右美托咪啶预处理对大鼠肾缺血再灌注后肝肾抗氧化能力的影响
2014-04-21冯泽国岳剑虹赵艳军颜光涛
鄢 娜,冯泽国,岳剑虹,赵艳军,颜光涛
解放军总医院,北京 100853 1麻醉手术中心;2基础医学研究所
右美托咪啶预处理对大鼠肾缺血再灌注后肝肾抗氧化能力的影响
鄢 娜1,冯泽国1,岳剑虹1,赵艳军1,颜光涛2
解放军总医院,北京 1008531麻醉手术中心;2基础医学研究所
目的 探究右美托咪啶(dexmedetomidine,DEX)预处理对大鼠肾缺血再灌注损伤后肾及肝抗氧化能力的影响。方法90只雄性SD大鼠随机分为3组(n=30),右美托咪啶预处理组(DEX组),缺血再灌注组(IRI组),假手术组(Sham组)。DEX组于建模前30 min腹腔注射DEX (100 μg/kg),其余两组腹腔注射等量0.9%氯化钠注射液,建立肾缺血再灌注模型,再灌注后2 h、8 h、24 h肝肾组织匀浆检测超氧化物歧化酶(serum levels of superoxide dismutase,SOD)、一氧化氮(nitric oxide,NO)、乳酸(lactate dehydrogenases,LD)、还原型谷胱甘肽(reduced glutathione,GSH)及总抗氧化能力(total antioxidant capacity,T-AOC)。结果与IRI组相比,DEX组肾匀浆SOD升高,总NO降低,T-AOC升高(P<0.05),LD再灌注8 h后降低明显(P<0.05);肝SOD升高,总NO升高,T-AOC升高(P<0.05),LD再灌注8 h、24 h降低显著(P<0.05)。结论右美托咪啶预处理可提高大鼠肾缺血再灌注后肾及肝的抗氧化能力,减少乳酸堆积。
右美托咪啶;缺血再灌注损伤;肾;肝;抗氧化;大鼠
急性肾损伤(acute kidney injury,AKI)是大型心血管手术、器官移植手术等围术期内的常见严重并发症,表现为急进性肾功能恶化,肾缺血再灌注损伤是最重要的原因[1-3]。本实验旨在讨论右美托咪啶预处理对大鼠肾缺血再灌注损伤后肾及肝的抗氧化能力的影响。
材料和方法
1 实验动物及材料 选择健康清洁型成年雄性SD大鼠90只(解放军总医院实验动物中心提供,许可证号为SCXK(京)2012-0001),体质量220 ~ 240 g。适应环境1周,分笼饲养,室温20 ~ 25℃,湿度60% ~ 70%,自由摄食、饮水。注射用盐酸右美托咪啶200 μg/支(批号:13080834,13082832,江苏恒瑞医药股份有限公司)。超氧化物歧化酶(serum levels of superoxide dismutase, SOD)、一氧化氮(nitric oxide,NO)、总抗氧化能力(total antioxidant capacity,T-AOC)C、乳酸(lactate dehydrogenases,LD)试剂盒均购自南京建成生物工程研究所。
2 分组 90只SD雄性大鼠随机分为3组(n=30),右美托咪啶预处理组(DEX组),缺血再灌注组(IRI组),假手术组(Sham组),每组按再灌注后时间点不同各自分为3个亚组。DEX组统一于建模前30 min腹腔注射DEX 100μg/kg,其余两组则注射等量的0.9%氯化钠注射液。
3 肾缺血再灌注损伤大鼠模型制备 参考Sugita等[4]介绍的方法建立肾缺血再灌注损伤模型。术前禁食12 h,自由饮水,大鼠称重后,3%硫喷妥钠50 mg/kg腹腔注射麻醉,待翻正反射消失后,俯卧位于温度恒定于38℃的体温毯上,背侧入路,于肋弓下缘0.5 cm、脊柱旁开1 cm处做两侧纵形切口,分离肾蒂,游离输尿管,切除右侧肾,左侧肾蒂使用无损伤血管夹夹闭45 min。根据左肾颜色来判断缺血情况,确认其由鲜红转为整体肾暗红色时即可。45 min后松开血管夹进行再灌注,待肾由暗红色转为鲜红色,恢复血液供应后再将其还纳入腹腔并缝合伤口。Sham组仅切除右肾并分离左侧肾蒂,不进行钳夹处理。
4 标本收集及检测 每组各随机选取10只大鼠,于再灌注后2 h、8 h及24 h 3个时间点,麻醉后统一获取左肾及肝左上叶,置于0.9%氯化钠注射液中,去除表面残血后液氮快速冻存。根据检测指标试剂盒要求制备肝及肾组织匀浆,测定肝及肾SOD、NO、LD及T-AOC含量。
5 统计学处理 采用SPSS17.0统计学软件进行分析,计量资料以表示,每组不同时间点指标比较采用双因素方差分析,组间比较采用单因素方差分析,P<0.05为差异有统计学意义。
结 果
1 肾抗氧化能力 与IRI组相比,再灌注后2 h、8 h、24 h,DEX组肾组织匀浆SOD、T-AOC含量均升高(P<0.05),总NO含量下降(P<0.05),LD含量于再灌注后8 h下降,且差异具有统计学意义(P<0.05);与Sham组相比,再灌注后2 h、8 h、24 h,DEX组T-AOC含量均升高,且差异具有统计学意义(P<0.05)。见图1。
2 肝抗氧化能力 与IRI组相比,再灌注后2 h、8 h、24 h,DEX组肝组织匀SOD、总NO、T-AOC含量升高(P<0.05),LD含量降低(P<0.05);与Sham组相比,再灌注后2 h、8 h、24 h,DEX组肝匀浆总NO含量升高(P<0.05),T-AOC含量升高(P<0.05), LD于再灌注后8 h升高明显(P<0.05)。见图2。
讨 论
肾缺血再灌注损伤是临床大型心血管、移植手术中常见的临床问题,严重时可导致急性肾损伤,降低了远期生存率[2-3,5-6]。肾缺血再灌注损伤引起体内炎症反应的发生,加之再灌注期间体内产生高浓度的活性氧簇(reactive oxygen species,ROS),从而导致远隔脏器,如肝的功能障碍,严重时可导致肝衰竭,增大术后并发症处理难度[7]。有研究表明右美托咪啶对肾缺血再灌注损伤具有保护作用。其抗缺血再灌注损伤作用机制可能与其能减弱交感神经兴奋性、抑制肾素释放、在再灌注期间阻止肾血管痉挛而起到增加肾脏血流、提高肾小球率过滤、增加钠水排泄相关,也与其通过激活相关促存活通路如PI3K/Akt、ERK1/2及HMGB-1/TLR-4信号通路,起到抗缺血再灌注损伤作用相关[8-15]。同时其本身具有镇痛作用减少了非甾体类抗炎药及吗啡的使用,降低了由于非甾体类抗炎药导致的急性肾损伤的风险及吗啡在急性肾损伤患者体内堆积所产生的危害[15]。然而关于DEX是否可以减轻肾缺血再灌注损伤后所致的肝损伤的研究甚少。
本实验结果表明,与IRI组相比,DEX组肾的SOD、T-AOC值于再灌注后2 h、8 h及24 h升高,表明DEX预处理后,大鼠肾的抗氧化能力有所提高;LD含量,IRI组于再灌注后8 h升高(P<0.05),推测DEX预处理可能通过减轻肾缺氧引起的糖酵解增加,或加强肾排泄消除堆积LD的能力来起到保护作用,致使乳酸在再灌注过程中维持相对平稳的水平;肾总NO结果显示,DEX预处理后肾脏总NO含量较IRI组降低,该结果与图2中肝总NO含量结果恰恰相反。肝总NO结果显示,DEX预处理后肝总NO含量较IRI组升高。机体的NO是由L-精氨酸与氧分子在不同一氧化氮合酶(NOS)的催化下生成的,目前研究发现的NOS有3种,内皮型NOS(eNOS)、神经型NOS(nNOS)及诱生型NOS(iNOS)。本实验仅针对肝、肾总NO含量进行测定,而未进行分型测定,故大鼠肝、肾中NO主要来源未知,有待进一步实验研究探索。图2结果表明,与IRI组相比,DEX组肝SOD、T-AOC含量升高(P<0.05),清除氧自由基能力增强;LD结果表明,大鼠肾缺血再灌注后,肝乳酸增多,DEX可以减轻肝乳酸堆积情况,进而产生肝的保护作用。
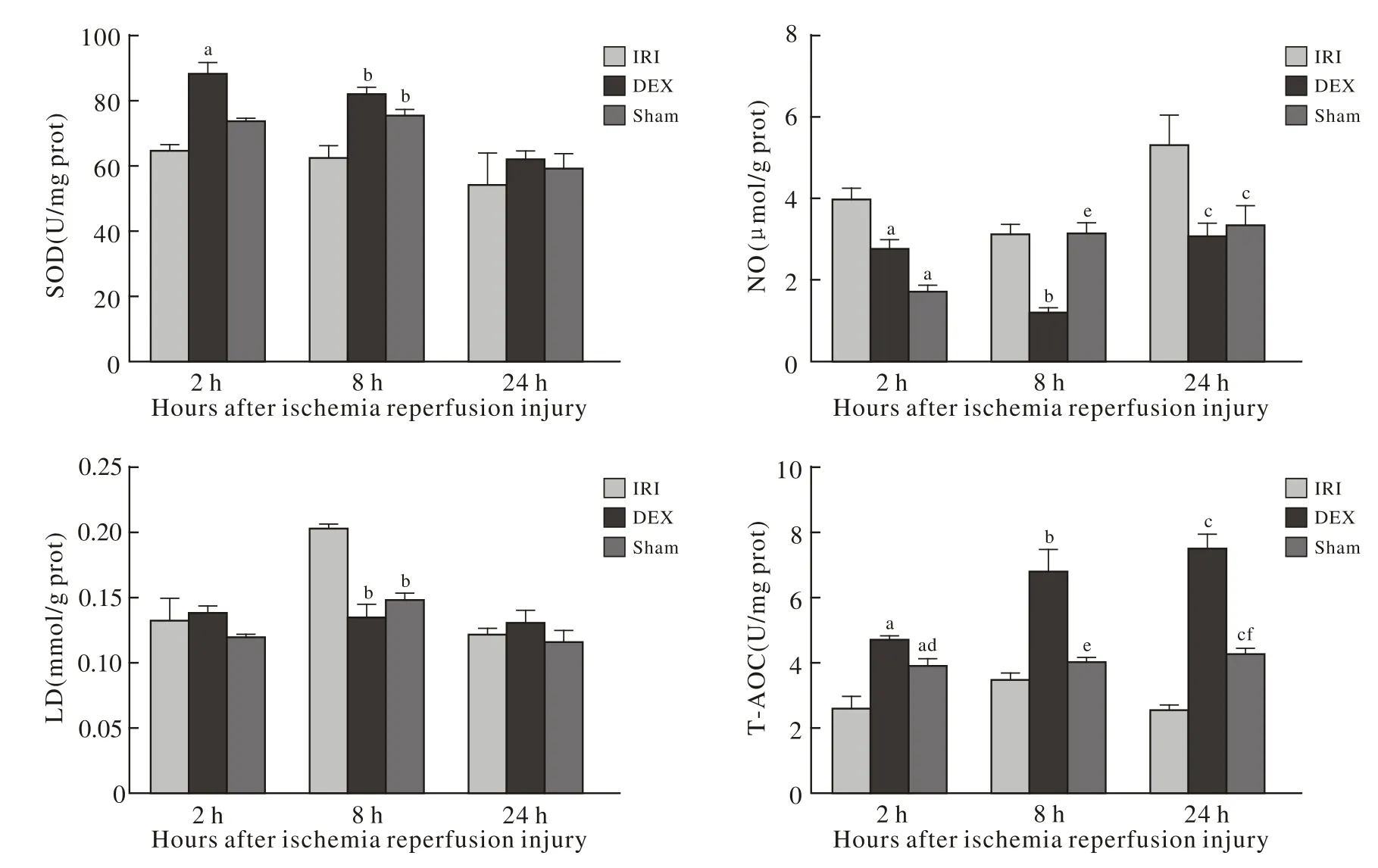
图 1 大鼠肾组织匀浆SOD、 NO、 LA、 T-AOC检测结果Fig. 1 Serum SOD, NO, LA levels and T-AOC in renal tissue of ratsP<0.05, vs I/R injury group at different time points; P<0.05, vs RHP at different time points
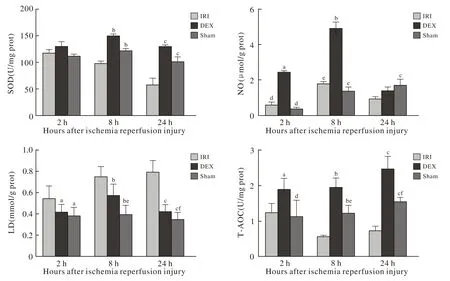
图 2 大鼠肝组织匀浆SOD、 NO、 LA、 T-AOC检测结果Fig. 2 Serum SOD, NO, LA levels and T-AOC in renal tissue of rats
综上所述,右美托咪啶预处理可以提高大鼠肾缺血再灌注后的肾及肝对氧自由基的清除能力,提高总抗氧化能力,减少乳酸堆积情况,进而起到对肾及肝的保护作用。
1 Perico N, Cattaneo D, Sayegh MH, et al. Delayed graft function in kidney transplantation[J]. Lancet, 2004, 364(9447):1814-1827.
2 Stafford-Smith M, Shaw A, Swaminathan M. Cardiac surgery and acute kidney injury: emerging concepts[J]. Curr Opin Crit Care,2009, 15(6): 498-502.
3 Balasubramanian G, Al-Aly Z, Moiz A, et al. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot study[J]. Am J Kidney Dis, 2011, 57(2): 228-234.
4 Sugita S, Okabe T, Sakamoto A. Continuous infusion of dexmedetomidine improves renal ischemia-reperfusion injury in rat kidney[J]. J Nippon Med Sch, 2013, 80(2): 131-139.
5 Akhtar MZ, Henderson T, Sutherland A, et al. Novel approaches to preventing ischemia-reperfusion injury during liver transplantation[J]. Transplant Proc, 2013, 45(6): 2083-2092.
6 Chen HH, Sundt TM, Cook DJ, et al. Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study[J]. Circulation, 2007, 116(11 Suppl): I134-I138.
7 Serteser M, Koken T, Kahraman A, et al. Changes in hepatic TNF-alpha levels, antioxidant status, and oxidation products after renal ischemia/reperfusion injury in mice[J]. J Surg Res, 2002, 107(2):234-240.
8 Tsutsui H, Sugiura T, Hayashi K, et al. Moxonidine prevents ischemia/reperfusion-induced renal injury in rats[J]. Eur J Pharmacol, 2009, 603(1/3): 73-78.
9 Billings FT, Chen SW, Kim M, et al. Alpha2-Adrenergic agonists protect against radiocontrast-induced nephropathy in mice[J]. Am J Physiol Renal Physiol, 2008, 295(3): F741-F748.
10 Morgan CJ, Gill PJ, Lam S, et al. Peri-operative interventions, but not inflammatory mediators, increase risk of acute kidney injury after cardiac surgery: a prospective cohort study[J]. Intensive Care Med, 2013, 39(5):934-941.
11 Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery[J]. Br J Anaesth, 2012, 109(S1):i29-i38.
12 Chi OZ, Hunter C, Liu X, et al. The effects of dexmedetomidine on regional cerebral blood flow and Oxygen consumption during severe hemorrhagic hypotension in rats[J]. Anesth Analg, 2011, 113(2):349-355.
13 Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery[J]. Cell, 1997, 91(2): 231-241.
14 O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling[J]. Nat Rev Immunol,2007, 7(5):353-364.
15 Gu J, Sun P, Zhao H, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice[J]. Crit Care, 2011, 15(3):R153.
Effect of dexmedetomidine pretreatment on antioxidant ability of kidney and liver in rats following renal ischemia/reperfusion injury
YAN Na1, FENG Ze-guo1, YUE Jian-hong1, ZHAO Yan-jun1, YAN Guang-tao2
1Anesthesia and Operation Center;2Institute of Basic Medical Sciences Chinese PLA General Hospital, Beijing 100853, China
FENG Ze-guo. Email:beijing_301@sina.com
ObjectiveTo study the effect of dexmedetomidine (DEX) pretreatment on antioxidant ability of kidney and liver in rats following renal ischemia/reperfusion (I/R) injury.MethodsNinety male SD rats were randomly divided into DEX pretreatment group, I/R injury group, and sham operation group (30 in each group). DEX (100 μg/kg) was injected into the abdominal cavity of rats in DEX pretreatment group 30 min before the model was established. A renal I/R injury model was established for I/R injury group and sham operation group, respectively, by injecting 0.9% sodium chloride (100 μg/kg). Serum levels of superoxide dismutase (SOD), nitric oxide (NO) and lactate dehydrogenases (LD), reduced glutathione (GSH) and total antioxidant capacity (T- AOC) in liver and renal tissues were measured 2, 8 and 24 h, respectively, after reperfusion.ResultsThe serum SOD level and T-AOC were higher and the serum NO level was lower in DEX pretreatment group than in I/R injury group (P<0.05). The serum LA level was signifcantly lower while the serum SOD and NO level and T-AOC were signifcantly higher in DEX pretreatment group than in I/ R injury group 8 h after reperfusion (P<0.05) and the serum LA level was signifcantly lower in DEX pretreatment group than in I/ R injury group 8 and 24 h after reperfusion (P<0.05).ConclusionDEX pretreatment can increase the antioxidant ability of kidney and liver in rats following renal I/R injury, thus reducing LA accumulation.
dexmedetomidine; reperfusion injure; kidney; liver; antioxidation; rats
R 361
A
2095-5227(2014)07-0755-04
10.3969/j.issn.2095-5227.2014.07.030
时间:2014-03-10 17:28
http://www.cnki.net/kcms/detail/11.3275.R.20140310.1728.005.html
2013-12-20
鄢娜,女,硕士。专业方向:麻醉学。Email:yanna871106@163.com
冯泽国,男,硕士生导师,主任医师。Email:beijing_301@sina.com
