Optimized One-Step Preparation of a Bioactive Natural Product, Guaiazulene-2,9-dione
2014-04-26CHENGCanlingLIPinglinWANGWeiSHIXuefengZHANGGangZHUHongyanWURongcuiTANGXuliandLIGuoqiang
CHENG Canling, LI Pinglin, WANG Wei, SHI Xuefeng ZHANG Gang ZHU Hongyan WU Rongcui TANG Xuli, and LI Guoqiang
1) Key Laboratory of Marine Drugs of Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, P. R. China
2) TCM and Ethnomedicine Innovation & Development Laboratory, School of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, P. R. China
3) College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, P. R. China
Optimized One-Step Preparation of a Bioactive Natural Product, Guaiazulene-2,9-dione
CHENG Canling1),#, LI Pinglin1),#, WANG Wei2), SHI Xuefeng1), ZHANG Gang1), ZHU Hongyan1), WU Rongcui1), TANG Xuli3),*, and LI Guoqiang1),*
1) Key Laboratory of Marine Drugs of Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, P. R. China
2) TCM and Ethnomedicine Innovation & Development Laboratory, School of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, P. R. China
3) College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, P. R. China
We previously isolated a natural product, namely guaiazulene-2,9-dione showing strong antibacterial activity against Vibrio anguillarum, from a gorgonian Muriceides collaris collected in South China Sea. In this experiment, guaiazulene-2,9-dione was quantitatively synthesized with an optimized one-step bromine oxidation method using guaiazulene as the raw material. The key reaction condition including reaction time and temperature, drop rate of bromine, concentration of aqueous THF solution, respective molar ratio of guaiazulene to bromine and acetic acid, and concentration of guaiazulene in aqueous THF solution, were investigated individually at five levels each for optimization. Combined with the verification test to show the absolute yield of each optimization step, the final optimal condition was determined as: when a solution of 0.025 mmol mL-1guaiazulene in 80% aqueous THF was treated with four volumes of bromine at a drop rate of 0.1 mL min-1and four volumes of acetic acid at -5℃ for three hours, the yield of guaiazulene-2,9-dione was 23.72%. This was the first report concerning optimized one-step synthesis to provide a convenient method for the large preparation of guaiazulene-2,9-dione.
azulene derivative; guaiazulene-2,9-dione; optimization; preparation
1 Introduction
The guaiazulene (GA) (Fig.1) derivatives, with an impressive azulene nucleus consisting of two fused fiveseven bicyclic aromatic rings, showed widely biological effects, such as anti-inflammatory, anti-spasmodic, antibiotic (Chen et al., 2012; Flori et al., 2011), relaxant property (Tanaka et al., 2000), lipid peroxidation-inhibiting (Kourounakis et al., 1997), hydroxyl radical-scavenging (Franchi et al., 2003), as well as cosmetic (Zheng et al., 2001) activities. GA and GA-derived products are very rich in natural resources (Fraga et al., 2008; Blunt et al., 2012), and our early studies have isolated guaiazulene-2,9-dione (Fig.1) as a new natural product from a gorgonian Muriceides collaris collected from South China Sea. Guaiazulene-2,9-dione was found to possess very strong antibacterial activity against Vibrio anguillarum (Shi et al., 2009), but the further studies on its broad spectrum antibacterial activity need a synthesis preparing process. However, there was only one report of Nozoe’s method (Nozoe et al., 1995) concerning the synthesis of guaiazulene-2,9-dione but with a very low yield of just 8%. In present work, we developed an optimized semi-synthesizing process for preparing guaiazulene-2,9-dione with one-step method reaching the yield up to 23.72%.
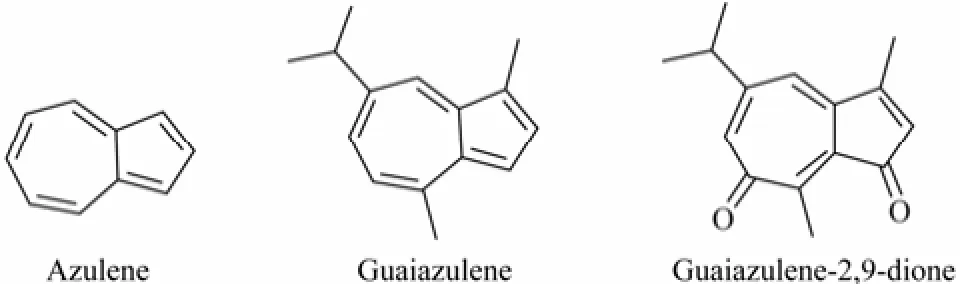
Fig.1 Chemical structure of azulene, guaiazulene and guaiazulene-2,9-dione.
2 Materials and Methods
2.1 Materials and Reagents
The raw material guaiazulene was purchased from Beijing Hengye Zhongyuan Chemical Co., LTD. It was imported with original packaging and the purity of more than 98% was verified by HPLC. The standard materialguaiazulene-2,9-dione was isolated from gorgonian Muriceides collaris collected at South China Sea, and was fully identified by HPLC and NMR. The conventional reagents used in this experiment were analytically pure, while the reagents used in HPLC were chromatography pure.
2.2 Trial Test
The reaction route was showed in Fig.2 as reported early (Nozoe et al., 1995). According to the findings in other studies (Nozoe et al., 1991a, 1991b, 1995), we preliminarily determined the quantity of reactants. The one-step operation process was performed as follows: a solution of guaiazulene (120 mg, 0.61 mmol) in aqueous THF (53%, 14 mL) was added into a solution of Br2(0.1 mL, 1.94 mmol) in THF (2 mL), and the mixture was stirred at -5℃ with acetic acid (0.1 mL) as catalyst. The reaction was monitored by TLC. The experiments were conducted for three times.

Fig.2 One-step synthesizing route of guaiazulene-2,9-dione with guaiazulene as the starting material.
Under the chromatographic condition (Elite, 5 µm, 4.6 × 250 mm, 25℃, λ = 254, 210, 230, 280, 320 nm, MeOH/ H2O 75:25, a flow rate of 1.0 mL min-1), guaiazulene-2,9-dione generated from the experiment was identified using the standard material as external and internal standard, respectively (Huang et al., 2008; Pei et al., 2011).
2.3 Post-Processing
A certain amount of ethyl acetate (1:1, v/v) was added to the reaction solution. At first, saturated aqueous Na-HSO3(1:1) was used to remove the excessive bromine, HBr, and acetic acid. Then pure water (1:1) was used to wash out the salts, and finally the ethyl acetate (1:1) was used to re-extract the water-phase. Every step was operated three times. The ethyl acetate phase was collected and concentrated using a rotary evaporator (model Heizbad HB digit, Heidolph) to obtain the raw guaiazulene-2,9-dione with the weight marked as Mt.
2.4 Analytical Method
In the seriatim-factorial experiment, yield of the target compound was calculated by the formula:
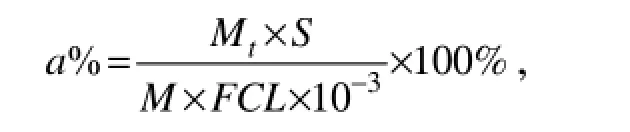
among which, F indicates the correction factor of the standard material, and S represents the peak area, while M refers to the theoretical quality of target compound. Samples to be detected were prepared with the same concentration (C) and injection volume (L). Yield of the target compound was directly proportional to MtS.
Under the chromatographic condition (Elite, 5 µm, 4.6 × 250 mm, 25℃, λ = 254 nm, MeOH/H2O 60:40, a flow rate of 1.0 mL min-1), six standard concentrations (0.1, 0.05, 0.025, 0.0125, 0.00625, and 0.003125 mg mL-1) were selected for constructing scatter diagram. In verification test, the content of target compound was calculated by analyzing the peak area with the standard curve equation.
2.5 Seriatim-Factorial Experiment
A seriatim-factorial experiment (Hu et al., 2011; Xiao et al., 2010) was used for the optimization study. Based on the findings in these experiments, we determined the levels of all the factors including reaction time (RT), reaction temperature (RTP), drop rate of bromine (DRBr), concentration of aqueous THF (CTHF), molar ratio of GA to bromine (MRGBr), molar ratio of GA to Ac (MRGA), and concentration of GA (CG). The optimized factors are summarized in Table 1.
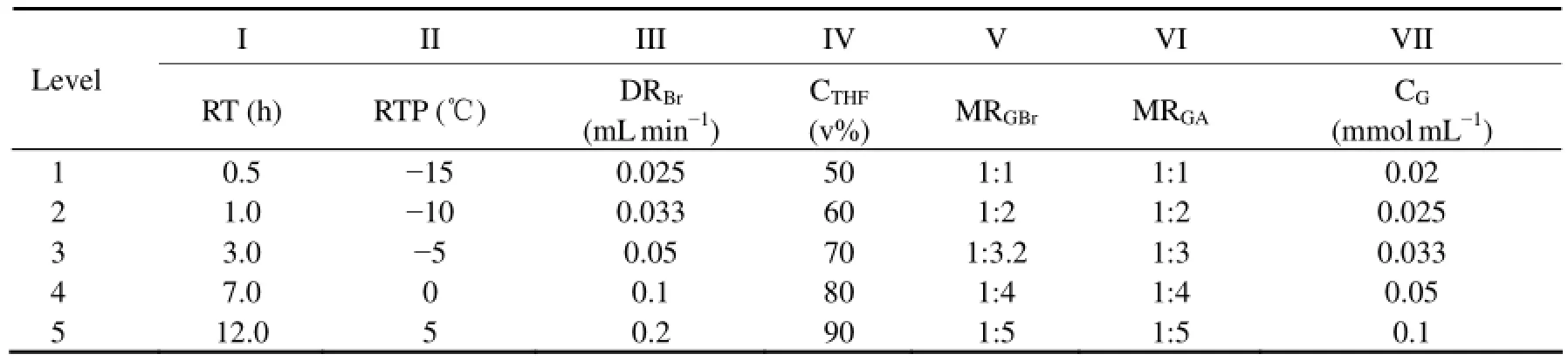
Table 1 Factors and levels of seriatim-factorial experiment
3 Results and Discussion
Chromatograms (Fig.3) indicated that guaiazulene-2,9-dione was successfully obtained. The UV (231, 248, 384 nm) and MS ([M+H]+:229.1) spectra of guaiazulene-2,9-dione were shown in Figs.4a and 4b, respectively.
There is a high linear correlation between the concentration and peak area of standard material (Fig.5). The linear regression formula was the concentration (mg mL-1) of standard material = 0.5822 Area × 10-4-1.755 × 10-4, R2= 0.9999.
As shown in Fig.6a, there was an obvious variation of target compound yield when the reaction time extended from 0.5 to 12.0 h. Such a tendency may be due to the underreaction in a short time or overreaction in a long time to produce more byproducts. Especially the byproducts increased fast from 0.5 h to 1.0 h. Thus, threehours of reaction time were needed for the highest yield of target compound. An optimal reaction temperature was determined as -5℃, although there was a very complex variation trend accompanied the reaction temperature change from -15℃ to 5℃ (Fig.6b). An apparent variation of target compound yield was observed when the drop rate of bromine accelerated from 0.033 to 0.2 mL min-1(Fig.6c). Considering the convenient for actual operation, 0.1 mL min-1was chosen as the optimal drop rate of bromine, although the highest yield of target compound was obtained at 0.025 mL min-1.
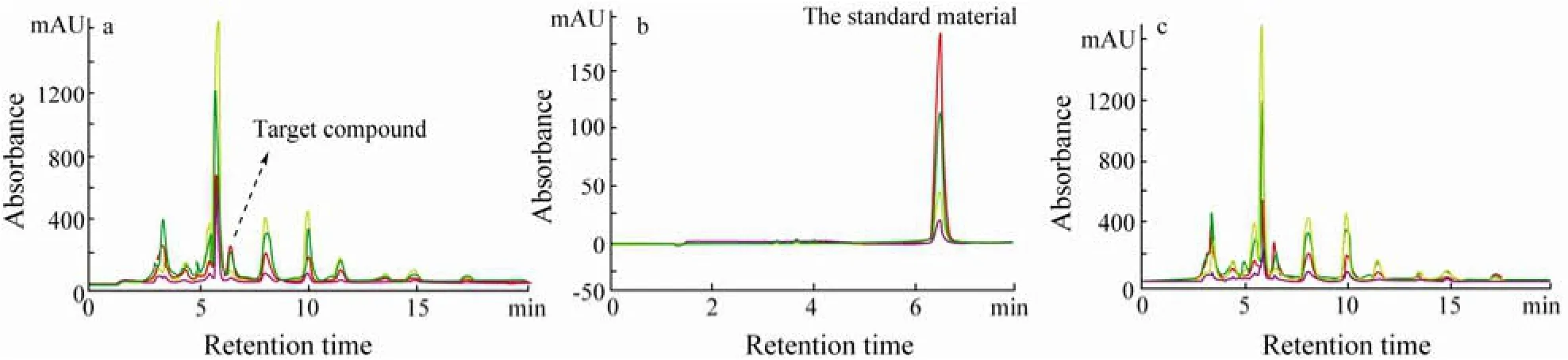
Fig.3 HPLC chromatograms of the trial experiment sample (a), standard material (b), and the sample with internal standard (c), respectively.
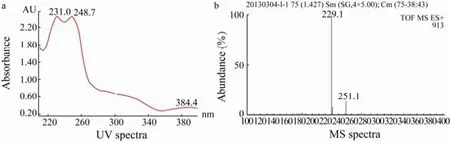
Fig.4 The UV (a) and MS (b) spectra of guaiazulene-2,9-dione.
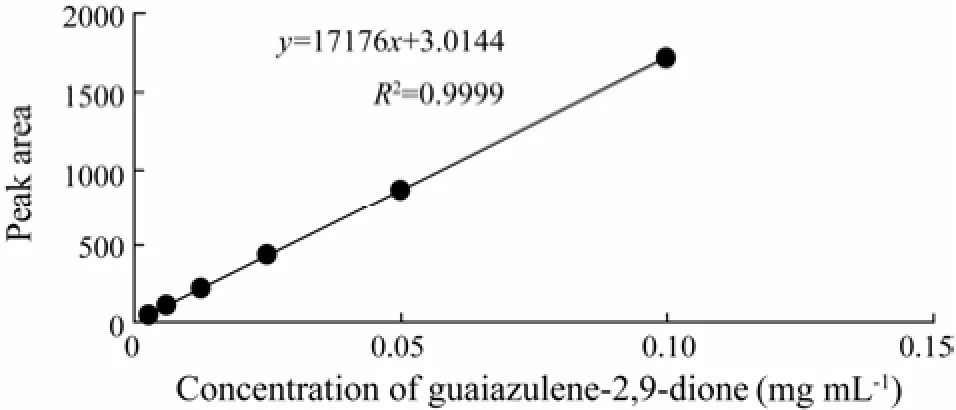
Fig.5 The standard curve of the concentration and peak area of guaiazulene-2,9-dione.
When the concentration of reactants was concerned, the variation trend of target compound yield was not obvious with regard to the aqueous THF concentration (Fig.7a), with just a relatively high yield obtained at 80%. However, there was a great yield change when the molar ratio of GA to bromine increased from 1:5 to 1:1 (Fig.7b). Nozoe et al. (1995) reported that the target compound can be obtained only when GA was treated with 3 volumes of bromine. In present study, the highest yield of target compound was obtained when the molar ratio was 1:4 (Fig.7b), probably due to the bromine volatilization, artificial operation errors, while more byproducts would be obtained if the volume of bromine increased more (Fig.7b). Fig.7c showed that the yield of target compound kept almost constant when the molar ratio of GA to Ac increased from 1:3 to 1:1, and a highest yield was obtained at a ratio of 1:4 (Fig.7c). Besides, there was also a great change of the yield when the concentration of GA in different volumes of 80% aqueous THF increased from 0.02 to 0.1 mmol mL-1, and the highest yield was obtained when the concentration of GA was 0.025 mmol mL-1(Fig.7d). Thus, each of the proper level range of the seven factors was preliminarily determined.
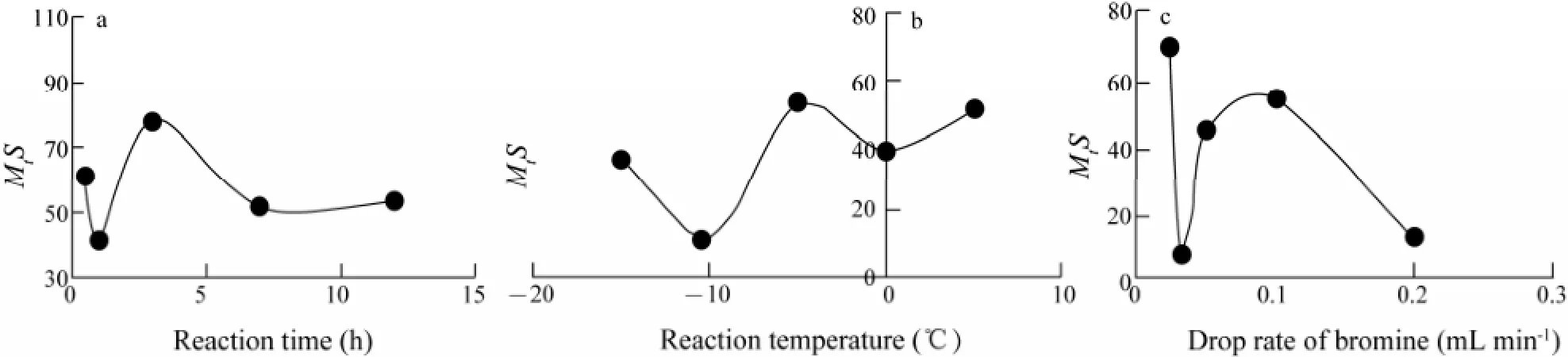
Fig.6 The scatter diagram of reaction time (a), reaction temperature (b) and drop rate of bromine (c).
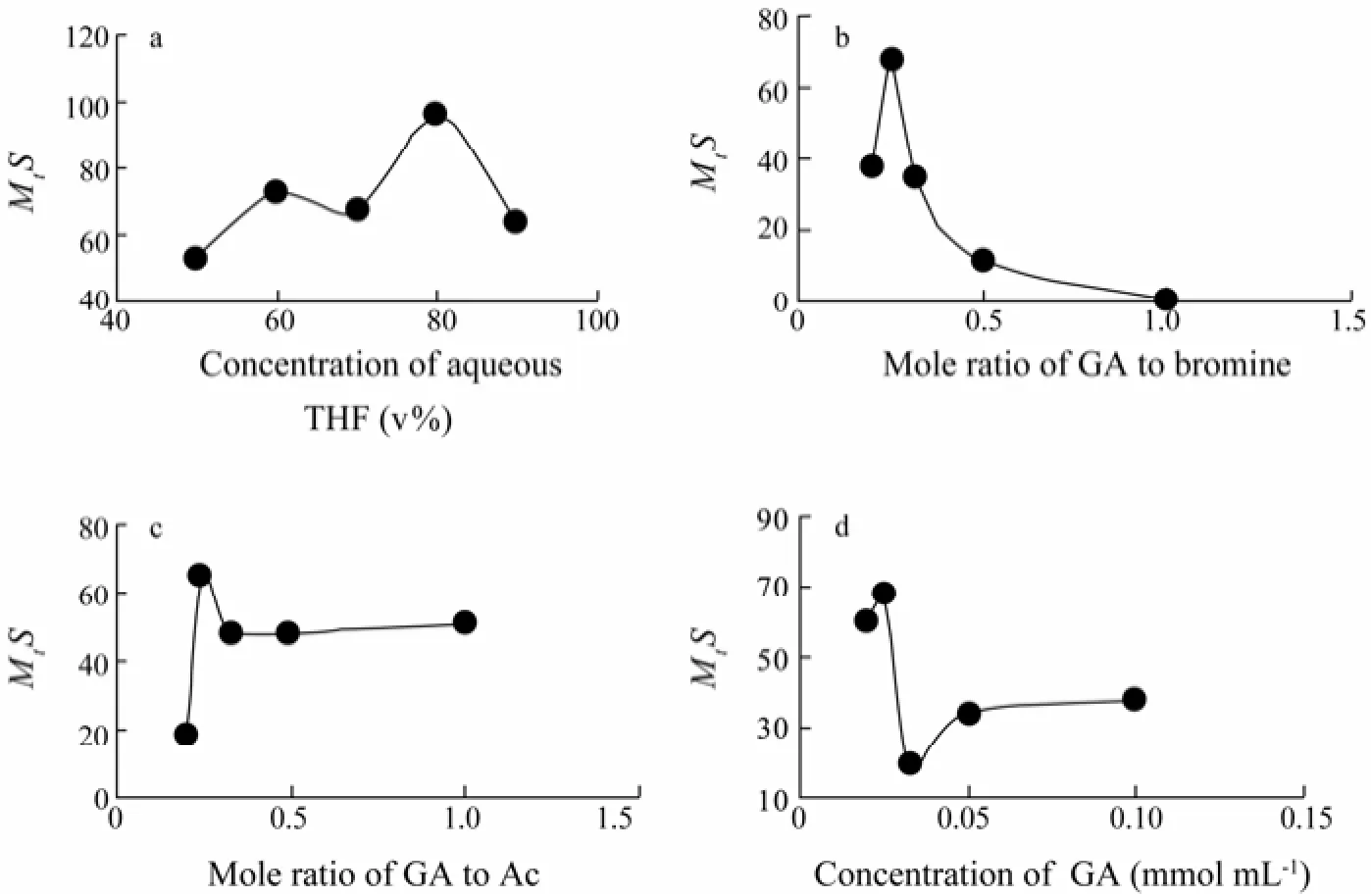
Fig.7 The scatter diagram of concentration of aqueous THF (a), molar ratio of GA to bromine (b), molar ratio of GA to Ac (c) and concentration of GA (d).
In verification test, the actual yield of target compound under each optimized conditions was obtained (Table 2; Fig.8), and all yields were higher than the reported early(Nozoe et al., 1995). Finally, a high yield up to 23.72%, almost 2 times higher than the reported, was obtained at an optimal level.
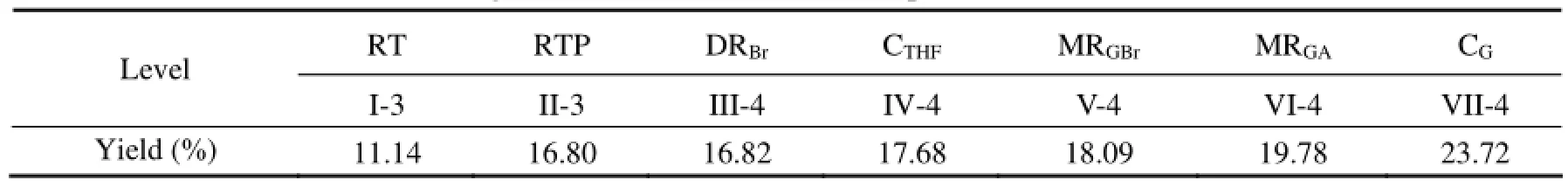
Table 2 Yield of guaiazulene-2,9-dione at the optimized level of factors each

Fig.8 The scatter diagram of the verification test.
4 Conclusio n
The strong antibacterial compound, guaiazulene-2,9-dione, was synthesized with one-step method. Under the optimized reaction condition, the yield of guaiazulene-2,9-dione reached 23.72%. That makes it feasible for deep investigating of guaiazulene-2,9-dione.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21102136) and Changjiang Scholars and Innovative Research Team in University (PCSIRT, No. IRT0944). Special thanks are given to Prof. Jixiang Chen (Faculty of Environmental Engineering, Petrol & Chemistry Department, Lanzhou University of Technology) for his assistance in bioactivity testing.
Blunt, J. W., Copp, B. R., Keyzers, R. A., Munro, M. H. G., and Prinsep, M. R., 2012. Marine natural products. Natural Product Reports, 29: 144-222.
Chen, D. W., Yu, S. J., Ofwegen, L. V., Proksh, P., and Lin, W. H., 2012. Anthogorgienes A-O, new guaiazulene-derived terpenoids from a Chinese gorgonian Anthogorgia species, and their antifouling and antibiotic activities. Journal of Agricultural and Food Chemistry, 60: 112-123.
Fraga, B. M., 2008. Natural sesquiterpenoids. Natural Product Reports, 25: 1180-1209.
Franchi, E., Ingrosso, G., Marchetti, F., and Pinzino, C., 2003. Guaiazulene-based phenolic radical scavengers: Synthesis, properties, and EPR studies of their reaction with oxygencentred radicals. Tetrahedron, 59: 5003-5018.
Flori, J., Teti, G., Gotti, R., Mazzotti, G., and Falconi, M., 2011. Cytotoxic activity of guaiazulene on gingival fibroblasts and the influence of light exposure on guaiazulene-induced cell death. Toxicology in Vitro, 25: 64-72.
Huang, C., Lu, L. M., and Liu, G. P., 2008. The comparison analysis on determination of blood alcohol concentration with ISTD and ESTD. Life Science Instruments,6: 41-43.
Hu, L. L., Xu, H. H., and Liao, M. D., 2011. Optimization of culture conditions for a strain of PS04 producing antifungal antibiotics. Journal of Huazhong Agricultural University,30(3): 276-279.
Kourounakis, A. P., Rekka, E. A., and Kourounakis, P. N., 1997. Antioxidant activity of guaiazulene and protection against paracetamol hepatoxicity in rats. Journal of Pharmacy and Pharmacology, 49: 938-942.
Nozoe, T., Ishikawa, S., Shindo, K., Wakabayashi, H., and Kurihara, T., 1991a. Unusual reactions of guaiazulene with N-bromosuccinimide and synthesis of variously functionalized azulene using these reactions. Collection of Czechoslovak Chemical Communications, 56: 991-1010.
Nozoe, T., Matsubara, Y., Takekuma, S., Yamamoto, H., and Nakano, T., 1991b. Oxidation of 4,6,8-trimethylazulene and guaiazulene with hydrogen peroxide in pyridine. Bulletin of the Chemical Society of Japan, 64: 3497-3499.
Nozoe, T., Shindo, K., Wakabayashi, H., Kurihara, T., and Uzawa, J., 1995. Reaction of guaiazulene with bromine in hexane and in aqueous tetrahydrofuran. Chemistry Letters, 8: 687-688.
Pei, C. Y., Wang, Z. Q., Jia, J. M., and Song, J., 2011. Determi-
nation of astragaloside Ⅳ of eight area s in astragali radix is by HPLC-ELSD internal standard method. China Journal of Chinese Materia Medica, 36 (14): 1982-1984.
Shi, X. F., 2009. Studies on bioactive constituents of the gorgonian Muriceides collaris. Master thesis, Ocean University of China, Qingdao.
Tanaka, Y., Kamibayashi, M., Yamaki, F., Saitoh, M., Nakazama, T., Tanaka, H., Noguchi, K., Hashimoto, K., and Shigenobu, K., 2000. Relaxant action of azulene-1-carboxamidine derivative N1, N1-dimethyl-N2-(2-pyridylmethyl)-5-isopropyl-3,8-dimethylazulene-1-carboxamidine (HNS-32) in pig coronary artery. Pharmacy Pharmacology Communication, 6: 397-404.
Xiao, H. Q., and Li, Y. Z., 2010. Optimization of enzymatic hydrolysis conditions of soybean protein isolated using seriatim-factorial experiment and taguchi OA approach. Food and Fermentation Technology, 46 (1): 61-64.
Zheng, Z. F., 2001. Development and application of S-guaiazulene and Na S-guaiazulene-3-sulfonate in Japan. Flavour Fragrance Cosmetics, 5: 18-22.
(Edited by Qiu Yantao)
(Received January 10, 2013; revised February 18, 2013; accepted April 4, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2014
# These authors made equal contributions to this paper.
* Corresponding authors. Tel: 0086-532-82032323
E-mail: liguoqiang@ouc.edu.cn, tangxuli@ouc.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- The Formation of Wind Curl in the Marine Atmosphere Boundary Layer over the East China Sea Kuroshio in Spring
- Wind Wave Characteristics and Engineering Environment of the South China Sea
- Prediction of the Mooring Force of a 2-D Floating Oil Storage Tank
- An Experimental Study on the Wave-Induced Pore Water Pressure Change and Relative Influencing Factors in the Silty Seabed
- Fe-Si-Mn-Oxyhydroxide Encrustations on Basalts at East Pacific Rise near 13˚N: An SEM – EDS Study
- Seasonal Changes in Phytoplankton Biomass and Dominant Species in the Changjiang River Estuary and Adjacent Seas: General Trends Based on Field Survey Data 1959 - 2009
