Stathmin destabilizing microtubule dynamics promotes malignant potential in cancer cells by epithelial-mesenchymal transition
2014-05-04YuLuChenLiuYongFengXuHeChengSiShiChunTaoWuandXianJunYu
Yu Lu, Chen Liu, Yong-Feng Xu, He Cheng, Si Shi, Chun-Tao Wu and Xian-Jun Yu
Shanghai, China
Stathmin destabilizing microtubule dynamics promotes malignant potential in cancer cells by epithelial-mesenchymal transition
Yu Lu, Chen Liu, Yong-Feng Xu, He Cheng, Si Shi, Chun-Tao Wu and Xian-Jun Yu
Shanghai, China
BACKGROUND:Stathmin is a ubiquitous cytosolic regulatory phosphoprotein and is overexpressed in different human malignancies. The main physiological function of stathmin is to interfere with microtubule dynamics by promoting depolymerization of microtubules or by preventing polymerization of tubulin heterodimers. Stathmin plays important roles in regulating many cellular functions as a result of its microtubuledestabilizing activity. Currently, the critical roles of stathmin in cancer cells, as well as in lymphocytes have been valued. This review discusses stathmin and microtubule dynamics in cancer development, and hypothesizes their possible relationship with epithelial-mesenchymal transition (EMT).
DATA SOURCES:A PubMed search using such terms as "stathmin", "microtubule dynamics", "epithelial-mesenchymal transition", "EMT", "malignant potential" and "cancer" was performed to identify relevant studies published in English. More than 100 related articles were reviewed.
RESULTS:The literature clearly documented the relationship between stathmin and its microtubule-destabilizing activity of cancer development. However, the particular mechanism is poorly understood. Microtubule disruption is essential for EMT, which is a crucial process during cancer development. As a microtubule-destabilizing protein, stathmin may promote malignant potential in cancer cells by initiating EMT.
CONCLUSIONS:We propose that there is a stathminmicrotubule dynamics-EMT (S-M-E) axis during cancer development. By this axis, stathmin together with itsmicrotubule-destabilizing activity contributes to EMT, which stimulates the malignant potential in cancer cells.
(Hepatobiliary Pancreat Dis Int 2014;13:386-394)
stathmin;
microtubule dynamics;
epithelial-mesenchymal transition;
malignant potential;
cancer
Introduction
Stathmin, also known as OP18 (oncoprotein 18), is one of the members of microtubule-destabilizing proteins expressed ubiquitously in vertebrates. Stathmin integrates diverse signaling pathways involved in cell proliferation, differentiation and other cell functions.[1]The microtubule-destabilizing activity of stathmin is mainly modulated by phosphorylation, while recent studies[2,3]also demonstrated that signal transducer and activator of transcription 3 (STAT3) can antagonize stathmin's activity by direct binding to its COOH-terminal. Microtubule constitutes one of the major components of the cytoskeleton of eukaryotic cells and is involved in many essential cellular processes, including cell division, ciliary and flagellar motility and intracellular transport.[4]A large number of researches[5-7]revealed that microtubule dynamics could contribute to oncogenic epithelial-mesenchymal transition (EMT). Stathmin, an important microtubule dynamics regulator, has been linked to the promotion of malignant potentials in cancer cells[8-10]as a result of its overexpression in a wide range of cancers. However, whether stathmin contributes to EMT, which plays a critical role in tumor invasiveness, distant dissemination and drug resistance,[11-13]is not clear. We hypothesize that stathmin-related microtubule dynamics might be associated with the malignant potentials of cancer cellsby promoting EMT in tumors such as the stroma-rich pancreatic cancer.[14,15]In this review, we focus on the relationship between stathmin and its microtubuledestabilizing activity in cancer development, as well as the possible correlation with EMT.
Microtubule dynamics
Microtubules are dynamic tubulin polymers and are required for a wide variety of central cellular functions, including mitosis, intracellular transport, polarity, and motility.[16-21]During the interphase of cell cycle, microtubule mainly contributes to intracellular trafficking of vesicles and organelles. When cell turns into mitosis, microtubules become the major component of mitotic spindle which enables correct chromosome segregation.[22]Microtubules are polarized structures composed of the combination of α/β-tubulin heterodimers.[23]The β-tubulin subunit always exposed to the fast growing plus end of microtubule and α-tubulin subunit exposed to the slow growing minus end.[24]There is a transition between growth and shrinkage within the polar ends of microtubule, which is referred to as dynamic instability. The transition from growth to shrinkage is called catastrophe, while a reverse transition is named rescue (Fig. 1A).[25]As is well-known, the growing plus end of microtubule contains a cap of tubulin with GTP in the β-tubulin unit. When GTPs in the cap hydrolyze into GDPs, catastrophe happens (Fig. 1B).[26,27]Highly regulated microtubule dynamics is essential to diverse of intracellular functions. Unbalanced dynamic properties of microtubule can give rise to abnormal mitotic spindle formation, and to the inability to properly segregate chromosomes, thereby leading to aneuploidy, and possibly tumorigenesis.[28]
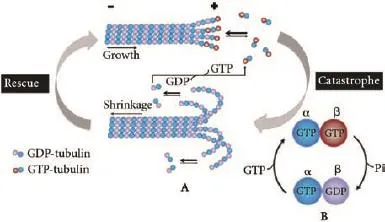
Fig. 1. Microtubule dynamics. A: There is a conformational transition between growth and shrinkage within the microtubule polar ends. The transition from growth to shrinkage is named catastrophe, the opposite event is named rescue. The tip of the growing plus end contains a GTP-cap in the β-tubulin subunit. Hydrolysis of the GTP-cap leads to microtubule catastrophe. B: GTP in α-tubulin subunit never hydrolyzes; GTP in the β-tubulin subunit can hydrolyze into GDP.
Stathmin and its microtubule-destabilizing activity
The dynamics of microtubule polymerization and depolymerization is regulated by two major classes of proteins: microtubule stabilizers and microtubule destabilizers.[29]As microtubule stabilizers, microtubuleassociated proteins were identified to bind the body of microtubule and enhance its stability. Microtubule destabilizers such as stathmin and XKCM1 were capable of binding free tubulin heterodimers or promoting catastrophe frequency.[30]
Stathmin is a 149 amino-acids cytosoluble protein which is highly conserved in vertebrates. There has been a debate over the specific mechanism of how stathmin destabilizing microtubules. Stathmin was initially found to act as a catastrophe promoter in regulating microtubule dynamics.[31]It was demonstrated that stathmin could sequester tubulin heterodimers into a tight complex and thus decrease the amount of free tubulin for microtubule assembly. The reduced free tubulin concentration slows down the growth rate of microtubule and thus, increases the catastrophe frequency within the microtubule polar ends. However, a further study indicated that stathmin sequestering tubulin slowed down the growth rate of microtubule but not promote catastrophes.[32]Later in vitro experiments performed by Howell et al[33]showed that the microtubule regulating activity of stathmin can be influenced by the buffer pH. At a pH of 6.8, stathmin could mainly function as a tubulin sequester. When the pH increases to 7.5, stathmin acts as a catastrophe promoter. At this point, stathmin primarily stimulates GTP hydrolysis by its direct binding to the plus end of microtubule (Fig. 2). Those authors also investigated the two roles of stathmin protein. NH2-terminal promotes catastrophe while COOH-terminal sequesters tubulin.
The microtubule-destabilizing activity of stathmin can be down-regulated in a multiple phosphorylation manner by a variety of kinases.[34]Four phosphorylation serine sites have been identified within the NH2-terminal regulatory domain: Ser16, Ser25, Ser38, and Ser63.[1]Ser16 is mainly phosphorylated by the Ca2+/ calmodulindependent kinase IV/Gr (CaMK IV/ Gr), and Ser25 by members of the mitogen-activated protein kinase (MAPK) family.[35,36]Ser38 is a target for phosphorylation by cyclin-dependent kinases (CDKs) and Ser63 is themajor site for protein kinase A (PKA).[37]It was revealed that phosphorylated Ser16 and Ser63 lead to a stronger impairment of stathmin microtubule-destabilizing activity than Ser25 and Ser38 do.[38]Non-phosphorylated stathmin (S) can sequester free α/β-tubulin (T) and generate a stable and "assembly incompetent" ternary T2S complex.[32,39]By combining tubulins in a curved complex that is not incorporated in microtubules, stathmin degrades the "assembly competent" tubulins[40]so as to prevent the polymerization of tubulin heterodimers or to promote depolymerization of microtubules. Meanwhile, microtubule assembles itself resulting in an increased stathmin phosphorylation. These processes might provide a positive feedback loop for microtubule formation.[41]A recent study[3]indicated that STAT3 could antagonize the tubulin-sequestering activity of stathmin by directly binding to its COOH-terminal (Fig. 3).
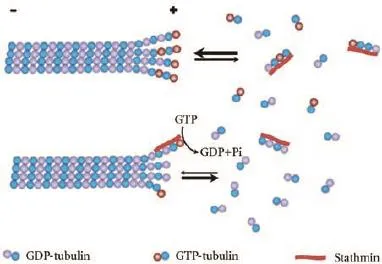
Fig. 2.Microtubule-destabilizing activity of stathmin. Stathmin is proposed to bind to free tubulin heterodimers so as to prevent their polymerization. Stathmin could also directly bind to the microtubule plus end and stimulate GTP hydrolysis so as to induce microtubule depolymerization.
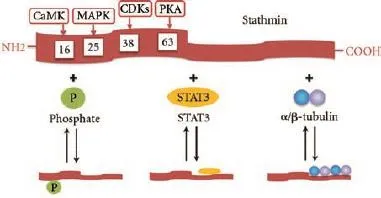
Fig. 3.Structure and regulating activity of stathmin. Stathmin can be phosphorylated at its four phosphorylation serine sites have been identified within its NH2-terminal domain. Stathmin can sequester free α/β-tubulin and generate a stable and "assembly incompetent" T2S complex by its COOH-terminal domain. Phosphorylated stathmin or STAT3-binding stathmin is incapable to sequester free α/β-tubulin.
Expression of stathmin in cancer
In recent years, many studies have confirmed that stathmin was overexpressed in different human malignancies, such as leukemia,[42,43]lymphoma,[44]cervical cancer,[45,46]breast cancer,[47-49]prostate cancer,[50,51]lung cancer,[9]osteosarcoma,[52]and gastrointestinal tumors.[53,54]In cervical carcinoma tissues, the expression levels of stathmin mRNA and protein were significantly higher than those in adjacent non-cancerous margin samples. Besides, a high stathmin expression was significantly related to the diameter of the primary tumor, the clinical TNM classification and the long-term survival rate, suggesting that stathmin was involved in cervical carcinogenesis and tumor progression. Those results indicated that stathmin could be used as a prognostic indicator in patients with cervical carcinoma.[46]Likewise, in breast carcinoma,[48]localized upper urinary tract urothelial carcinoma,[55]hepatocellular carcinoma,[56]oral squamous-cell carcinoma,[57]medulloblastoma[58]and adenoid cystic carcinoma of the salivary glands,[59]high stathmin expression is associated with cancer progression and prognosis.
Stathmin and cell cycle
Stathmin plays an critical role in the cell cycle progress.[60]In interphase, microtubule nucleation rate from centrosome was greatly influenced by stathmin. High stathmin level impaired nucleation, whereas low stathmin level enhanced this process.[61]At the beginning of mitotic phase, phosphorylated stathmin was significantly increased in the K562 erythroleukemia cells.[62]A recent study[63]showed that stathmin phosphorylation is remarkably lower in silent cells than that in proliferating cells. They also demonstrated that phosphorylation of stathmin can be regulated by P34cdc2, a dominating protein kinase controlling the entry of mitosis in eukaryotic cells. Marklund et al[64]investigated the effects of stathmin overexpression of wild type and P34cdc2-target site deficient mutant on the morphological changes of the mitotic spindle, the results demonstrated that induced expression of wild-type stathmin mainly results in microtubule depolymerization in interphase. Phosphorylation switches off stathmin microtubuledepolymerizing activity and promotes the assembly of the mitotic spindle at the onset of mitosis.[64,65]All those observations suggested that stathmin is inactivatedby phosphorylation which plays an important role in the regulation of mitotic spindle formation during cell cycle progression. Mistry and Atweh[66]examined the effects of okadaic acid, an inhibitor of serine/threonine protein phosphatases, on parameters of mitosis with or without inhibition of stathmin. They found that dephosphorylation of stathmin is necessary for mitotic spindle disassembly and the proper exit from mitosis.
Recent reports[67-69]also illustrated that stathmin was related to p53 in regulating cell cycles. In HCT116 cell lines and Hela cells, depletion of stathmin by siRNA or shRNA leads to a G2/M blockade while depletion of both p53 and stathmin displayed a blockade in G2 phase. It is worth noting that up-regulated p53 restrains stathmin expression and induces the G2/M delay,[70]both stathmin and p53 are necessary for the G2/M cell cycle progression.
Stathmin and cell proliferation/differentiation
High expression of stathmin was found in undifferentiated multipotent cells or initial stage tissues.[71-73]However, stathmin expression decreased dramatically in most adult tissues. Similar results were found in the liver regeneration model.[74,75]Stathmin was rarely expressed in mature liver tissue; partial hepatectomy significantly increases the level of stathmin. At a peek around 60 hours postoperation, the expression decreased in the next few days. It appears that stathmin expression prevents uncontrolled cell growth before initiation of cell differentiation. The finding seems to be inconsistent with the fact that stathmin was overexpressed in high proliferative tumors. With the theories above, we propose that the expression of stathmin may prevent the normal cell from overproliferation, this effect is not sufficient enough in cancer cells. The specific molecular mechanism remains unclear. Recent studies[45,76,77]revealed a relationship between stathmin phosphorylation and the PI3K pathway, which is involved in cellular functions such as cell growth, proliferation/differentiation and apoptosis. Stathmin may be a component of the PI3K pathway in cell proliferation and differentiation.
Stathmin and cell motility
Studies[21,78]showed that microtubule stability takes part in the regulation of cell motility. Therefore, as a critical microtubule-destabilizing protein, stathmin is involved in the regulation of cell motility, particularly in cancer cells. Belletti et al[10]evaluated the stathmin expression in human sarcomas and demonstrated that stathmin stimulated cell motility via extracellular matrixin vitroand contributed to the local invasion and distant dissemination. Besides, a range of cellular proteins were reported to be in concert with stathmin to influence the cell motility-stimulating activity.[8]Singer et al[9]considered the far upstream sequence element-binding protein-1 as a key molecule in the induction of different stathmin family members. Their studies indicated that far upstream sequence elementbinding protein-1 and overexpression of microtubuledestabilizing factors such as stathmin is a pivotal process to destabilize microtubule dynamics and further increases proliferative and motile ability of malignant cells.
Stathmin might contribute to the activation and migration of T cells. T cell activation and polarization were involved in intracellular material transportations, promoting the formation of the immunological synapse and/or facilitating the directed cytokines secretion.[79-81]Reposition of microtubule-organizing center is critical for the activation of T cells. Filbert et al[82]demonstrated that activated extracellular signal-regulated kinase which located in immunological synapses could contribute to microtubule-organizing center polarization as a result of inducing phosphorylation of stathmin. Deletion of stathmin leads to detention of microtubuleorganizing center reposition and thus impairs cytotoxic T lymphocyte cytolysis. Verma et al[2]reported that STAT3 is critical for T cell migration by interacting with stathmin and interfering its microtubule-destabilizing activities.
Stathmin and drug resistance
Anti-cancer chemotherapeutics used in the clinic such as taxanes and vinca alkaloids are classified as microtubule stabilizers or destabilizers.[83-88]These drugs inhibit mitosis through a similar mechanism of slowing microtubule dynamics, resulting in mitotic arrest and apoptosis, thus limiting the cell proliferation. As a microtubule-destabilizing protein, stathmin plays a special role in the drug resistance. Using a set of human breast cancer cell lines, Alli et al[89]found that there are at least two different ways for stathmin to influence the action of anti-microtubule chemotherapy drugs, i.e. directly interfere drug-tubulin binding and growth arrest at G2/M phase during a cell cycle. Combining antisense to target certain proteins and chemotherapeutic agents, Benner and colleagues demonstrated that some proteins are essential for the malignant phenotype.[90]
A hypothetical S-M-E axis
Overexpression of stathmin and its microtubule-destabilizing activity contribute to cell proliferation, differentiation, motility and drug resistance during cancer development. However, the underlying mechanism is still poorly understood.
Microtubule is an essential component of cytoskeleton. Highly organized microtubules are required for many cellular functions including the maintenance of cell morphology, intracellular material transportation, cell motility and differentiation.[10]Microtubule disruption leads to the break-down of basement membrane, which is the first step of EMT process.[5]EMT is a crucial mechanism for embryogenesis, forming fibroblasts and/or mesenchymal cells in injured tissues, and activating metastasis of epithelial cancer cells.[91]Abnormal activation of EMT contributes to pathologic changes, including fibrosis, neoplasia and cancer development.[92-96]During the EMT process, epithelial cells usually lose the capability of intercellular adhesion and acquire a mesenchymal phenotype,[97-99]which requires the modification in cellular architecture, morphology, adhesion, and migration capacity.[100,101]The key point of EMT is the down-regulation of E-cadherin expression, a crucial step reducing the cell-cell adhesion and leading to remolding of the cytoskeleton.[102,103]Moreover, the acquisition of a motile and invasiveness may result in the tumor resistance to chemotherapy.[104-106]For instance, pancreatic cancer is characterized by an abundant tumor stroma resulted from EMT. Tumor stroma contributes to a majority of malignant biological features, such as dissemination at early stages and resistance to chemotherapeutics.[13-15]As a microtubule-destabilizing protein that involved with various oncogenic functions, stathmin might promote the EMT process through regulating microtubule dynamics in the development of cancer.
Direct proof on the contribution of stathmin to EMT is difficult to conduct. Li et al[7]performed a series of researches utilizing Siva1 to examine the role of stathmin and microtubule dynamics in promoting EMT. They proved that Siva1 counteracts stathmin by impeding the interaction between stathmin and tubulin and/or promoting CaMK II-mediated phosphorylation of stathmin at Ser16. They further investigated the role of Siva1 in EMT and found that Siva1 overexpression suppresses cell migration and EMT, whereas knockdown of Siva1 reverses this effect. However, this EMT-regulating activity of Siva1 vanished when stathmin was knocked-out. These findings suggest that Siva1 may suppress EMT by attenuate the activity of stathmin. This is an indirect proof of the role of stathmin in promoting EMT.
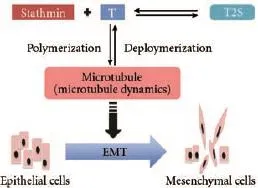
Fig. 4.The hypothetical S-M-E axis. Stathmin may promote microtubule disruption by its microtubule-destabilizing activity; microtubule disruption causes the depolarization of cell shape, cell locomotion which is involved in EMT process thereby contributing to high invasive and metastatic characteristics of cancer. The dashed line with arrow indicates the progress is putative or involves multiple steps.
We suppose that there is a stathmin-microtubule dynamics-EMT (S-M-E) axis in the development of cancer (Fig. 4). The axis might work mainly in three steps. First, up-regulated non-phosphorylated stathmin sequesters tubulin heterodimers so as to promote depolymerization of microtubules or prevent polymerization of tubulin heterodimers. Second, such microtubule destabilizing activity of stathmin promotes EMT in cells. Third, EMT contributes to the aggressive features of malignant cells, including high invasiveness and drug resistance.
S-M-E axis as a potential target for cancer therapy
Besides the high invasiveness and metastasis propensity, the poor response to existing treatments contributes a large part to the lethality of human malignancies.[107]As mentioned above, stathmin was overexpressed in many cancers and significantly related to the prognosis of cancer patients. Stathmin and its microtubuledestabilizing activity contribute to malignant potentials in cancer cells. All those facts strongly suggest that stathmin is a promising target for more effective therapeutic interventions.[108-110]Microtubule disruption contributes greatly to cellular morphological changes, and to the EMT process, which is involved in the development of diverse solid human malignancies.[111-113]As a result, targeting microtubule and EMT may also provide novel anti-cancer therapies. Clinically, multiple anti-cancer drugs like taxanes and vinca alkaloids targeting microtubule dynamics were widely used.[83-88]The microtubule-destabilizing activity of stathmin contributes to the resistance of tumors to chemotherapy,[89]and can be targeted by numerous ofchemotherapy agents. The S-M-E axis was supposed to be an important pathway in cancer development, including the invasiveness and drug resistance. Consequently, we speculate that the S-M-E axis may be a potential target in anti-cancer therapies.
Conclusion and perspectives
The microtubule-destabilizing protein stathmin was involved in cancer development. However, the specific molecular mechanism remains unclear. We reviewed the available studies and summarized the role of stathmin and its microtubule destabilizing activity in the process of cancer development. Microtubule disruption causes the depolarization of cell shape, cell locomotion which is involved in EMT process. All of these suggest that stathmin destabilizes microtubule which promotes malignant potential in cancer cells. We proposed a stathmin-microtubule dynamics-EMT (S-M-E) axis: stathmin and its microtubule-depolymerizing activity lead to the EMT progress during cancer development. The S-M-E axis can be involved in tumor invasion, metastasis and drug resistance. Further studies are required to investigate the specific mechanism of this axis. Targeting the S-M-E axis may provide novel therapy for human malignancies.
Contributors:LY and LC wrote the main body of the article and contributed equally to this article. YXJ revised the manuscript. All authors contributed to the design and interpretation of the study and to further drafts. YXJ is the guarantor.
Funding:This work was supported by grants from the National Natural Science Foundation of China (81172276, 81001058, 8110156, Sino-German GZ857) and the Shanghai Committee of Science and Technology, China (11JC1402500).
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Curmi PA, Gavet O, Charbaut E, Ozon S, Lachkar-Colmerauer S, Manceau V, et al. Stathmin and its phosphoprotein family: general properties, biochemical and functional interaction with tubulin. Cell Struct Funct 1999;24:345-357.
2 Verma NK, Dourlat J, Davies AM, Long A, Liu WQ, Garbay C, et al. STAT3-stathmin interactions control microtubule dynamics in migrating T-cells. J Biol Chem 2009;284:12349-12362.
3 Ng DC, Lin BH, Lim CP, Huang G, Zhang T, Poli V, et al. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J Cell Biol 2006;172: 245-257.
4 Vasiliev JM, Samoylov VI. Regulatory functions of microtubules. Biochemistry (Mosc) 2013;78:37-40.
5 Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol 2008;10:765-775.
6 Oyanagi J, Ogawa T, Sato H, Higashi S, Miyazaki K. Epithelial-mesenchymal transition stimulates human cancer cells to extend microtubule-based invasive protrusions and suppresses cell growth in collagen gel. PLoS One 2012;7: e53209.
7 Li N, Jiang P, Du W, Wu Z, Li C, Qiao M, et al. Siva1 suppresses epithelial-mesenchymal transition and metastasis of tumor cells by inhibiting stathmin and stabilizing microtubules. Proc Natl Acad Sci U S A 2011;108:12851-12856.
8 Ringhoff DN, Cassimeris L. Gene expression profiles in mouse embryo fibroblasts lacking stathmin, a microtubule regulatory protein, reveal changes in the expression of genes contributing to cell motility. BMC Genomics 2009;10:343.
9 Singer S, Malz M, Herpel E, Warth A, Bissinger M, Keith M, et al. Coordinated expression of stathmin family members by far upstream sequence element-binding protein-1 increases motility in non-small cell lung cancer. Cancer Res 2009;69: 2234-2243.
10 Belletti B, Nicoloso MS, Schiappacassi M, Berton S, Lovat F, Wolf K, et al. Stathmin activity influences sarcoma cell shape, motility, and metastatic potential. Mol Biol Cell 2008;19: 2003-2013.
11 Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelialmesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 2006;172:973-981.
12 Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010;29:4741-4751.
13 Sarkar FH, Li Y, Wang Z, Kong D. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir 2009;64:489-500.
14 Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012;148:349-361.
15 Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem 2007;101:887-907.
16 Avila J. Microtubule functions. Life Sci 1992;50:327-334.
17 Minton K. Mitosis: Microtubule nucleation branches out. Nat Rev Mol Cell Biol 2013;14:192-193.
18 Zhu H, Fang K, Fang G. Mechanism, function and regulation of microtubule-dependent microtubule amplification in mitosis. Mol Cells 2009;27:1-3.
19 Guzik BW, Goldstein LS. Microtubule-dependent transport in neurons: steps towards an understanding of regulation, function and dysfunction. Curr Opin Cell Biol 2004;16:443-450.
20 Scholey JM. Cell motility: multiple microtubule motors. Nature 1990;343:118-120.
21 Satir P, Goltz JS, Wolkoff AW. Microtubule-based cell motility: the role of microtubules in cell motility and differentiation. Cancer Invest 1990;8:685-690.
22 Risinger AL, Giles FJ, Mooberry SL. Microtubule dynamics as a target in oncology. Cancer Treat Rev 2009;35:255-261.
23 Lopez-Fanarraga M, Avila J, Guasch A, Coll M, Zabala JC. Review: postchaperonin tubulin folding cofactors and theirrole in microtubule dynamics. J Struct Biol 2001;135:219-229.
24 Wade RH. On and around microtubules: an overview. Mol Biotechnol 2009;43:177-191.
25 Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 2003;40: 209-227.
26 Quiniou E, Guichard P, Perahia D, Marco S, Mouawad L. An atomistic view of microtubule stabilization by GTP. Structure 2013;21:833-843.
27 Bowne-Anderson H, Zanic M, Kauer M, Howard J. Microtubule dynamic instability: a new model with coupled GTP hydrolysis and multistep catastrophe. Bioessays 2013;35: 452-461.
28 Beghin A, Galmarini CM, Dumontet C. Tubulin folding pathways: implication in the regulation of microtubule dynamics. Curr Cancer Drug Targets 2007;7:697-703.
29 Walczak CE. Microtubule dynamics and tubulin interacting proteins. Curr Opin Cell Biol 2000;12:52-56.
30 McNally FJ. Microtubule dynamics: Controlling split ends. Curr Biol 1999;9:R274-276.
31 Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell 1996;84:623-631.
32 Curmi PA, Andersen SS, Lachkar S, Gavet O, Karsenti E, Knossow M, et al. The stathmin/tubulin interaction in vitro. J Biol Chem 1997;272:25029-25036.
33 Howell B, Larsson N, Gullberg M, Cassimeris L. Dissociation of the tubulin-sequestering and microtubule catastrophepromoting activities of oncoprotein 18/stathmin. Mol Biol Cell 1999;10:105-118.
34 Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol 2002;14:18-24.
35 Melander Gradin H, Marklund U, Larsson N, Chatila TA, Gullberg M. Regulation of microtubule dynamics by Ca2+/ calmodulin-dependent kinase IV/Gr-dependent phosphorylation of oncoprotein 18. Mol Cell Biol 1997;17:3459-3467.
36 Marklund U, Brattsand G, Shingler V, Gullberg M. Serine 25 of oncoprotein 18 is a major cytosolic target for the mitogenactivated protein kinase. J Biol Chem 1993;268:15039-15047.
37 Beretta L, Dobránsky T, Sobel A. Multiple phosphorylation of stathmin. Identification of four sites phosphorylated in intact cells and in vitro by cyclic AMP-dependent protein kinase and p34cdc2. J Biol Chem 1993;268:20076-20084.
38 Manna T, Thrower DA, Honnappa S, Steinmetz MO, Wilson L. Regulation of microtubule dynamic instability in vitro by differentially phosphorylated stathmin. J Biol Chem 2009;284:15640-15649.
39 Jourdain L, Curmi P, Sobel A, Pantaloni D, Carlier MF. Stathmin: a tubulin-sequestering protein which forms a ternary T2S complex with two tubulin molecules. Biochemistry 1997;36:10817-10821.
40 Gigant B, Martin-Barbey C, Curmi PA, Sobel A, Knossow M. The stathmin-tubulin interaction and the regulation of the microtubule assembly. Pathol Biol (Paris) 2003;51:33-38.
41 Küntziger T, Gavet O, Manceau V, Sobel A, Bornens M. Stathmin/Op18 phosphorylation is regulated by microtubule assembly. Mol Biol Cell 2001;12:437-448.
42 Melhem R, Hailat N, Kuick R, Hanash SM. Quantitative analysis of Op18 phosphorylation in childhood acute leukemia. Leukemia 1997;11:1690-1695.
43 Sellin ME, Holmfeldt P, Stenmark S, Gullberg M. Op18/ Stathmin counteracts the activity of overexpressed tubulindisrupting proteins in a human leukemia cell line. Exp Cell Res 2008;314:1367-1377.
44 Meyer N, Prentice DA, Fox MT, Hughes JP. Prolactin-induced proliferation of the Nb2 T-lymphoma is associated with protein kinase-C-independent phosphorylation of stathmin. Endocrinology 1992;131:1977-1984.
45 Wang X, Ren JH, Lin F, Wei JX, Long M, Yan L, et al. Stathmin is involved in arsenic trioxide-induced apoptosis in human cervical cancer cell lines via PI3K linked signal pathway. Cancer Biol Ther 2010;10:632-643.
46 Xi W, Rui W, Fang L, Ke D, Ping G, Hui-Zhong Z. Expression of stathmin/op18 as a significant prognostic factor for cervical carcinoma patients. J Cancer Res Clin Oncol 2009;135:837-846.
47 Bièche I, Lachkar S, Becette V, Cifuentes-Diaz C, Sobel A, Lidereau R, et al. Overexpression of the stathmin gene in a subset of human breast cancer. Br J Cancer 1998;78:701-709.
48 Brattsand G. Correlation of oncoprotein 18/stathmin expression in human breast cancer with established prognostic factors. Br J Cancer 2000;83:311-318.
49 Baquero MT, Hanna JA, Neumeister V, Cheng H, Molinaro AM, Harris LN, et al. Stathmin expression and its relationship to microtubule-associated protein tau and outcome in breast cancer. Cancer 2012;118:4660-4669.
50 Mistry SJ, Bank A, Atweh GF. Targeting stathmin in prostate cancer. Mol Cancer Ther 2005;4:1821-1829.
51 Ghosh R, Gu G, Tillman E, Yuan J, Wang Y, Fazli L, et al. Increased expression and differential phosphorylation of stathmin may promote prostate cancer progression. Prostate 2007;67:1038-1052.
52 Zhang HZ, Gao P, Yan L, Lin F. Significance of stathmin gene overexpression in osteosarcoma cells. Ai Zheng 2004;23:493-496.
53 Ji H, Baldwin GS, Burgess AW, Moritz RL, Ward LD, Simpson RJ. Epidermal growth factor induces serine phosphorylation of stathmin in a human colon carcinoma cell line (LIM 1215). J Biol Chem 1993;268:13396-13405.
54 Tan HT, Wu W, Ng YZ, Zhang X, Yan B, Ong CW, et al. Proteomic analysis of colorectal cancer metastasis: stathmin-1 revealed as a player in cancer cell migration and prognostic marker. J Proteome Res 2012;11:1433-1445.
55 Lin WC, Chen SC, Hu FC, Chueh SC, Pu YS, Yu HJ, et al. Expression of stathmin in localized upper urinary tract urothelial carcinoma: correlations with prognosis. Urology 2009;74:1264-1269.
56 Yuan RH, Jeng YM, Chen HL, Lai PL, Pan HW, Hsieh FJ, et al. Stathmin overexpression cooperates with p53 mutation and osteopontin overexpression, and is associated with tumour progression, early recurrence, and poor prognosis in hepatocellular carcinoma. J Pathol 2006;209:549-558.
57 Kouzu Y, Uzawa K, Koike H, Saito K, Nakashima D, Higo M, et al. Overexpression of stathmin in oral squamous-cell carcinoma: correlation with tumour progression and poor prognosis. Br J Cancer 2006;94:717-723.
58 Kuo MF, Wang HS, Kuo QT, Shun CT, Hsu HC, Yang SH, et al. High expression of stathmin protein predicts a fulminant course in medulloblastoma. J Neurosurg Pediatr 2009;4:74-80.
59 Nakashima D, Uzawa K, Kasamatsu A, Koike H, Endo Y, Saito K, et al. Protein expression profiling identifies maspin andstathmin as potential biomarkers of adenoid cystic carcinoma of the salivary glands. Int J Cancer 2006;118:704-713.
60 Cassimeris L. Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr Opin Cell Biol 1999;11:134-141.
61 Ringhoff DN, Cassimeris L. Stathmin regulates centrosomal nucleation of microtubules and tubulin dimer/polymer partitioning. Mol Biol Cell 2009;20:3451-3458.
62 Luo XN, Mookerjee B, Ferrari A, Mistry S, Atweh GF. Regulation of phosphoprotein p18 in leukemic cells. Cell cycle regulated phosphorylation by p34cdc2 kinase. J Biol Chem 1994;269:10312-10318.
63 Brattsand G, Marklund U, Nylander K, Roos G, Gullberg M. Cell-cycle-regulated phosphorylation of oncoprotein 18 on Ser16, Ser25 and Ser38. Eur J Biochem 1994;220:359-368.
64 Marklund U, Larsson N, Gradin HM, Brattsand G, Gullberg M. Oncoprotein 18 is a phosphorylation-responsive regulator of microtubule dynamics. EMBO J 1996;15:5290-5298.
65 Rubin CI, Atweh GF. The role of stathmin in the regulation of the cell cycle. J Cell Biochem 2004;93:242-250.
66 Mistry SJ, Atweh GF. Stathmin inhibition enhances okadaic acid-induced mitotic arrest: a potential role for stathmin in mitotic exit. J Biol Chem 2001;276:31209-31215.
67 Alli E, Yang JM, Hait WN. Silencing of stathmin induces tumor-suppressor function in breast cancer cell lines harboring mutant p53. Oncogene 2007;26:1003-1012.
68 Sonego M, Schiappacassi M, Lovisa S, Dall'Acqua A, Bagnoli M, Lovat F, et al. Stathmin regulates mutant p53 stability and transcriptional activity in ovarian cancer. EMBO Mol Med 2013;5:707-722.
69 Johnsen JI, Aurelio ON, Kwaja Z, Jörgensen GE, Pellegata NS, Plattner R, et al. p53-mediated negative regulation of stathmin/Op18 expression is associated with G(2)/M cellcycle arrest. Int J Cancer 2000;88:685-691.
70 Carney BK, Cassimeris L. Stathmin/oncoprotein 18, a microtubule regulatory protein, is required for survival of both normal and cancer cell lines lacking the tumor suppressor, p53. Cancer Biol Ther 2010;9:699-709.
71 Doye V, Kellermann O, Buc-Caron MH, Sobel A. High expression of stathmin in multipotential teratocarcinoma and normal embryonic cells versus their early differentiated derivatives. Differentiation 1992;50:89-96.
72 Koppel J, Boutterin MC, Doye V, Peyro-Saint-Paul H, Sobel A. Developmental tissue expression and phylogenetic conservation of stathmin, a phosphoprotein associated with cell regulations. J Biol Chem 1990;265:3703-3707.
73 Doye V, Le Gouvello S, Dobransky T, Chneiweiss H, Beretta L, Sobel A. Expression of transfected stathmin cDNA reveals novel phosphorylated forms associated with developmental and functional cell regulation. Biochem J 1992;287:549-554.
74 Koppel J, Loyer P, Maucuer A, Rehák P, Manceau V, Guguen-Guillouzo C, et al. Induction of stathmin expression during liver regeneration. FEBS Lett 1993;331:65-70.
75 Okazaki T, Himi T, Peterson C, Mori N. Induction of stathmin mRNA during liver regeneration. FEBS Lett 1993;336:8-12.
76 Andersen JN, Sathyanarayanan S, Di Bacco A, Chi A, Zhang T, Chen AH, et al. Pathway-based identification of biomarkers for targeted therapeutics: personalized oncology with PI3K pathway inhibitors. Sci Transl Med 2010;2:43ra55.
77 Karst AM, Levanon K, Duraisamy S, Liu JF, Hirsch MS, Hecht JL, et al. Stathmin 1, a marker of PI3K pathway activation and regulator of microtubule dynamics, is expressed in early pelvic serous carcinomas. Gynecol Oncol 2011;123:5-12.
78 Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic 2004;5:470-477.
79 Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol 2006;7:247-255.
80 Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature 2006;443:462-465.
81 Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 2001;15:751-761.
82 Filbert EL, Le Borgne M, Lin J, Heuser JE, Shaw AS. Stathmin regulates microtubule dynamics and microtubule organizing center polarization in activated T cells. J Immunol 2012;188:5421-5427.
83 Matesanz R, Rodríguez-Salarichs J, Pera B, Canales A, Andreu JM, Jiménez-Barbero J, et al. Modulation of microtubule interprotofilament interactions by modified taxanes. Biophys J 2011;101:2970-2980.
84 Cortes J, Vidal M. Beyond taxanes: the next generation of microtubule-targeting agents. Breast Cancer Res Treat 2012;133:821-830.
85 Warfield RK, Bouck GB. Microtubule-macrotubule transitions: intermediates after exposure to the mitotic inhibitor vinblastine. Science 1974;186:1219-1221.
86 Sajó I. Vinblastine inhibition of microtubule assembly in vitro. Acta Biochim Biophys Acad Sci Hung 1977;12:259-261.
87 Morgan EH, Iacopetta BJ. Vinblastine but not other microtubule inhibitors block transferrin endocytosis and iron uptake by reticulocytes. Clin Exp Pharmacol Physiol 1987;14:119-126.
88 Durkó I, Juhász A. Effects of two microtubuledepolymerizing drugs, vincristine and vinblastine, on porphyrin production by primary neural tissue cultures. Neurochem Res 1990;15:1135-1139.
89 Alli E, Bash-Babula J, Yang JM, Hait WN. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res 2002;62:6864-6869.
90 Benner E, Bishop MR, Agarwal N, Iversen P, Joshi SS. Combination of antisense oligonucleotide and low-dose chemotherapy in hematological malignancies. J Pharmacol Toxicol Methods 1997;37:229-235.
91 Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest 2009;119:1417-1419.
92 López-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 2009;1:303-314.
93 Zhou C, Liu J, Tang Y, Liang X. Inflammation linking EMT and cancer stem cells. Oral Oncol 2012;48:1068-1075.
94 Hofman P, Vouret-Craviari V. Microbes-induced EMT at the crossroad of inflammation and cancer. Gut Microbes 2012;3: 176-185.
95 Neilson EG. Mechanisms of disease: Fibroblasts--a new look at an old problem. Nat Clin Pract Nephrol 2006;2:101-108.
96 Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003;112:1776-1784.
97 Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 2004;118:277-279.
98 Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007;7:415-428.
99 Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 2009;119:1438-1449.
100 Kalluri R, Weinberg RA. The basics of epithelialmesenchymal transition. J Clin Invest 2009;119:1420-1428.
101 Stracke ML, Aznavoorian SA, Beckner ME, Liotta LA, Schiffmann E. Cell motility, a principal requirement for metastasis. EXS 1991;59:147-162.
102 Guarino M. Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol 2007;39:2153-2160.
103 Kee SH, Steinert PM. Microtubule disruption in keratinocytes induces cell-cell adhesion through activation of endogenous E-cadherin. Mol Biol Cell 2001;12:1983-1993.
104 Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, et al. Chronic oxaliplatin resistance induces epithelial-tomesenchymal transition in colorectal cancer cell lines. Clin Cancer Res 2006;12:4147-4153.
105 Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res 2009;69:5820-5828.
106 Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q, Tang F, et al. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res 2009;15: 2657-2665.
107 Shi S, Yao W, Xu J, Long J, Liu C, Yu X. Combinational therapy: new hope for pancreatic cancer? Cancer Lett 2012;317:127-135.
108 Nemunaitis J. Stathmin 1: a protein with many tasks. New biomarker and potential target in cancer. Expert Opin Ther Targets 2012;16:631-634.
109 Rana S, Maples PB, Senzer N, Nemunaitis J. Stathmin 1: a novel therapeutic target for anticancer activity. Expert Rev Anticancer Ther 2008;8:1461-1470.
110 Dong B, Mu L, Qin X, Qiao W, Liu X, Yang L, et al. Stathmin expression in glioma-derived microvascular endothelial cells: a novel therapeutic target. Oncol Rep 2012;27:714-718.
111 Shintani Y, Okimura A, Sato K, Nakagiri T, Kadota Y, Inoue M, et al. Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in nonsmall cell lung cancer. Ann Thorac Surg 2011;92:1794-1804.
112 Foroni C, Broggini M, Generali D, Damia G. Epithelialmesenchymal transition and breast cancer: role, molecular mechanisms and clinical impact. Cancer Treat Rev 2012;38: 689-697.
113 Liu C, Cheng H, Shi S, Cui X, Yang J, Chen L, et al. MicroRNA-34b inhibits pancreatic cancer metastasis through repressing Smad3. Curr Mol Med 2013;13:467-478.
Received April 1, 2013
Accepted after revision September 23, 2013
Author Affiliations: Pancreatic Cancer Institute, Fudan University; Department of Pancreatic and Hepatobiliary Surgery, Fudan University Shanghai Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China (Lu Y, Liu C, Xu YF, Cheng H, Shi S, Wu CT and Yu XJ)
Xian-Jun Yu, MD, PhD, Pancreatic Cancer Institute, Fudan University; Department of Pancreatic and Hepatobiliary Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, China (Tel: +86-21-64175590ext1307; Fax: +86-21-64031446; Email: yuxianjun88@hotmail.com)
© 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60038-2
Published online March 27, 2014.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Effect of external beam radiotherapy on patency of uncovered metallic stents in patients with inoperable bile duct cancer
- Ultrasonic integrated backscatter in assessing liver steatosis before and after liver transplantation
- Liver transplantation using organs from deceased organ donors: a single organ transplant center experience
- Prostacyclin decreases splanchnic vascular contractility in cirrhotic rats
- A matched-pair analysis of laparoscopic versus open pancreaticoduodenectomy: oncological outcomes using Leeds Pathology Protocol
- Effects of melatonin on the oxidative damage and pancreatic antioxidant defenses in ceruleininduced acute pancreatitis in rats
