade GM, Rao VS, et al. The natural flavonoid quercetin ameliorates cerulein-induced acute pancreatitis in mice. Biol Pharm Bull 2010;33:1534-1539.
30 Lima PR, de Melo TS, Carvalho KM, de Oliveira íB, Arruda BR, de Castro Brito GA, et al. 1,8-cineole (eucalyptol) ameliorates cerulein-induced acute pancreatitis via modulation of cytokines, oxidative stress and NF-κB activity in mice. Life Sci 2013;92:1195-1201.
31 Kuliaviene I, Gulbinas A, Cremers J, Pundzius J, Kupcinskas L, Dambrauskas Z, et al. Fatty acids of erythrocyte membrane in acute pancreatitis patients. World J Gastroenterol 2013;19: 5678-5684.
32 Qi W, Tan DX, Reiter RJ, Kim SJ, Manchester LC, Cabrera J, et al. Melatonin reduces lipid peroxidation and tissue edema in cerulein-induced acute pancreatitis in rats. Dig Dis Sci 1999;44:2257-2262.
33 Sener G, Tosun O, Sehirli AO, Kaçmaz A, Arbak S, Ersoy Y, et al. Melatonin and N-acetylcysteine have beneficial effects during hepatic ischemia and reperfusion. Life Sci 2003;72: 2707-2718.
34 Atilgan D, Parlaktas BS, Uluocak N, Erdemir F, Firat F, Erkorkmaz U, et al. Effects of melatonin on partial unilateral ureteral obstruction induced oxidative injury in rat kidney. Urol Ann 2012;4:89-93.
Received January 27, 2014
Accepted after revision April 20, 2014
Author Affiliations: Department of Physiology, Neuroimmunophysiology and Chrononutrition Research Group, University of Extremadura, Badajoz 06006, Spain (Carrasco C, Rodríguez AB and Pariente JA)
José A Pariente, PhD, Neuroimmunophysiology and Chrononutrition Research Group; Department of Physiology, Faculty of Science, University of Extremadura, Badajoz 06006, Spain (Tel: +34-924-289300ext86956; Fax: +34-924-289388; Email: pariente@unex.es)
© 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60271-X
Published online June 23, 2014.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Prostacyclin decreases splanchnic vascular contractility in cirrhotic rats
- A modified protocol with rituximab and intravenous immunoglobulin in emergent ABO-incompatible liver transplantation for acute liver failure
- Ultrasonic integrated backscatter in assessing liver steatosis before and after liver transplantation
- Liver transplantation using organs from deceased organ donors: a single organ transplant center experience
- Pancreaticoduodenectomy and pancreaticoduodenectomy combined with superior mesentericportal vein resection for elderly cancer patients
- A matched-pair analysis of laparoscopic versus open pancreaticoduodenectomy: oncological outcomes using Leeds Pathology Protocol
