HPLC Pigment Profiles of 31 Harmful Algal Bloom Species Isolated from the Coastal Sea Areas of China
2014-04-26LIUShuxiaYAOPengYUZhigangLIDongDENGChunmeiandZHENYu
LIU Shuxia, YAO Peng, YU Zhigang,, LI Dong, DENG Chunmei, and ZHEN Yu
1) Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Qingdao 266100, P. R. China
2) Ocean College, Zhejiang University, Hangzhou 310058, P. R. China
3) Qingdao Collaborative Innovation Center of Marine Science and Technology, Qingdao 266100, P. R. China
4) Institute of Marine Organic Geochemistry, Ocean University of China, Qingdao 266100, P. R. China
5) College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, P. R. China
6) Key Laboratory of Marine Spill Oil Identification and Damage Assessment Technology, State Oceanic Administration, Qingdao 266033, P. R. China
7) Key Laboratory of Marine Environment and Ecology, Ministry of Education, Qingdao 266100, P. R. China
HPLC Pigment Profiles of 31 Harmful Algal Bloom Species Isolated from the Coastal Sea Areas of China
LIU Shuxia1),2), YAO Peng1),3),4), YU Zhigang1),4),*, LI Dong1),5), DENG Chunmei6), and ZHEN Yu7)
1) Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Qingdao 266100, P. R. China
2) Ocean College, Zhejiang University, Hangzhou 310058, P. R. China
3) Qingdao Collaborative Innovation Center of Marine Science and Technology, Qingdao 266100, P. R. China
4) Institute of Marine Organic Geochemistry, Ocean University of China, Qingdao 266100, P. R. China
5) College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, P. R. China
6) Key Laboratory of Marine Spill Oil Identification and Damage Assessment Technology, State Oceanic Administration, Qingdao 266033, P. R. China
7) Key Laboratory of Marine Environment and Ecology, Ministry of Education, Qingdao 266100, P. R. China
Chemotaxonomy based on diagnostic pigments is now a routine tool for macroscopic determination of the composition and abundance of phytoplankton in various aquatic environments. Since the taxonomic capability of this method depends on the relationships between diagnostic pigments and chlorophyll a of classified groups, it is critical to calibrate it by using pigment relationships obtained from representative and/or dominant species local to targeted investigation area. In this study, pigment profiles of 31 harmful algal bloom (HAB) species isolated from the coastal sea areas of China were analyzed with high performance liquid chromatography (HPLC). Pigment compositions, cellular pigment densities and ratios of pigments to chlorophyll a were determined and calculated. Among all these species, 25 kinds of pigments were detected, of which fucoxanthin, peridinin, 19’-butanoyloxyfucoxanthin, 19’-hexanoyloxyfucoxanthin, violaxanthin, and antheraxanthin were diagnostic pigments. Cellular pigment density was basically independent of species and environmental conditions, and therefore was recommended as a bridge to compare the results of HPLC-CHEMTAX technique with the traditional microscopy method. Pigment ratios of algal species isolated from the coast of China, especially the diagnostic pigment ratios, were higher than those from other locations. According to these results, pigment ratio ranges of four classes of phytoplankton common off the coast of China were summarized for using in the current chemotaxonomic method. Moreover, the differences of pigments ratios among different species under the same culturing conditions were consistent with their biological differences. Such differences have the potential to be used to classify the phytoplankton below class, which is meaningful for monitoring HABs by HPLC-CHEMTAX.
harmful algal bloom species; high performance liquid chromatography; chemotaxonomy; cellular pigment density; pigment ratios
1 Introduction
Harmful algal blooms (HABs) have been spreading and increasing along coastal regions worldwide in the last decades. This phenomenon has gained much attention from researchers and local regulatory authorities due to the negative impacts of HABs on ecosystem and human health (Tomas and Smayda, 2008). One of the tasks for HAB studies is to convey complete information on phytoplankton communities and their relationships with environmental parameters, which can reflect the successsion between species during triggering and vanishing process of HABs, and help to understand the possible physical and chemical mechanisms that drive and sustain the HABs (Abdenadher et al., 2012; Cohu et al., 2013). This is meaningful for forecasting the occurrence of HABs.
The classical method to determine phytoplankton communities relies on light microscopic enumeration. This method allows species identification and biometrics, which can be converted into species-specific biovolume or biomass. However, microscopy method not only is time-consuming, but also requires a thorough taxonomic expertise. Additionally, some small and fragile species can become distorted during sample preservation (Galluzzi et al., 2008), making their identification and detec-tion even more difficult. Some recent techniques are recommended to be combined with microscopy for determination of phytoplankton composition, of which the chemotaxonomy of phytoplankton based on diagnostic pigments is considered to be the tool for this aim (e.g., de Souza et al., 2012; van Oostende et al., 2012).
Initially, diagnostic pigments were just used as semiquantitative indicators of phytoplankton biomass (Gieskes and Kraay, 1986; Klein and Sournia, 1987). The development of matrix factorization software (CHEM-TAX) by Mackey et al. (1996) has made great progress on the determination of phytoplankton group abundance separately. With this software, pigment concentrations determined by high performance liquid chromatography (HPLC) can be successfully used to calculate the contribution of different phytoplankton groups to total chlorophyll a (Chl a).
In CHEMTAX, an initial pigment ratio matrix for targeted algal groups is input as a ‘seed’ for subsequent iterative calculation to give an estimation of individual algal group to the total chlorophyll a pool from unknown samples. The taxonomic capability of CHEMTAX partly depends on this initial pigment ratios input (Mackey et al., 1996). Those pigment ratios of species common to the investigation area are very helpful to obtain accurate results (Lewitus et al., 2005). Although a number of pigment ratios of algal species have been published in recent years, the fact is that most of them were obtained from cultures of several famous culture centers (e.g., Llewellyn and Gibb, 2000; Zapata et al., 2004). The scarcity of pigment ratios local to targeted environments obviously restricts the application of CHEMTAX, especially for HAB species. In addition, pigment analysis of different algal species, especially those grown under the same condition, will reveal the differences of pigment patterns within a single phytoplankton class (Zapata et al., 2004; 2011; Yao et al., 2011). This would allow the classification of phytoplankton groups under a lower level than class. For example, Wright et al. (2010) successfully divided the diatoms in the Southern Ocean into two groups using chlorophyll c3as a diagnostic pigment, and the contributions of the two forms of Phaeocystis antarctica with high and low Fe to total chlorophyll a were also distinguished with the differences in chlorophyll c3/Chl a, fucoxanthin/Chl a and 19’- hexanoyloxyfucoxanthin/Chl a ratios.
In this study, pigments of 31 HAB species (IOCUNESO; Yan et al., 2002; Yu et al., 2007; Hou et al., 2008), including 12 species of diatoms, 15 species of dinophyceae, 3 species of raphidophyceae and 1 specimen of haptophyceae, isolated from the coast of China were systematically determined using the HPLC method developed by Zapata et al. (2000). This method uses C8column and pyridine as mobile phase modifier which can completely separate several important pigment pairs, such as chlorophyll c1and c2, 4-keto-19’-hexanoyloxyfucoxanthin and 19’-hexanoyloxyfucoxanthin, and partially separate zeaxanthin and lutein. The power of separating these pigment pairs is of benefit not only to defining pigment types within algal groups but also to obtaining accurate pigment ratios without interference of unresolved pigments. The aim of this study is to provide useful pigment profiles of these species, which will be helpful to promote the application of HPLC-CHEMTAX method in the coastal areas of China for HAB researches.
2 Materials and Methods
2.1 Algal Cultures
The information of HAB species in this study is listed in Table 1. All species were obtained from the Research Center for Harmful Algae and Aquatic Environment Laboratory of Jinan University in Guangzhou, China. Unialgal, non-axenic cultures were grown at a salinity of 30 and different temperatures recommended by CCMP (Provasoli-Guillard National Center for Culture of Marine Phytoplankton, USA) (Table 1). Natural water from the East China Sea was filtered through 0.45 μm acetate cellulose filter (Xidoumen, Hangzhou, China) and then sterilized at 120℃ for 30 min in a pressure vapor sterilizer (AC-002, SANYO, Japan). Sterilized seawater enriched with different medium described in Table 1 was used as culture medium. Triplicate samples were grown under light intensities of 40 μmol photons m-2s-1(6000 lux) in conical Erlenmeyer flasks containing 150 mL of culture medium under a light : dark cycle (12 h:12 h). Light was supplied by cool white fluorescent lamps (Philips, Shanghai, China) and measured by a luxmeter (ZDS-10F-2D, Shanghai, China).
About 5-30 mL (depending on cell density) of cultures in exponential phase were harvested by gentle filtration onto 25 mm Whatman GF/F glass fiber filters (UK) under low light condition. Residue water in the filters was absorbed by tissue. The filters were frozen at -30℃ prior to analysis within 12 h.
2.2 Pigment Extraction and Analyses
Frozen substances in filters were extracted in 10 mL centrifuge tubes with 3-5 mL 95% methanol in water by sonication for 5 min in an ice water bath (SK250H, Kudos, China). Extracts were then filtered through 4 mm diameter PTFE syringe-filters (0.45 μm pore size, Whatman, UK) to remove cells and filter debris prior to instrument analysis. An aliquot of the extracts was injected immediately into the HPLC after filtration. HPLC analyses were performed according to Zapata et al. (2000). The chromatographic equipment was a Waters Alliance HPLC system, which has a 2695-separations module with an autosampler, a Waters 2996 photodiode array detector (DAD; wavelength range: 350-750 nm; 1.2 nm optical resolution) and a Waters 2475 multi fluorescence detector (Ex (Excitation): 440 nm, Em (Emission): 650 nm; GAIN=1; Emission/Energy Units Full Scale (EUFS) = 1000) (Waters, Milford, USA). The C8column (Waters Symmetry, 150 mm × 4.6 mm, 3.5 μm particle size, 10 nm pore size) was maintained at 25℃ by means of a column oven. Pigments were eluted by a linear gradientfrom 0 to 40% B in 22 min, followed by an increase to 95% for 6 min and isocratically held for a further 10 min, and then initial conditions were reestablished by reversed linear gradient within 2 min, where A=methanol: acetonitrile:aqueous pyridine solution (0.25 mol L-1pyridine, see detail in Zapata et al., 2000) (50:25:25) and B=methanol: acetonitrile:acetone (20:60:20). HPLC-grade organic solvents (Merck, Germany), reagent grade pyridine (Sangon, Shanghai, China) and Milli-Q water were used to prepare the mobile phases. Mobile phases were filtered through 0.45 μm GHP filter (Pall, USA) before ultrasonic degassing. The sampler was auto purged after each running with a loop volume of methanol:water (30:70). Flow rate was 1 mL min-1.
Chlorophylls and carotenoids were detected by absorbance at 440 nm and identified by comparison of retention times and absorption spectra with literatures and external standards, which were obtained commercially: chlorophyll a, lutein and β, β-carotene from Sigma-Aldrich Inc. (St. Louis, USA); chlorophyll c2, diadinoxanthin, diatoxanthin, fucoxanthin, 19’-hexanoyloxyfucoxanthin, peridinin, zeaxanthin and dinoxanthin from the International Agency for14C determination, DHI Water & Environment (Denmark). The molar extinction coefficients obtained from Jeffrey (1997) were used for quantification of pigments without external standards. The chlorophyll c2-MGDG was quantified by the molar extinction coefficient of chlorophyll c2, and the molar extinction coefficient of 19’-hexanoyloxyfucoxanthin was used for its 4-keto derivative (Seoane et al., 2009).
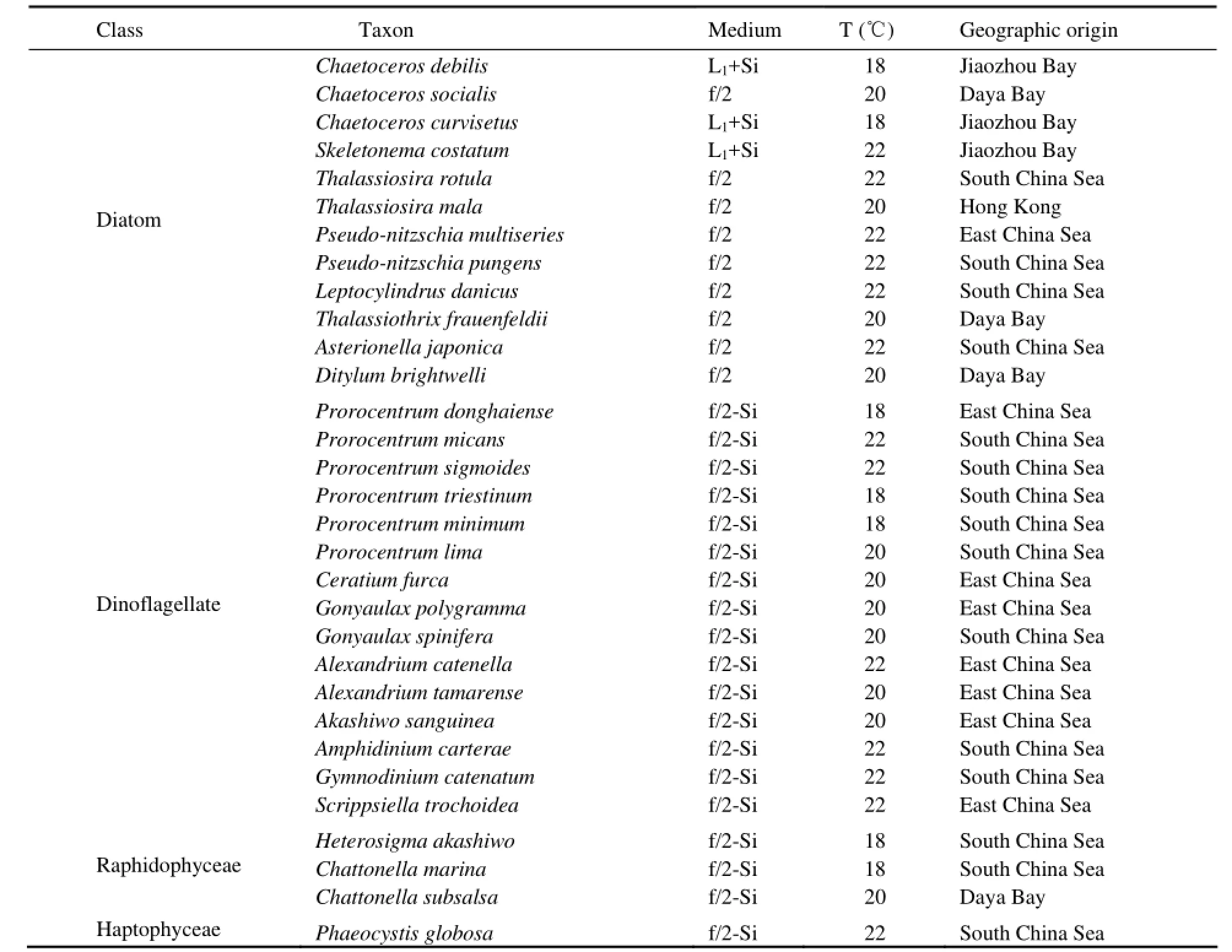
Table 1 Harmful algal species, culture media, growth temperature and geographic origins of 31 species examined
2.3 Calculation of Pigment Profiles
Cell volume was calculated according to the size measured by microscopy with the method of Sun et al. (1999). Cellular pigment densities were then obtained by dividing the cellular pigment concentrations by the cell volumes. The cellular pigment concentrations used to calculate the cellular pigment densities were obtained by dividing the pigment concentrations by the cell numbers. Since algal cells usually keep definite positions on the slide when the centre of gravity is low, this makes it difficult to calculate cell volumes for some algal species, such as the species of Chaetoceros (prism on elliptic base girdle view), Pseudo-nitzschia (prism on parallelogrambase), Thalassinema (rectangular box) and Asterionella (rectangular box). Therefore, the cell volumes and cellular pigment densities of only 19 species in this study were calculated.
Total chlorophyll a (Tchl a) was defined as the sum of Chl a and its derivatives (Chlide a, Me-chlide a, Phide a, Chl a allomer and Chl a epimer). All the pigment datawere normalized to Tchl a to obtain the ratios of pigment to total chlorophyll a on a weight basis (g g-1).
3 Results
3.1 Pigment Compositions and Types
Fig.1 shows the representative chromatograms of the HAB species isolated from the coast of China detected by absorbance at 440 nm. In these species, 25 different pigments were detected, in which 11 of them were chlorophylls and 14 of them were carotenoids. The information of these pigments, including pigment name, abbreviation, retention time and maximum absorbance wavelength, is shown in Table 2.
Among all pigments detected, only Chl c2, Chl a and Caro were contained by all algal species in this study. Besides these three kinds of pigments, Chl c1, Fuco, Dd and Dt were also contained in all diatoms. This pigment composition was consistent with that of pigment type 1 of diatoms defined by Jeffrey and Wright (2006). MgDVP was present only in 2 species of diatoms (Skeletonema costatum and Asterionella japonica) with low amount. The Chl a derivatives (Chl a allomer, Chlide a, Me-Chlide a, Phide a) in diatoms were more complex than other classes in the present study. This was probably induced by the high activity of chlorophyllase during the filtration and/or extraction processes (Jeffrey and Hallegraeff, 1987).
The pigment composition showed minor difference among different dinophyceae species. Most of them contained Peridnol, Chl c2, Perid, Ddchr, Dd, Dt, Chl a and Caro. Compared with diatoms, dinophyceae contained minimum Chl a derivatives. Only two kinds of Chl a derivatives, i.e., Chl a allomer and Chl a epimer were detected in this class. Most dinophyceae species analyzed in this study belong to dinophyceae pigment type 1 defined by Jeffrey and Wright (2006) with the simplest definitive pigment composition: Perid, Chl c2, Dd, Dt and Caro. The detection of Chl c1in Prorocentrum micans, Prorocentrum sigmoides and Gonyaulax spinifera made these three species have different pigment types from those defined by Jeffrey and Wright (2006).
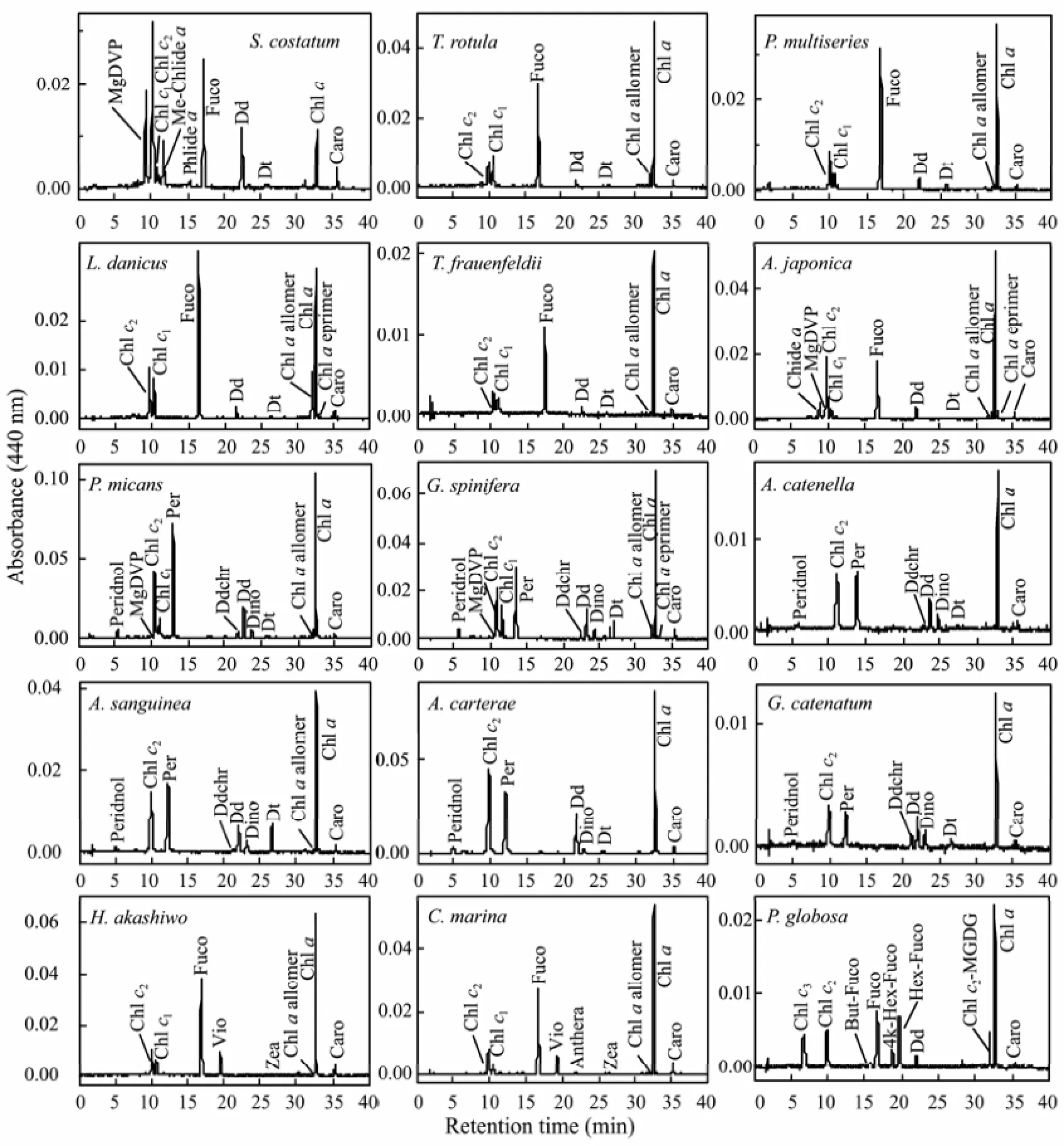
Fig.1 Representative HPLC chromatograms of HAB species isolated from the coast of China (440 nm).
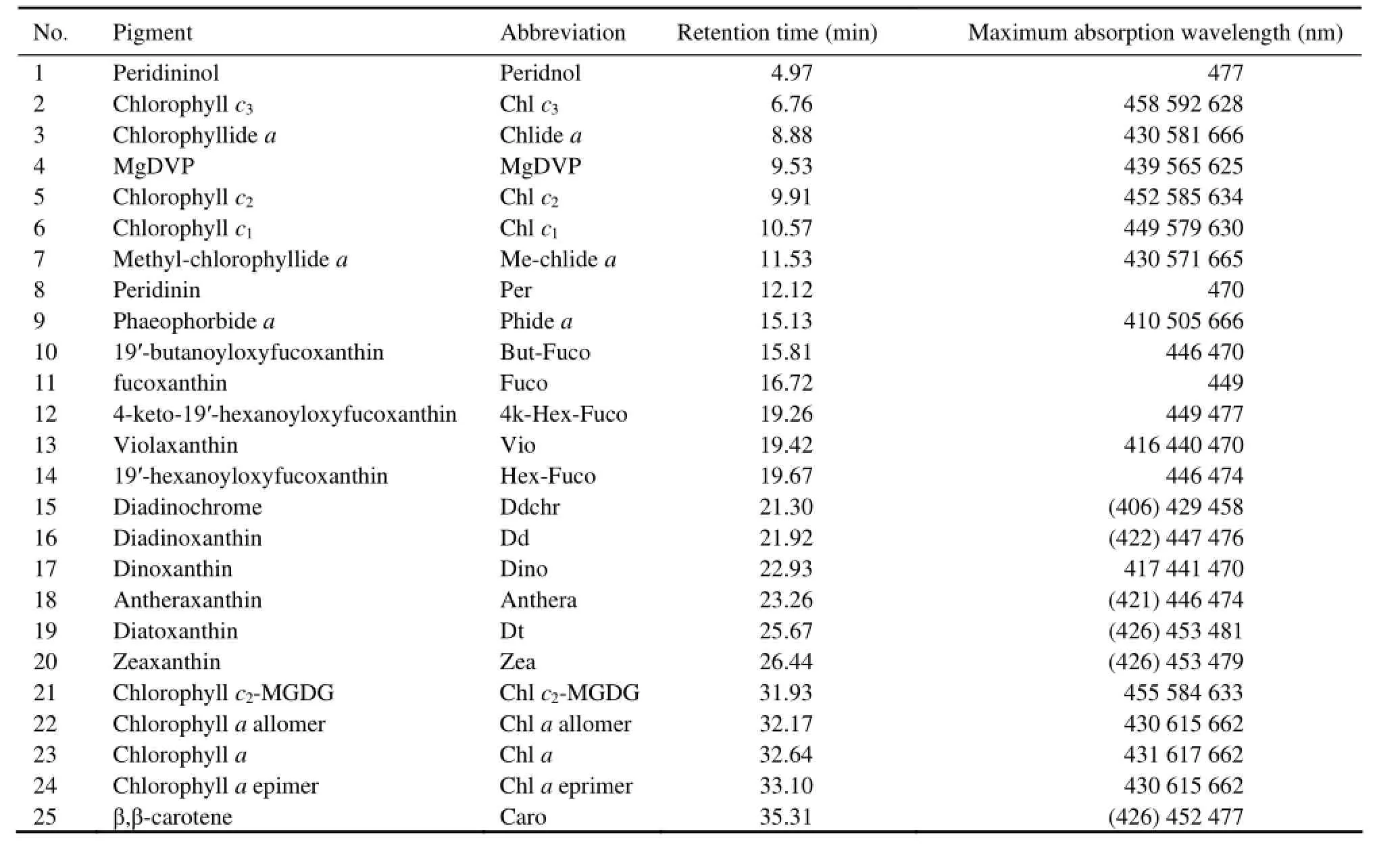
Table 2 Peak identification, retention times, and spectral absorbance maxima of phytoplankton pigments in eluent from 31 HAB species isolated from the coast of China (Wavelengths given in parentheses denote shoulders)
Phaeocystis globosa was classified into haptophyceae type 8 according to specific combination of Chls c and fucoxanthin-type carotenoids by Zapata et al. (2004). For Chls c, it has Chl c3, MgDVP, Chl c2and Chl c2-MGDG [18:4/14:0], and for fucoxanthin-type carotenoids, it has Fuco, But-Fuco, 4k-Hex-Fuco and Hex-Fuco.
3.2 Cell Volume and Cellular Pigment Density
The cell volumes of 19 HAB species in this study are shown in Table 3. The volumes varied from 33 μm3of Phaeocystis globosa to 73005 μm3of Akashiwo sanguinea. On average, the cell volume of raphidophyceae (15819 ± 6518 μm3, n=3) was the highest, followed by dinophyceae (15614 ± 24055 μm3, n=9), diatoms (2858 ± 3377μm3, n=6) and haptophyceae (33 μm3). Cell volumes varied significantly within the same class, for example, it varied from 128 μm3(in Skeletonema costatum) to 8252 μm3(in Ditylum brightwelli) in diatoms, and from 402 μm3(in Prorocentrum donghaiense) to 73005 μm3(in Akashiwo sanguine) in dinophyceae.
Table 4 shows the cellular pigment densities of 19 HAB species. In general, the cellular pigment density varied slightly with species, especially within the same class. The cellular Tchl a densities ranged from 1.48 fg μm-3(in Skeletonema costatum) to 6.50 fg μm-3(in Thalassiosira mala) in diatoms, from 1.05 fg μm-3(in Gonyaulax polygramma) to 6.92 fg μm-3(in Gymnodinium catenatum) in dinophyceae, and from 0.71 fg μm-3(in Heterosigma akashiwo) to 2.63 fg μm-3(in Chattonella subsalsa) in raphidophyceae. The cellular density of Per varied by a factor of 7 among 9 species of dinophyceae from 0.92 fg μm-3to 6.13 fg μm-3. The maximum of cellular density of Fuco in diatoms was 8.08 fg μm-3(in Thalassiosira mala), which was only three times higher than the minimum value 1.85 fg μm-3(in Skeletonema costatum).
Moreover, the cellular densities of chlorophylls showed more variations between species than those of carotenoids. The cellular densities of Chl c2varied from 0.02 fg μm-3(in Chattonella subsalsa) to 1.28 fg μm-3(in Alexandrium catenella), increased by 64 times, and the cellular densi-ties of Tchl a varied from 0.71 fg μm-3(in Heterosigma akashiwo) to 6.92 fg μm-3(in Gymnodinium catenatum), increased by 10 times, while the cellular densities of Fuco varied from 1.85 fg μm-3(in Skeletonema costatum) to 8.08 fg μm-3(in Thalassiosira mala) in diatoms, increased by only 4 times.
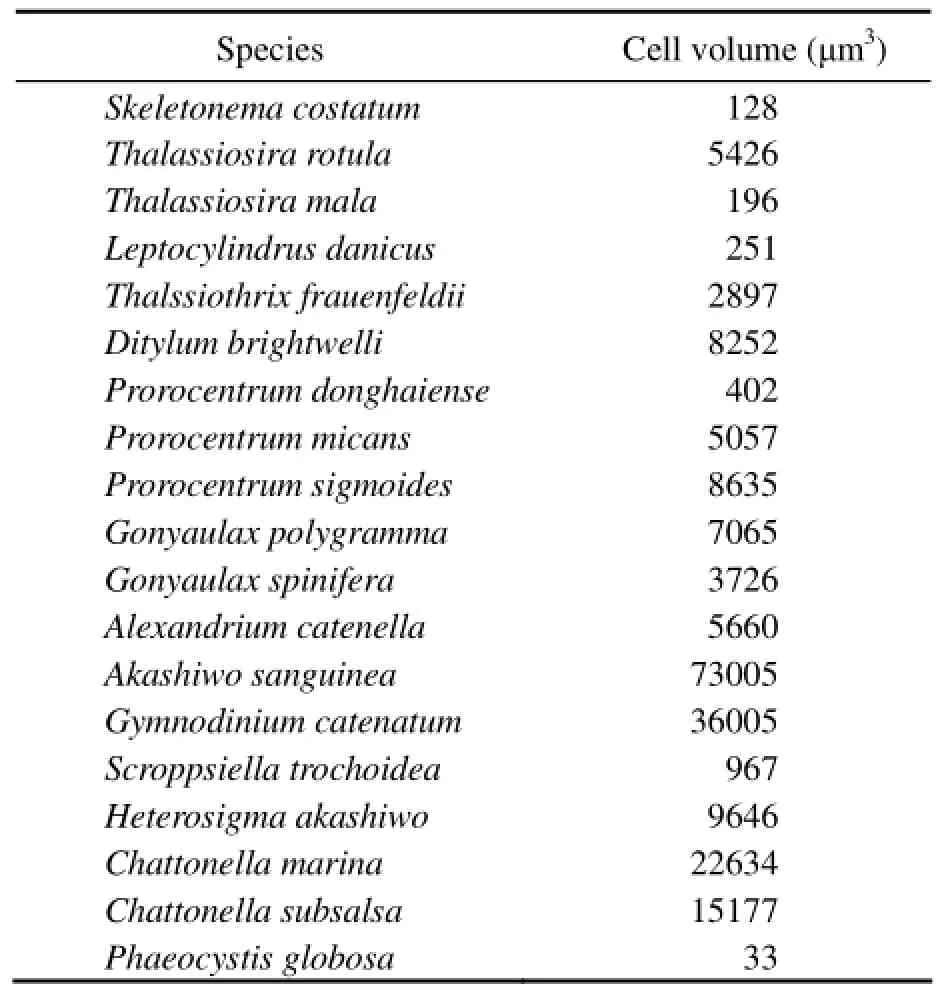
Table 3 Cell volumes (μm3) of 19 species of harmful algae isolated from the coast of China
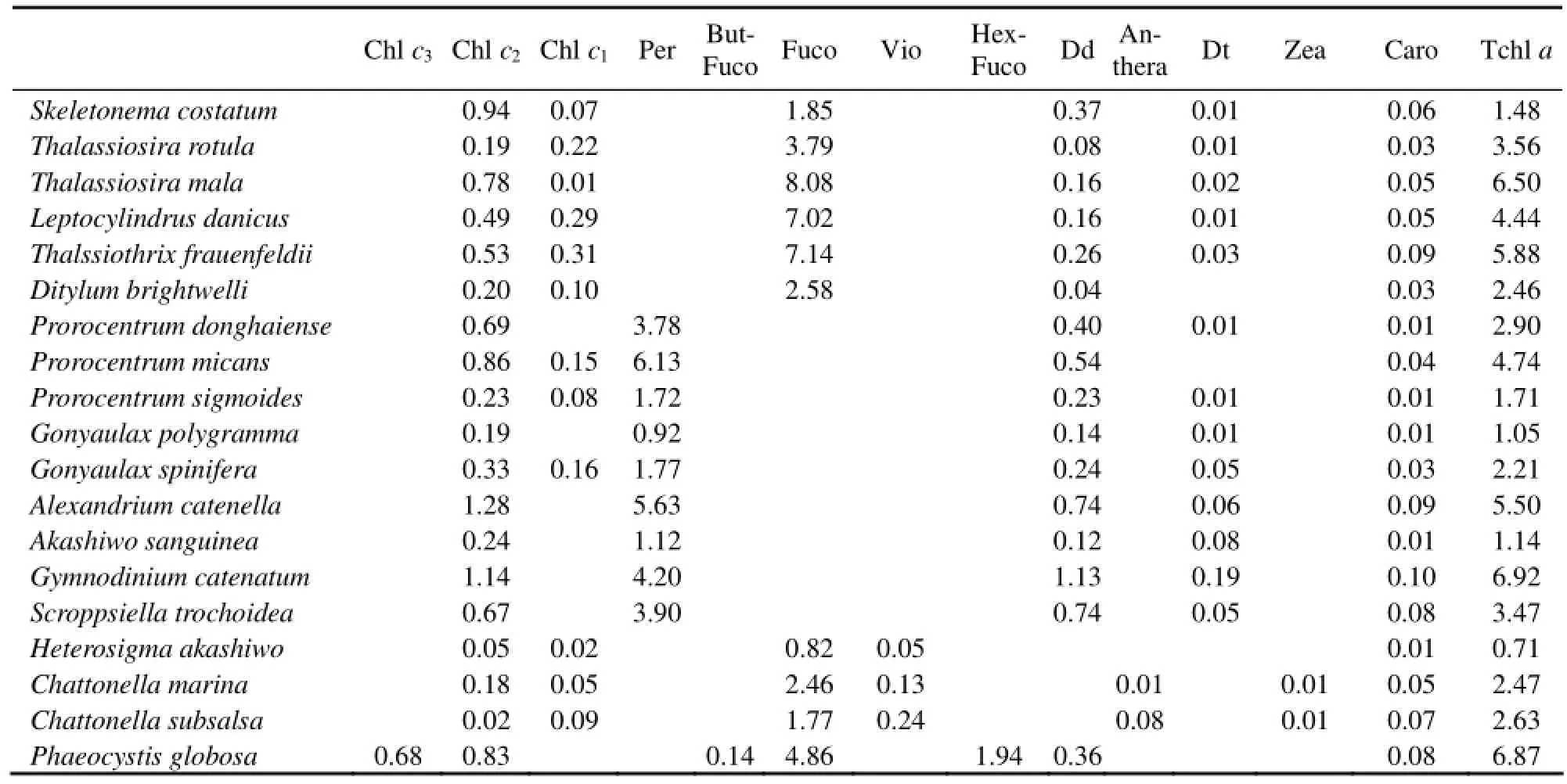
Table 4 Cellular pigment density (fg μm-3) of 19 species of harmful algae isolated from the coast of China
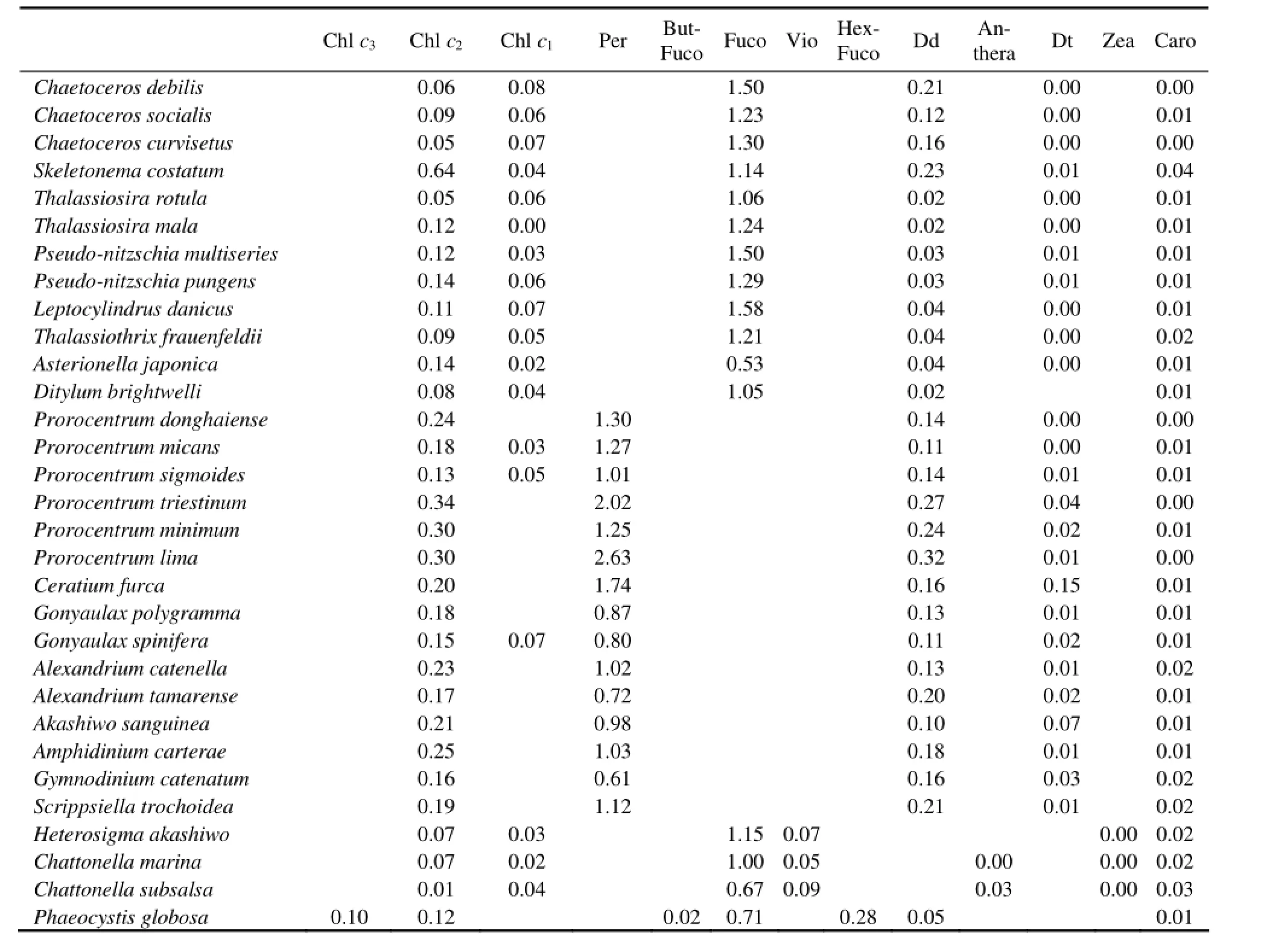
Table 5 Pigment ratios (pigments to total chlorophyll a (Tchl a)) of 31 harmful algae species isolated from the coast of China
3.3 Pigment Ratios
The major pigments to Tchl a ratios obtained from the 31 HAB species are presented in Table 5. The diagnostic pigments to Tchl a ratios differed less among different species compared with the non-diagnostic pigments to Tchl a ratios. The ratio of diagnostic pigments of diatoms (Fuco) to Tchl a ranged from 0.53 (in Asterionella japonica) to 1.58 (in Leptocylindrus danicus) with an average of 1.22, while it ranged from 0.67 (in Chattonella subsalsa) to 1.15 (in Heterosigma akashiwo) in raphidophyceae. The Per (diagnostic pigment of dianophyceae) to Tchl a ratios ranged from 0.61 (in Gymnodinium catenatum) to 2.63 (in Prorocentrum lima) with an average of 1.22.
Among the non-diagnostic pigments to Tchl a ratios, the Dd to Tchl a ratios differed the most among different algal species. These ratios ranged from 0.02 (in Ditylum brightwelli, Thalassiosira rotula and Thalassiosira mala) to 0.23 (in Skeletonema costatum) with an average of 0.08 in diatoms and ranged from 0.10 (in Akashiwo sanguinea) to 0.32 (in Prorocentrum lima) with an average of 0.17 in dinophyceae. The Chl c2to Tchl a ratios ranged from 0.05 (in Chaetoceros curvisetus and Thalassiosira rotula) to 0.64 (in Skeletonema costatum) with an average of 0.14 in diatoms and ranged from 0.13 (in Prorocentrum sigmoides) to 0.34 (in Prorocentrum triestium) with an average of 0.22 in dinophyceae. Within the three species of raphidophyceae, these ratios ranged from 0.01 (in Chattonella subsalsa) to 0.07 (in Chattonella marina) with an average of 0.05.
4 Discussion
4.1 Cell Volume and Cellular Pigment Density
As aforementioned, HPLC-CHEMTAX technique has become a routine approach to estimate the class abundance of phytoplankton in various aquatic systems (Lewitus et al., 2005; Wright et al., 2010; de Souza et al., 2012). However, since the accuracy of this method depends on the initial pigment ratio matrix, the results obtained with this method are usually compared with those obtained by traditional microscopy method. The result of HPLC-CHEMTAX is expressed as the contribution of certain classes/species to Tchl a while that of microscopy is expressed as the cell numbers per liter. In fact, the results of these two methods cannot be compared directly. Cellular pigment concentration/density is therefore used as a bridge to connect the results of these two methods. Apparently, the variations of these parameters due to genetic diversity within the same class and environmental factors will influence the result of comparison to some extent. Previous studies have shown that the cellular pigment concentration varies greatly among different species within a same class (Goericke et al., 1998). Moreover, both light intensity and growth rate can influence the cellular concentrations of Chl a of most algal species. In light-limited microalgae cultures cellular concentration of Chl a usually varies linearly as a function of log-irradiance (Falkowski, 1980), and in nutrient-limited cultures it usually changes linearly as a function of growth rate (Laws and Bannister, 1980). In our previous study the cellular concentrations of Chl a of Thalassiosira rotula ranged from 4.33 to 9.81 pg cell–1with light intensity (40 - 150 μmol photons m–2s–1) and ranged from 5.98 to 20.05 pg cell-1during growth (Liu et al., 2011). Considering the variation of environmental parameters during a bloom event, the instability of the cellular concentration of Chl a seems to weaken its applicability as a bridge to compare the results of HPLC-CHEMTAX method with traditional microscopic method in HAB monitoring.
Alternatively, a good agreement between microscope and HPLC-derived phytoplankton community assemblages has been observed when cellular pigment density is used as a bridge (Eker-Develi et al., 2012; de Souza et al., 2012). In general, the results of microscopy expressed in cells L-1must be converted to biovolume (mm3L-1) first when compared with the results of HPLC-CHEM- TAX using the cellular pigment density as a bridge. Therefore, the cell volume is also important for this process besides cell pigment density itself.
From our results, the cell volumes showed a big variation among different species even within the same class. Moreover, the cell volumes obtained in this study were significantly lower than those reported by Sun et al. (1999), e.g., the cell volumes of Thalassiosira rotula, Leptocylindrus danicus, Ditylum brightwelli, and Prorocentrum mican in the present study were 5426, 251, 8252, and 5057 μm3, respectively, while the corresponding cell volumes in Sun et al. (1999) were 15904, 6322, 123889, and 11550 μm3, respectively. However, the cell volumes in our study were close to those reported by Llewellyn and Gibb (2000) and Ruivo et al. (2011). In fact, cell volume is a parameter which varies with different growth conditions (Llewellyn and Gibb, 2000), e.g. the cell volume of Chaetoceros debilis in exponential phase and stationary phase were 125 μm3and 47 μm3, respectively, in Llewellyn and Gibb (2000). The species employed in this study and those in the studies of Llewellyn and Gibb (2000) and Ruivo et al. (2011) are all laboratory cultures, while in the study of Sun et al. (1999), the species were collected from the field directly. So, the different culturing conditions are presumed to be the main cause of differences in cell volumes between these studies.
Compared with cell volumes, the cellular pigment density shows less variation among species and with environmental parameters. Although the light intensity for culturing in this study (40 μmol photons m–2s–1) is different from that in Llewellyn and Gibb (2000) (98-146 μmol photons m–2s–1), the cellular pigment densities of Skeletonema costatum in these two studies are similar to each other. For example, the cellular densities of Chl c (Chl c2plus c1), Fuco, Dd and Tchl a of Skeletonema costatum in this study were 1.01, 1.85, 0.37 and 1.48 fg μm-3, respectively, and these values in the study of Llewellyn and Gibb (2000) were 1.68, 1.18, 0.17 and 8.23 fg μm-3(in exponential phase), respectively. Llewellyn and Gibb(2000) studied the cellular densities of Chl a of 20 species at exponential and stationary time and found that this parameter varied by a factor of above 2 in 6 species only. All these studies showed the minor variation of cellular pigment density under different growth conditions. The relative stability of this parameter makes it more suitable as a bridge than cellular pigment concentration.
From these results, we can see that the cellular pigment density is a stable parameter. The variation of cell volumes with species and environmental conditions is the main factor influencing the usage of cellular pigment density as a bridge to convert the results between microscopy method and HPLC-CHEMTAX technique. However, compared with the cellular pigment concentration, cell volume can easily be determined. The cell volume with similar reality can be obtained from captured images by a camera attached to the microscope or during observation, and then, biovolume is estimated using the most similar geometric shape (Hillebrand et al., 1999). Therefore, we recommend cellular pigment density as a bridge to compare the results of these two different methods.
4.2 Pigment Ratios
In general, the pigment ratios obtained in this study are in the range reported by Higgins et al. (2011), in which the pigment to Tchl a ratios for freshwater, estuarine, coastal and oceanic environments from 66 cultures and field studies have been reviewed. For example, the ranges of ratios of Chl c2:Tchl a, Chl c1:Tchl a, Fuco:Tchl a, Dd:Tchl a, Dt:Tchl a and Caro:Tchl a in diatoms obtained in this study were 0.05-0.64, 0-0.08, 0.53-1.58, 0.02-0.23, 0-0.01, and 0-0.04, respectively. The corresponding values reported by Higgins et al. (2011) were 0.012-0.310, 0-0.057, 0.191-1.710, 0.011-0.784, 0.002-0.296, and 0.001-0.111, respectively; The ratios Chl c2: Tchl a, Dd:Tchl a, Dt:Tchl a, and Caro: Tchl a in dinophyceae obtained in this study ranged from 0.13 to 0.34, from 0.10 to 0.32, from 0 to 0.15, and from 0 to 0.02, respectively. The corresponding values in previous studies ranged from 0.090 to 0.568, from 0.123 to 0.456, from 0 to 0.242, and from 0.002 to 0.119, respectively (Higgins et al., 2011). The Per:Tchl a ratios in dinophyceae in this study were higher than those in previous studies, e.g., the maximum of this value in previous studies was 1.028 while most of Per:Tchl a ratios in this study were above this value, and the maximum of Per:Tchl a ratio in this study was 2.63 (in Prorocentrum lima).
Differences for the same species among different studies also exist. The pigment ratios, especially the diagnostic pigment ratios for the species isolated from the coast of China are higher than those from other isolations. For example, the Fuco:Tchl a ratio in S. costatum in this study is 1.14 while it is 0.143 in Llewellyn and Gibb (2000) (CCAP-1077/5) and 0.384 in Laviale and Neveux (2011) (RCC-70), respectively. However, the Fuco/Tchl a ratio in S. costatum in this study was very close to that in the same species with the same isolation reported by Yao et al. (2006) (1.171). The Fuco:Tchl a ratio in Ditylum brightwellii in this study (1.05) was also higher than that reported by Schlüter et al. (2000) (0.522). The same cases occur in C. debilis. The Fuco:Tchl a ratio in this species obtained in this study was 1.50 while it was 0.395 in Llewellyn and Gibb (2000).
Similarly, the diagnostic pigment ratios of dinophyceae species isolated from the coast of China are also higher compared with others. For example, the Per:Tchl a ratio in Prorocentrum micans in this study is 1.27 while it ranged from 0.375 to 0.442 at different light intensities (23-554 μmol photons m–2s–1) as reported by Schlüter et al. (2000) (SCCAP, K-0335). For Prorocentrum minimum, this pigment ratio obtained by the present study (1.25) is also higher than those obtained by Rodríguez et al. (2006) (0.320 at 35 μmol photons m-2s-1; 0.285 at 500 μmol photons m-2s-1) and Laviale and Neveux (2011) (0.406). However, the other pigment ratios including Chl c2:Tchl a, Dd:Tchl a and Dt:Tchl a in Prorocentrum minimum obtained by Rodríguez et al. (2006) and this study are very close to each other.
Differences of pigment ratios among different studies are relatively small for raphidophyceae and haptophyceae. The ratios of Chl c3:Tchl a, Chl c2:Tchl a, But-Fuco:Tchl a, Fuco:Tchl a and Hex-Fuco:Tchl a in Phaeocystis globosa obtained in this study are 0.10, 0.12, 0.02, 0.71 and 0.28 (g g-1), respectively. The corresponding values in previous studies range from 0.115-0.233, 0.145-0.198, 0.000- 0.040, 0.090-1.035 and 0.022-0.227 (g g-1), respectively (Zapata et al., 2004; Rodríguez et al., 2006; Seoane et al., 2009); The pigment ratios in Heterosigma akashiwo obtained by this study are also similar to those reported in Rodríguez et al. (2006).
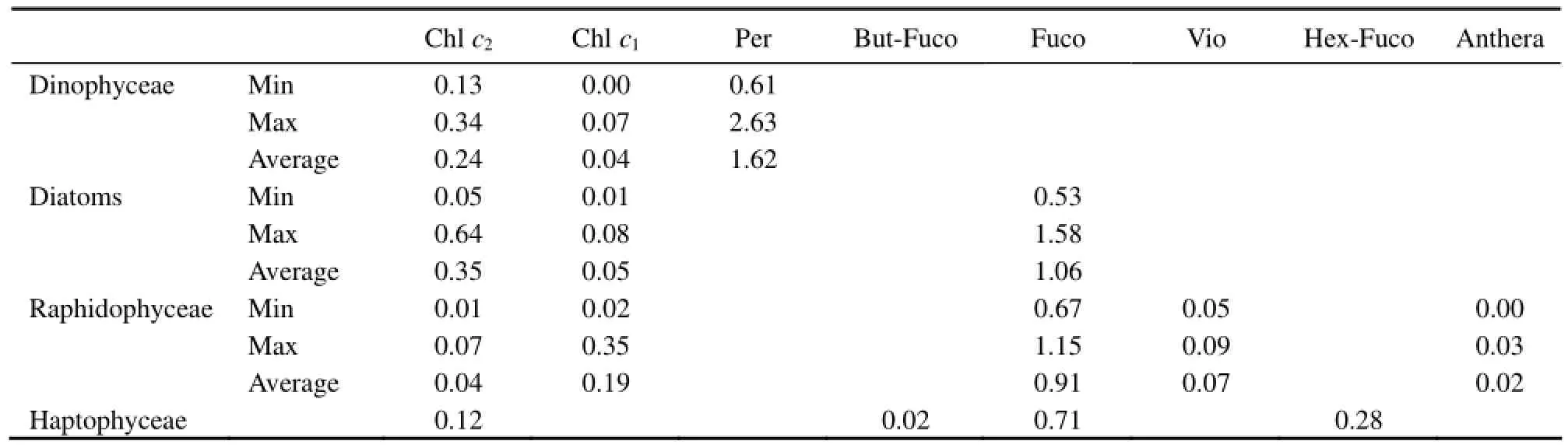
Table 6 Ranges of pigment to Chl a ratios in this study
Although pigment ratio is influenced by a series of environmental factors (Schlüter et al., 2000; Rodríguez et al., 2006), differences in pigment:Chl a ratios for the same species with different isolations underline the importance of obtaining pigment data from regional isolates. The average value and range of pigment to Chl a ratios of four classes based on this work are summarized in Table 6. We believe that the ratios within the range in this table are more suitable as initial pigment ratios of the corresponding classes for the application of HPLC-CHEM- TAX method in the coast of China.
4.3 Pigment Profiles and Phylogenetic Trees for Prorocentrum Genus
In this study, we found significant differences for the Per/Tchl a ratio among different dinophyceae species even within the same genus. The Per/Tchl a ratios range from 1.01 of Prorocentrum sigmoides to 2.63 of Prorocentrum lima in Prorocentrum, increased by 2.6 times. Such differences, if not occurring unoccasionally, have the potential to realize the classification of phytoplankton groups under a lower level than class by HPLCCHEMTAX technique (Wright et al., 2010). In order to further interpret the differences of pigment ratios existing in this study, we compared these differences among different Prorocentrum species with the phylogetic trees.
Wang et al. (2007) cloned and sequenced the 28S rDNA partial sequences of several species of Prorocentrum and constructed the phylogenetic trees using the methods of NJ (neighbour-joining) and ME (minimum evolution). Among those species, P. donghaiense, P. micans, P. minimum and P. triestinum are the same strains we used in this study. The differences of the Per/Tchl a ratios among different Prorocentrum species are consistent with those of the genetic relationships they found. This is a convincing proof that the differences did not occur randomly. In fact, the consistence between the differences of pigments among different species under the same culturing conditions and the biological differences had been proved by other studies (Latasa et al., 2004; Zapata et al., 2004). Such differences can be potentially applied to distinguish the contribution of a species, not a class of phytoplankton to total chlorophyll a. This is especially meaningful to use the HPLC-CHEMTAX technique to monitor HABs.
5 Conclusions
In this study, pigment profiles of 31 harmful algal species isolated from the coast of China were studied. The cellular pigment density is less affected by species and environmental conditions. It is more suitable as a bridge to compare the results of HPLC-CHEMTAX technique with the traditional microscopy method. However, cautions must be observed because of the variation of cell volume under different physical conditions. Compared with literatures, there are obvious geographical characteristics for pigment ratios, which underline the importance of obtaining pigment data from regional isolates. The consistence between the differences of pigments among different species under the same culturing conditions and the biological differences highlight the potential to develop a method to classify phytoplankton at a lower level than class, which is meaningful to monitor HABs with the HPLC-CHEMTAX technique.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 41176063 and 41221004) and the National Marine Public Welfare Research Project of China (No. 201205031). The authors are grateful to Prof. Songhui Lü and Dr. Rongguo Su for providing some of the algal cultures used in this study, and Ms. Yan Zheng, Ms. Xia Zhu, and Mr. Jian Yuan for laboratory assistance. This is MCTL contribution No.50.
Abdenadher, M., Hamza, A., Fekih, W., Hannachi, I., Bellaaj, A. Z., Bradai, M. N., and Aleya, L., 2012. Factors determining the dynamics of toxic blooms of Alexandrium minutum during a 10-year study along the shallow southwestern Mediterranean coasts. Estuarine, Coastal and Shelf Science, 106: 102-111.
Cohu, S., Mangialajo, L., Thibaut, T., Blanfuné, A., Marro, S., and Lemée, R., 2013. Proliferation of the toxic dinoflagellate Ostreopsis cf. ovata in relation to depth, biotic substrate and environmental factors in the North West Mediterranean Sea. Harmful Algae, 24: 32-44.
de Souza, M. S., Mende, C. R. B., Garcia, V. M. T., Pollery, R., and Brotas, V., 2012. Phytoplankton community during a coccolithophorid bloom in the Patagonian Shelf: Microscopic and high-performance liquid chromatography pigment analyses. Journal of the Marine Biological Association of the UK, 92 (1): 13-27.
Eker-Develi, E., Berthon, J., Canuti, E., Slabakova, N., Moncheva, S., Shtereva, G., and Dzhurova, B., 2012. Phytoplankton taxonomy based on CHEMTAX and microscopy in the northwestern Black Sea. Journal of Marine Systems, 94: 18-32.
Falkowski, P. G., 1980. Primary Productivity in the Sea. Plenum Press, New York, 99-119.
Goericke, R., and Montoya, J. P., 1998. Estimating the contribution of microalgal taxa to chlorophyll a in the field-variations of pigment ratios under nutrient- and light-limited growth. Marine Ecology Progress Series, 169: 97-112.
Galluzzi, L., Bertozzini, E., Penna, A., Perini, F., Pigalarga, A., Granéli, E., and Magnani, M., 2008. Detection and quantification of Prymnesium parvum (Haptophyceae) by real-time PCR. Letters in Applied Microbiology, 46: 261-266.
Gieskes, W. W. C., and Kraay, G. W., 1986. Floristic and physiological differences between the shallow and the deep nanophytoplankton community in the euphotic zone of the open tropical Atlantic revealed by HPLC analysis of pigments. Marine Biology, 91: 567-576.
Hou, J. J., Huang, B. Q., Hu, J., Lin, L. Z., and Hong, H. S., 2008. Fourteen FITC-conjugated lectins as a tool for the recognition and differentiation of some harmful algae in Chinese coastal waters. Journal of Applied Phycology, 20: 35-46.
Hillebrand, H., Dürselen, C. D., Kirschtel, D., Pollingher, U., and Zohary, T., 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology,35: 403-424.
Higgins, H. W., Wright, S. W., and Schlüter, L., 2011. Quantitative interpretation of chemotaxonomic pigment data. In: Phytoplankton Pigments Characterization, Chemotaxonomy and Applications in Oceanography. Roy, S., et al., eds., Cambridge University Press, Cambridge, 257-313.
Jeffrey, S. W., and Hallegraeff, G. W. 1987. Chlorophyllase distribution in ten classes of phytoplankton: a problem for chlorophyll analysis. Marine Ecology Progress Series,35: 293-304.
Jeffrey, S. W., 1997. Chlorophyll and carotenoid extinction coefficients. In: Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods. Jeffrey, S. W., et al., UNESCO Publishing, Paris, 595-596.
Jeffrey, S. W., and Wright, S. W., 2006. Photosynthetic pigments in marine microalgae: insights from cultures and the sea. In: Algal Cultures, Analogues of Blooms and Applications. Subba Rao, D. V., ed., Science Publishers, Enfield, NH, 33-90.
Klein, B., and Sournia, A., 1987. A daily study of the diatom spring bloom at Roscoff, France, in 1985. II. Phytoplankton pigment composition studied by HPLC analysis. Marine Ecology Progress Series,37: 265-275.
Latasa, M., Scharek, R., Gall, F. L., and Guillou, L., 2004. Pigment suites and taxonomic groups in prasinophyceae. Journal of Phycology,40: 1149-1155.
Laviale, M., and Neveux, J., 2011. Relationships between pigment ratios and growth irradiance in 11 marine phytoplankton species. Marine Ecology Progress Series,425: 63-77.
Laws, E. A., and Bannister, T. T., 1980. Nutrient- and lightlimited growth of Thalassiosira fluviatilis in continuous culture, with implications for phytoplankton growth in the ocean. Limnolgy Oceanography,25: 457-473.
Lewitus, A. J., White, D. L., Tymowski, R. G., Geesey, M. E., Hymel, S. N., and Noble, P. A., 2005. Adapting the CHEMTAX method for assessing phytoplankton taxonomic composition in Southeastern U.S. Estuaries. Estuaries,28(1): 160-172.
Liu, S. X., Yu, Z. G., Yao, P., Zheng, Y., and Li, D., 2011. Effects of irradiance on pigment signatures of harmful algae during growth process. Acta Oceanologica Sinica,30(6): 46-57.
Llewellyn, C. A., and Gibb, S. W., 2000. Intra-class variability in the carbon, pigment and biomineral content of prymnesiophytes and diatoms. Marine Ecology Progress Series,193: 33-44.
Mackey, M. D., Mackey, D. J., Higgins, H. W., and Wright, S. W., 1996. CHEMTAX - a program for estimating class abundances from chemical markers: Application to HPLC measurements of phytoplankton. Marine Ecology Progress Series,144: 265-283.
Rodríguez, F., Chauton, M., Johnsen, G., Andresen, L., Olsen, L. M., and Zapata, M., 2006. Photoacclimation in phytoplankton: Implications for biomass estimates, pigment functionality and chemotaxonomy. Marine Biology,148: 963-971.
Ruivo, M., Amorim, A., and Cartaxana, P., 2011. Effects of growth phase and irradiance on phytoplankton pigment ratios: Implications for chemotaxonomy in coastal waters. Journal of Plankton Research,33(7): 1012-1022.
Schlüter, L., Møhlenberg, F., Havskum, H., and Larsen, S., 2000. The use of phytoplankton pigments for identifying and quantifying phytoplankton groups in coastal areas: Testing the influence of light and nutrients on pigment/chlorophyll a ratios. Marine Ecology Progress Series,192: 49-63.
Seoane, S., Zapata, M., and Orive, E., 2009. Growth rates and pigment patterns of haptophytes isolated from estuarine waters. Journal of Sea Research,62: 286-294.
Sun, J., Liu, D. Y., and Qian, S. B., 1999. Study on phytoplankton biomass I. Phytoplankton measurement biomass from cell volume or plasma volume. Acta Oceanologica Sinica,21(2): 75-85 (in Chinese with English abstract).
Tomas, C. R., and Smayda, T. J., 2008. Red tide blooms of Cochlodinium polykrikoides in a coastal cove. Harmful Algae,7: 308-317.
van Oostende, N., Harlay, J., Vanelslander, B., Chou, L., Vyverman, W., and Sabbe, K., 2012. Phytoplankton community dynamics during late spring coccolithophore blooms at the continental margin of the Celtic Sea (North East Atlantic, 2006-2008). Progress in Oceanography,104: 1-16.
Wang, B., Mi, T. Z., Lü, S. H., Sun, J., Li, R. X., and Yu, Z. G., 2007. Cloning and analysis of 28S rDNA partial sequences of several strains of Prorocentrum. Acta Oceanologica Sinica,29(1): 120-126 (in Chinese with English abstract).
Wright, S. W., van den Enden, R. L., Pearce, I., Davidson, A. T., Scott, F. J., and Westwood, K. J., 2010. Phytoplankton community structure and stocks in the Southern Ocean (30–80˚E) determined by CHEMTAX analysis of HPLC pigment signatures. Deep-Sea Research II,57: 758-778.
Yao, P., Yu, Z. G., and Deng, C. M., 2006. Pigment signatures of some diatoms isolated from China seas. Acta Oceanologica Sinica,25(1): 108-118.
Yao, P., Yu, Z. G., Deng, C. M., Liu, S. X., and Zhen, Y., 2011. Classification of marine diatoms using pigment ratio suites. Chinese Journal of Oceanology and Limnology,29(5): 1075-1085.
Yan, T., Zhou, M. J., and Zou, J. Z., 2002. A national report on harmful algal blooms in China. In: Harmful Algal Blooms in the PICES Region of the North Pacific. Taylor, F. J. R., and Trainer, V. L., eds., Secretariat, 21-32.
Yu, J., Tang, D. L., Oh, I. S., and Yao, L. J., 2007. Response of harmful algal blooms to environmental changes in Daya Bay, China. Terrestrial, Atmospheric and Oceanic Sciences,18(5): 1011-1027.
Zapata, M., Jefrrey, S. W., Wright, S. W., Rodríguez, F., Garrido, J. L., and Clementson, L., 2004. Photosynthetic pigments in 37 species (65 strains) of Haptophyta: Implications for oceanography and chemotaxonomy. Marine Ecology Progress Series,270: 83-102.
Zapata, M., Rodríguez, F., Fraga, S., Barra, L., and Ruggiero, M. V., 2011. Chlorophyll c pigment patterns in 18 species (51 strains) of the genus Pseudo-nitzschia (Bacillariophyceae). Journal of Phycology,47: 1274-1280.
Zapata, M., Rodríguez, F., and Garrido, J. L., 2000. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8column and pyridine-containing mobile phases. Marine Ecology Progress Series,195: 29-45.
(Edited by Ji Dechun)
(Received August 1, 2013; revised April 10, 2014; accepted June 2, 2014)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-532-66781006
E-mail: zhigangyu@ouc.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- The Formation of Wind Curl in the Marine Atmosphere Boundary Layer over the East China Sea Kuroshio in Spring
- Wind Wave Characteristics and Engineering Environment of the South China Sea
- Prediction of the Mooring Force of a 2-D Floating Oil Storage Tank
- An Experimental Study on the Wave-Induced Pore Water Pressure Change and Relative Influencing Factors in the Silty Seabed
- Fe-Si-Mn-Oxyhydroxide Encrustations on Basalts at East Pacific Rise near 13˚N: An SEM – EDS Study
- Seasonal Changes in Phytoplankton Biomass and Dominant Species in the Changjiang River Estuary and Adjacent Seas: General Trends Based on Field Survey Data 1959 - 2009
