Staging the axilla in women with breast cancer: the utility of preoperative ultrasound-guided needle biopsy
2014-03-29NehmatHoussamiRobinTurner
Nehmat Houssami, Robin M. Turner
Screening and Test Evaluation Program (STEP), School of Public Health, Sydney Medical School, University of Sydney, Sydney 2006, Australia
Staging the axilla in women with breast cancer: the utility of preoperative ultrasound-guided needle biopsy
Nehmat Houssami, Robin M. Turner
Screening and Test Evaluation Program (STEP), School of Public Health, Sydney Medical School, University of Sydney, Sydney 2006, Australia
Preoperative staging of the axilla in women with invasive breast cancer using ultrasound-guided needle biopsy (UNB) identi fi es approximately 50% of patients with axillary nodal metastases prior to surgical intervention. Although moderately sensitive, it is a highly speci fi c staging strategy that is rarely falsely-positive, hence a positive UNB allows patients to be triaged to axillary lymph-node dissection (ALND) avoiding potentially unnecessary sentinel node biopsy (SNB). In this review, we extend our previous work through an updated literature search, focusing on studies that report data on UNB utility. Based on data for 10,934 breast cancer patients, sourced from 35 studies, a positive UNB allowed triage of 1,745 cases (simple proportion 16%) to axillary surgical treatment: the utility of UNB was a median 19.8% [interquartile range (IQR) 11.6%-26.7%] across these studies. We also modelled data from a subgroup of studies, and estimated that amongst patients with metastases to axillary nodes, the odds ratio (OR) for high nodal disease burden for a positive UNB versus a negative UNB was 4.38 [95% confidence interval (95% CI): 3.13, 6.13], P<0.001. From this model, the estimated proportion with high nodal disease burden was 58.9% (95% CI: 50.2%, 67.0%) for a positive UNB, whereas the estimated proportion with high nodal disease burden was 24.6% (95% CI: 17.7%, 33.2%) if UNB was negative. Overall, axillary UNB has good clinical utility and a positive UNB can e ff ectively triage to ALND. However, the evolving landscape of axillary surgical treatment means that UNB will have relatively less utility where surgeons have modi fi ed their practice to omission of ALND for minimal nodal metastatic disease.
Breast cancer; axillary staging; node metastases; test utility; ultrasound-guided needle biopsy (UNB)
Introduction
Surgical management of the axilla in women with invasive breast cancer has changed considerably in the last two decades: sentinel node biopsy (SNB) has replaced axillary lymph node dissection (ALND) as the primary surgical staging approach1,2with selective ALND based on the status of the sentinel node(s). More recently, evidence from a landmark randomized trial (Z0011)3has shown that omission of ALND may be appropriate in de fi ned groups of patients (clinical stage T1-2and N0patientshaving breast conservation and whole-breast radiation) with minimal sentinel node disease burden. It is not surprising then that the evolution of surgical treatment of the axilla has shaped the role of preoperative staging, specifically the use of preoperative axillary ultrasound with selective ultrasoundguided needle biopsy (UNB). In this review, we estimate and discuss the utility of UNB highlighting the role of preoperative UNB and its consequences on surgical management of the axilla.
Background on clinical utility of a test
Test accuracy describes the ability of a test to rule in or out a disease or to assess disease severity. On the other hand clinical utility of a test represents the capacity to use the information from the test to enable a decision to adopt, or to reject, a therapy or an intervention. Test utility expresses4to what extent testingcontributes to improving health outcomes. Bossuyt et al.4report that key features of test clinical utility are that use of the test improves health outcomes, and that the test forms part of a strategy whereby health outcomes are generated ‘not only by using the test but also by a management strategy that starts with testing but includes all downstream consequences of subsequent clinical management’4. In the specific scenario of the axilla in invasive breast cancer, knowledge of the status of axillary nodes prior to surgical intervention can affect treatment planning. Two meta-analyses5,6, each based on a large number of original studies and hence large datasets, have reported that a preoperative strategy of ultrasound with selective UNB of abnormal-appearing or suspicious axillary node(s) correctly identifies approximately 50% of breast cancer patients who have node metastases. Diepstraten et al.6estimated this strategy to have a sensitivity of 50% [95% con fi dence interval (95% CI): 43%, 57%] and Houssami et al.5reported it as a median 55.2% (IQR 41.8%-68.2%) across primary studies. On this basis exists the potential utility of preoperative UNB whereby ultrasound-directed needle biopsy can con fi rm metastases to the axillary nodes, enabling triage to ALND and a single axillary operation.
Background on ultrasound accuracy
Using ultrasound with selective UNB (based on ultrasound features of nodes) for preoperative staging of the axilla in newly diagnosed breast cancer patients has been practiced for many years5,7,8, however it is noteworthy that the progressive use of this approach is not solely related to accuracy. It has been partly due to the relative efficiency and modest cost of this combination strategy, the long-established use of ultrasound in breast diagnosis, and because breast ultrasound is a patient-friendly form of image-guided intervention. It should also be noted that ultrasound on its own yields moderate and variable accuracy: meta-analysis5of data (4,313 subjects) from 21 studies8-28found a median ultrasound sensitivity of 61.4% (IQR 51.2%-79.4%) and a median ultrasound specificity of 82.0% (IQR 76.9%-89.0%). Therefore the addition of UNB (directed by axillary ultrasound features) is intended to improve both the sensitivity and the specificity of preoperative staging, and in particular to substantially improve its speci fi city such that a positive UNB can be used to plan surgical treatment of the axilla. So, using data from the same meta-analysis, it can be shown that for the subset of 1,733 patients who were selected to UNB, the median UNB sensitivity is 79.4% (IQR 68.3%-88.9%) and the median UNB specificity is 100% (IQR 100%-100%)5. Various criteria have been used to define abnormal nodes, including morphologic features and/or node size (enlarged nodes), and to select patients to UNB; some of the most frequently reported morphologic features12,13,16,17,24,29-31de fi ning suspicious nodes are:
■ thickening of the cortex (primary studies have used various thresholds to define thickening, usually 2-3 mm, but some studies have used a wider mm threshold to define thickening); cortical thickening may be di ff use or focal;
■ cortex shape/appearance: eccentric or irregular; asymmetric; lobulated (uni- or multi-lobulation);
■ absence/loss of central fatty hilum (this criterion is predictive of metastases but it is not frequently present so may be insensitive);
■ rounded nodes (ratio of the longitudinal and transverse dimensions).
Review methods
We previously reported a systematic evidence review on the accuracy and utility of UNB5. In the present review, we extend our previous work focusing on studies that report data on UNB utility. Because the accuracy of ultrasound and UNB has been comprehensively reported in our meta-analysis5and also in another more recent meta-analysis from Diepstraten et al.6, we will not repeat analyses of ultrasound and UNB accuracy. Instead, we present a summary table of findings from these previous meta-analyses (Table 1) to inform readers of reported accuracy estimates. For the present analysis, we extended our previous review by updating the literature search strategy described by Houssami et al.5, and performed this at January 2014 (Medline and Pre-Medline search). Studies were eligible for the updated review if they provided data on ultrasoundguided fine needle aspiration biopsy (FNAB) or core needle biopsy (CNB) of axillary nodes (collectively referred to as UNB) in women with invasive breast cancer, and if they provided data that quantify or allow estimation of clinical utility. We defined utility as the proportion of women triaged to axillary surgery or axillary treatment as previously defined5. Amongst all eligible studies (from the previous and the updated search) we also looked for data that would allow investigation of UNB results in relation to nodal disease burden.
Statistical analysis
Descriptive statistics (median and IQR) were used to describe UNB utility, which was calculated as the simple proportion of women triaged to axillary surgery or axillary treatment, from all subjects included in the study (therefore the denominator for this calculation was not restricted to women who had UNBbut included all cases). Because we previously found evidence of a positive linear correlation between UNB utility and the underlying prevalence (study-specific proportion) of axillary node metastases across studies, we also calculated descriptive statistics for underlying prevalence of axillary node metastases. We used a bubble plot to demonstrate the relationship between UNB utility and the underlying prevalence of axillary node metastases across all studies.
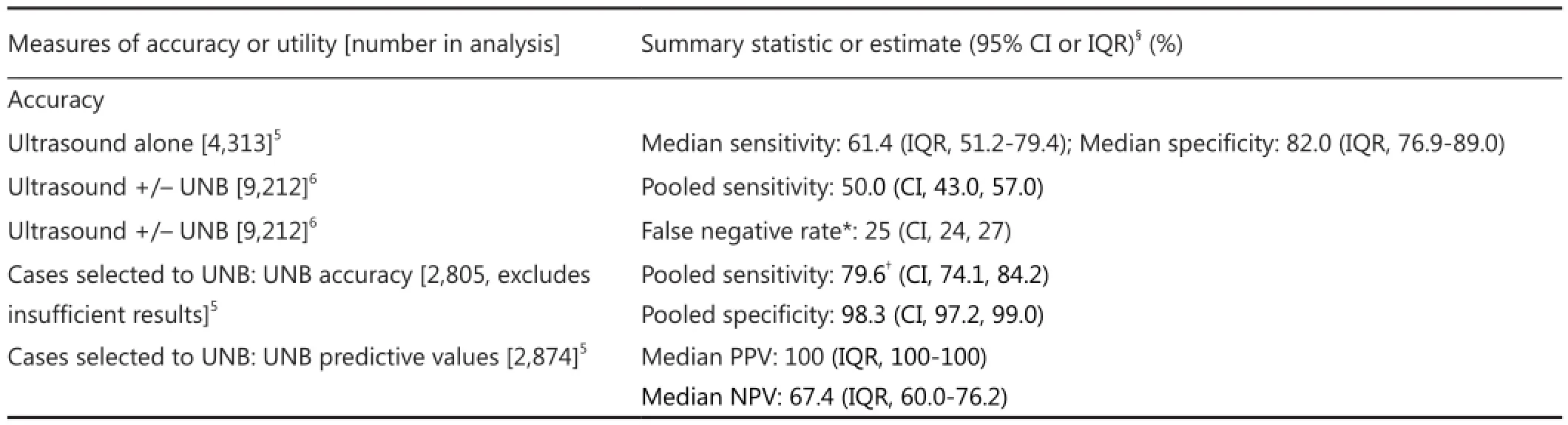
Table 1 Accuracy of preoperative ultrasound & UNB for staging the axilla in invasive breast cancer based on two meta-analyses
For the subset of studies that provided data on UNB results in relation to node disease burden, we used logistic regression modelling incorporating a random-e ff ect for study to investigate nodal disease burden according to whether UNB was positive versus negative. Node disease burden was examined in the model by analysing the proportion of patients with high nodal disease burden (de fi ned as >3 metastatic nodes in the majority of studies) from all patients with axillary node metastases (total of low and high node disease burden), by UNB result. Therefore the model estimated the odds ratio (OR) and corresponding 95% CI for high nodal disease burden in patients with a positive UNB versus those with a negative UNB.
Results
Our updated search yielded 35 eligible studies9,11-17,21-23,25-28,30,31,33-50providing data on 10,934 patients with breast cancer in whom a positive UNB result allowed triage of 1,745 cases (simple proportion 16%) to axillary surgical treatment: the utility of UNB was a median 19.8% (IQR 11.6%-26.7%) across all stu dies9,11-17,21-23,25-28,30,31,33-50. Axillary treatment consisted of triage directly to ALND for the vast majority (and avoidance of SNB) but in some studies UNB was used to a ff ect neoadjuvant therapy prior to ALND9,31,41,47.e median prevalence of node metastases (proportion of patients found to have node metastases on surgical histology) across the 35 studies was 43.2% (IQR 38.7%-51.2%)9,11-17,21-23,25-28,30,31,33-50. In Figure 1, the bubble plot (bubble size reflects study size) displays study-specific proportion of utility (proportion of subjects triaged to axillary surgery based on UNB result) in relation to study-specific underlying prevalence of node metastases.

Figure 1 Bubble plot shows study-speci fi c UNB utility (proportion of subjects triaged to axillary surgery based on UNB result) in relation to underlying prevalence of node metastases.
Seven studies20,34,35,38,42,43,48contributed to the model that estimated the OR for high nodal disease burden in patients with a positive UNB versus those with a negative UNB: studyspeci fi c and pooled estimates are shown in Figure 2. Based on the model, the OR for high nodal disease burden for a positive UNB (versus negative UNB) was 4.38 (95% CI: 3.13, 6.13), P<0.001.e estimated proportion of patients with high nodal disease burden (>3 nodes) from this model was 58.9% (95% CI: 50.2%, 67.0%) for a positive UNB result, whereas the estimated proportion with high nodal disease burden was 24.6% (95% CI: 17.7%, 33.2%) if UNB was negative.
Discussion
In this review, we summarize the clinical utility of axillary UNB as a median 19.8% (IQR 11.6%-26.7%) across 35 primary studies, based on 10,934 patients with breast cancer in whom a positive UNB result allowed triage to axillary surgical treatment (and hence avoidance of two-stage axillary surgery) for those harbouring nodal metastases. This median proportion has been calculated from all subjects included in these studies, therefore it also includes all those who had axillary ultrasound but did not proceed to needle biopsy. The UNB utility % is essentially unchanged from our earlier meta-analysis which reported a utility ranging between 17.7% and 19.8% (based on data for 4,941 patients) representing the proportion of all subjects who are triaged, or could be triaged, directly to ALND5thus avoiding SNB. The UNB utility proportions we describe represent good clinical utility in the context of a management framework whereby patients with UNB-con fi rmed axillary node metastases can proceed to ALND given the high specificity of the test (and assuming at least moderate sensitivity as estimated in meta-analyses5,6). However, when interpreting UNB utility, it should be noted that many of the studies had moderate to high underlying prevalence of node metastases (Figure 1) implying that published studies may have selected patients at relatively higher risk of harbouring metastatic nodes, which could overestimate UNB utility.
Another utility for preoperative ultrasound and UNB, described in several studies, relates to axillary staging in patients who are to receive neoadjuvant therapy9,13,22,24,26,31,47,51,52. For example, one study52reported that UNB for axillary staging can support planning of neoadjuvant therapy in breast cancer patients, and other studies included in our analysis reported use of this axillary staging strategy to affect neoadjuvant therapy followed by axillary surgery9,31,41,47.

Figure 2 Estimated OR for high nodal disease burden in patients with a positive UNB vs. those with a negative UNB amongst patients with axillary node metastases. Study-speci fi c and pooled estimates shown in plot; pooled OR=4.38 (95% CI: 3.13, 6.13); a positive UNB refers to positive ultrasound/positive needle biopsy, a negative UNB refers to positive ultrasound/negative needle biopsy amongst patients subsequently shown to have nodal metastases on SNB and/or ALND; high nodal disease burden refers to >3 nodes (relative to 1-3 nodes) however the study from Garcia-Fernandez 2011 used ≥2 nodes to classify higher node disease burden. UNB, ultrasound-guided needle biopsy; SNB, sentinel node biopsy; OR, odds ratio; ALND, axillary lymph-node dissection.
In this review, we considered studies that provided data on ultrasound-guided FNAB or CNB of axillary nodes, which wecollectively referred to as UNB; however, clinicians may want to know whether there are di ff erences in the accuracy between FNAB and CNB for preoperative axillary staging. In our earlier work we compared sensitivity for studies using FNAB with those using CNB but we did not find statistically significant differences5—this is most likely because the vast majority of published studies used FNAB, or used FNAB or CNB in the same study but most subjects had FNAB, which limits meaningful statistical comparisons. Of note, when we examined data in our previous meta-analysis and included insufficient UNB (as negative test result), we found some di ff erence in sensitivity of FNAB (72.2%; 95% CI: 63.9%, 79.3%) and CNB (83.3%; 95% CI: 70.0%, 91.4%) than estimates that excluded insu ffi cient data from the analysis, but this di ff erence between FNAC and CNB sensitivity was not statistically signi fi cant (P=0.13)5.erefore, either FNAB or CNB of the axilla provide good accuracy in this clinical context and can be used to manage patients. In the updated literature review, we identi fi ed one study that uniquely performed both FNAB and CNB on the (same) suspicious node in each of 66 patients, meaning that each suspicious axillary node was sampled within-patient using both FNAB and CNB. In this study, Rautiainen et al.30reported that the sensitivities of FNAB and CNB were 72.5% and 88.2% respectively (P=0.008), and speci fi city was 100% for FNAB and for CNB (as was PPV). Although this was a relatively small study, it showed that CNB is more sensitive than FNAB, however, each of these tests are highly specific in the preoperative axillary staging setting and hence either of these axillary UNB tests would confer good utility for triaging to axillary surgery.
Because the accuracy of UNB has been comprehensively reported in our meta-analysis5and also in another recent metaanalysis from Diepstraten et al.6, we did not repeat analyses of UNB accuracy in this paper, instead we summarized this information (Table 1). However, it was noted through our updated literature search that some studies reported data showing that UNB is much more likely to be correctly positive(more sensitive) if macro metastases are present in the axillary nodes than if only micrometastases (<2 mm) are present16,31,43,49. Brion et al.16reported UNB sensitivity as 60.3% for macro metastases and as <30% for micrometastases; and Garcia-Ortega31reported a sensitivity of UNB of 71% for macro metastases whereas none of 12 cases (0%) with micrometastases were correctly diagnosed using UNB.
We extended our previous work to describe UNB utility as the proportion of women (from all subjects) triaged to ALND, based on published studies at January 2014. It is noteworthy that we had also examined a related measure of UNB utility in our earlier review5, de fi ned as the median proportion of women with metastatic axillary nodes triaged or potentially triaged directly to ALND if UNB is used routinely in patients with invasive breast cancer: the previous analysis was based on 2,162 UNBs in 4,451 subjects, and showed that UNB utility measure differed by (study-level) median tumor size5. We reported that the median proportion of women with metastatic axillary nodes triaged was 42.2% (IQR 30.6%-49.2%) for studies with a median tumor size <21 mm, and 65.6% (IQR 48.9%-69.7%) for studies with median tumor size ≥21 mm, and the OR for the proportion triaged in median tumor size ≥21 mm relative to <21 mm studies was 2.57 (95% CI: 1.29, 5.09), P=0.0095. This indicates that axillary UNB has signi fi cantly higher utility in women with larger cancers, which is not surprising given that they have a relatively higher likelihood of having nodal metastases than women with smaller cancers.
While the above data and discussion to this point highlight the clinical utility of axillary UNB, given the evidence from the Z0011 trial3,53and its apparent impact on practice54, raises the issue of whether UNB remains useful at present and whether it will have utility in breast cancer staging in the future. In the subgroup of patients de fi ned by the Z0011 criteria (clinical stage T1-2and N0patients having breast conservation and whole-breast radiation)3,53there may be relatively less utility for UNB because patients with node metastases in only 1-2 sentinel nodes would not necessarily be managed with ALND: hence the utility of axillary ultrasound with UNB will depend on whether or not the surgeon has adopted omission of ALND in patients with minimal sentinel node disease. Surgeons who have modi fi ed their practice according to the Z0011 trial3,53may find preoperative axillary ultrasound with UNB of limited or questionable utility, because there is little evidence that axillary UNB can differentiate between minimal and more advanced nodal disease.
We therefore interrogated existing data on UNB in an aempt to gain further insights regarding UNB outcome and nodal disease burden. However, only seven studies identified in our review20,34,35,38,42,43,48provided relevant information allowing us to model data in a subgroup analysis—we estimated that amongst patients with axillary nodal metastases, the OR for high nodal disease burden in those with a positive UNB versus those with a negative UNB was 4.38 (95% CI: 3.13, 6.13), P<0.001. This means that the odds of harbouring high nodal disease burden are signi fi cantly increased in patients who have a positive UNB relative to those who have a negative UNB. From this model, the estimated proportion of patients with high nodal disease burden was 58.9% for a positive UNB, whereas the estimated proportion with high nodal disease burden was 24.6% if UNB was negative.ese fi ndings indicate that amongst those with a positive UNB there is a relatively higher proportion of patients with high nodaldisease burden but still a substantial proportion (approximately 40%) will have low nodal disease burden. So overall, axillary UNB has good clinical utility and a positive UNB can e ff ectively triage to ALND. However, the changing landscape of axillary surgical treatment means that a positive UNB may have relatively less utility where surgeons have modified their practice to omission of ALND for minimal nodal metastatic disease. Also, our model is limited by the paucity of studies contributing data to this subgroup analysis.
Although the utility of UNB appears questionable if there is broader and progressive adoption of SNB-only for minimal axillary node metastases, it is possible that the reverse may occur. Because the algorithm for axillary surgical management in invasive breast cancer is evolving, this could result in a potentially more pragmatic approach to the application of axillary ultrasound with UNB. For example, axillary ultrasound might be used to look for multiple abnormal nodes, and to triage those with multiple metastatic nodes to ALND. Other more novel possibilities include enhanced application of ultrasound, either through refined systematic scanning of the axilla (as shown by Brion et al.55), or through technologic developments, for example contrast-enhanced ultrasound56,57, mayallow precise UNB-sampling of the sentinel node(s). Britton and colleagues have described systematic scanning of the axilla, with emphasis on level I nodes and with particular aention to identifying the lowest 1-2 nodes, and have reported that use of that approach can lead to UNB of sentinel nodes in 64% of patients55. Because false negative ultrasound and UNB may be due to failure to find and sample the sentinel node, or may be due to failure to adequately sample metastatic disease within correctly identi fi ed diseased sentinel node(s), Sever et al. have investigated contrastenhanced ultrasound with microbubbles (injected intradermally in the periareolar region)56,57: this research showed the potential to improve identification as well as sampling of the sentinel node(s) through targeted UNB of the microbubble enhancing axillary lymph node57. Such novel approaches to preoperative axillary staging, that support identi fi cation and/or sampling of sentinel nodes, could mean that many patients may not require any axillary intervention in future (in the context of the results of Z0011 trial3,53). So further research to develop and evaluate these staging strategies would be worthy and could contribute to future practice.
An important study in this fi eld is a trial currently in progress in Europe: a prospective randomized controlled trial using axillary ultrasound to decide surgical management of the axilla [Sentinel node vs. Observation aer axillary Ultra-Sound (SOUND) trial] in patients with early breast cancer (tumors ≤2 cm and clinically node-negative axillae) who are candidates for breast-conserving surgery58. Patients will have axillary ultrasound to assess whether or not they have suspicious nodal involvement, and those shown to have a negative ultrasound or (for a single abnormal node) negative UNB will be randomized to SNB or no further axillary surgery. The SOUND trial represents yet another possibility for a potential shiin axillary management towards less intervention and may see an extended role for axillary ultrasound with selective UNB in breast cancer staging in future58.
Conclusion
Preoperative ultrasound-based staging of the axilla using ultrasound with selective UNB is moderately sensitive but highly speci fi c and provides a staging strategy that allows patients to be triaged to ALND (based on a positive result); this helps avoid unnecessary two-stage axillary surgery, whereas those with a negative UNB proceed to standard SNB for staging. A large number of non-randomised studies report that UNB provides good clinical utility for axillary surgical management, quanti fi ed in this paper as a median utility of 19.8% (IQR 11.6%-26.7%) of breast cancer patients (across 35 studies) who can be triaged to axillary surgery based on a positive UNB, and without SNB. However, ongoing evolution of axillary surgical treatment may render preoperative axillary UNB less useful depending on local surgical practice, and specifically on whether omission of ALND in patients with minimal nodal metastatic burden has been adopted into practice. Future research that allows enhanced application of ultrasound with UNB to identify and target sentinel node(s) and/or to discriminate between minimal versus advanced axillary nodal metastatic involvement is likely to contribute substantially towards management of the axilla in invasive breast cancer.
Acknowledgements
This work is partly funded by National Health and Medical Research Council (NHMRC) program (Grant No. 633003) to the Screening & Test Evaluation Program, Australia.
Con fl ict of interest statement
No potential con fl icts of interest are disclosed.
1. Kell MR, Burke JP, Barry M, Morrow M. Outcome of axillary staging in early breast cancer: a meta-analysis. Breast Cancer Res Treat 2010;120:441-447.
2. Wang Z, Wu LC, Chen JQ. Sentinel lymph node biopsy compared with axillary lymph node dissection in early breast cancer: a metaanalysis. Breast Cancer Res Treat 2011;129:675-689.
3. Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-575.
4. Bossuyt PM, Reitsma JB, Linnet K, Moons KG. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem 2012;58:1636-1643.
6. Diepstraten SC, Sever AR, Buckens CF, Veldhuis WB, van Dalen T, van den Bosch MA, et al. Value of preoperative ultrasound-guided axillary lymph node biopsy for preventing completion axillary lymph node dissection in breast cancer: a systematic review and meta-analysis. Ann Surg Oncol 2014;21:51-59.
7. Verbanck J, Vandewiele I, De Winter H, Tytgat J, Van Aelst F, Tanghe W. Value of axillary ultrasonography and sonographically guided puncture of axillary nodes: a prospective study in 144 consecutive patients. J Clin Ultrasound 1997;25:53-56.
8. Bonnema J, van Geel AN, van Ooijen B, Mali SP, Tjiam SL, Henzen-Logmans SC, et al. Ultrasound-guided aspiration biopsy for detection of nonpalpable axillary node metastases in breast cancer patients: new diagnostic method. World J Surg 1997;21:270-274.
10. Popli MB, Sahoo M, Mehrotra N, Choudhury M, Kumar A, Pathania OP, et al. Preoperative ultrasound-guided fi ne-needle aspiration cytology for axillary staging in breast carcinoma. Australas Radiol 2006;50:122-126.
11. Davis JT, Brill YM, Simmons S, Sachleben BC, Cibull ML, McGrath P, et al. Ultrasound-guided fi ne-needle aspiration of clinically negative lymph nodes versus sentinel node mapping in patients at high risk for axillary metastasis. Ann Surg Oncol 2006;13:1545-1552.
12. Deurloo EE, Tanis PJ, Gilhuijs KG, Muller SH, Kröger R, Peterse JL, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer 2003;39:1068-1073.
14. Sapino A, Cassoni P, Zanon E, Fraire F, Croce S, Coluccia C, et al. Ultrasonographically-guided fi ne-needle aspiration of axillary lymph nodes: role in breast cancer management. Br J Cancer 2003;88:702-706.
15. Brancato B, Zappa M, Bricolo D, Catarzi S, Risso G, Bonardi R, et al. Role of ultrasound-guided fi ne needle cytology of axillary lymph nodes in breast carcinoma staging. Radiol Med 2004;108:345-355.
17. Podkrajsek M, Music MM, Kadivec M, Zgajnar J, Besic N, Pogacnik A, et al. Role of ultrasound in the preoperative staging of patients with breast cancer. Eur Radiol 2005;15:1044-1050.
18. Bedrosian I, Bedi D, Kuerer HM, Fornage BD, Harker L, Ross MI, et al. Impact of clinicopathological factors on sensitivity of axillary ultrasonography in the detection of axillary nodal metastases in patients with breast cancer.Ann Surg Oncol 2003;10:1025-1030.
19. Cowher MS, Erb KM, Poller W, Julian TB. Correlation of the use of axillary ultrasound and lymph node needle biopsy with surgical lymph node pathology in patients with invasive breast cancer. Am J Surg 2008;196:756-759.
20. Damera A, Evans AJ, Cornford EJ, Wilson AR, Burrell HC, James JJ, et al. Diagnosis of axillary nodal metastases by ultrasoundguided core biopsy in primary operable breast cancer. Br J Cancer 2003;89:1310-1313.
21. van Rijk MC, Deurloo EE, Nieweg OE, Gilhuijs KG, Peterse JL, Rutgers EJ, et al. Ultrasonography and fi ne-needle aspiration cytology can spare breast cancer patients unnecessary sentinel lymph node biopsy. Ann Surg Oncol 2006;13:31-35.
22. Boughey JC, Middleton LP, Harker L, GarreB, Fornage B, Hunt KK, et al. Utility of ultrasound and fi ne-needle aspiration biopsy of the axilla in the assessment of invasive lobular carcinoma of the breast. Am J Surg 2007;194:450-455.
23. Genta F, Zanon E, Camanni M, Delteo F, Drogo M, Gallo R, et al. Cost/accuracy ratio analysis in breast cancer patients undergoing ultrasound-guided fi ne-needle aspiration cytology, sentinel node biopsy, and frozen section of node. World J Surg 2007;31:1155-1163.
24. Koelliker SL, Chung MA, Mainiero MB, Steinho ff MM, Cady B. Axillary lymph nodes: US-guided fi ne-needle aspiration for initial staging of breast cancer--correlation with primary tumor size. Radiology 2008;246:81-89.
25. Altomare V, Guerriero G, Carino R, Baista C, Primavera A, Altomare A, et al. Axillary lymph node echo-guided fi ne-needle aspiration cytology enables breast cancer patients to avoid a sentinel lymph node biopsy. Preliminary experience and a reviewof the literature. Surg Today 2007;37:735-739.
26. Jain A, Hais fi eld-Wolfe ME, Lange J, Ahuja N, Khouri N, Tsangaris T, et al.e role of ultrasound-guided fi ne-needle aspiration of axillary nodes in the staging of breast cancer. Ann Surg Oncol 2008;15:462-471.
27. Gilissen F, Oostenbroek R, Storm R, Westenend P, Plaisier P. Prevention of futile sentinel node procedures in breast cancer: ultrasonography of the axilla and fi ne-needle aspiration cytology are obligatory. Eur J Surg Oncol 2008;34:497-500.
28. Luparia A, Campanino P, Coi R, Lucarelli D, Durando M, Mariscoi G, et al. Role of axillary ultrasound in the preoperative diagnosis of lymph node metastases in patients a ff ected by breast carcinoma. Radiol Med 2010;115:225-237.
29. Duchesne N, Ja ff ey J, Florack P, Duchesne S. Rede fi ning ultrasound appearance criteria of positive axillary lymph nodes. Can Assoc Radiol J 2005;56:289-296.
30. Rautiainen S, Masarwah A, Sudah M, Sutela A, Pelkonen O, Joukainen S, et al. Axillary lymph node biopsy in newly diagnosed invasive breast cancer: comparative accuracy of fi neneedle aspiration biopsy versus core-needle biopsy. Radiology 2013;269:54-60.
31. Garcia-Ortega MJ, Benito MA, Vahamonde EF, Torres PR, Velasco AB, Paredes MM. Pretreatment axillary ultrasonography and core biopsy in patients with suspected breast cancer: diagnostic accuracy and impact on management. Eur J Radiol 2011;79:64-72. 32. Hackney L, Williams S, Bajwa S, Morley-Davies AJ, Kirby RM, Brion I. In fl uence of tumor histology on preoperative staging accuracy of breast metastases to the axilla. Breast J 2013;19:49-55. 33. de Kanter AY, van Eijck CH, van Geel AN, Kruijt RH, Henzen SC, Paul MA, et al. Multicentre study of ultrasonographically guided axillary node biopsy in patients with breast cancer. Br J Surg 1999;86:1459-1462.
34. Kuenen-Boumeester V, Menke-Pluymers M, de Kanter AY, Obdeijn IM, Urich D, Van Der Kwast TH. Ultrasound-guided fi ne needle aspiration cytology of axillary lymph nodes in breast cancer patients. A preoperative staging procedure. Eur J Cancer 2003;39:170-174.
36. Sahoo S, Sanders MA, Roland L, Pile N, Chagpar AB. A strategic approach to the evaluation of axillary lymph nodes in breast cancer patients: analysis of 168 patients at a single institution. Am J Surg 2007;194:524-526.
38. Hinson JL, McGrath P, Moore A, Davis JT, Brill YM, Samoilova E, et al.e critical role of axillary ultrasound and aspiration biopsy in the management of breast cancer patients with clinically negative axilla. Ann Surg Oncol 2008;15:250-255.
39. Baruah BP, Goyal A, Young P, Douglas-Jones AG, Mansel RE. Axillary node staging by ultrasonography and fi ne-needle aspiration cytology in patients with breast cancer. Br J Surg 2010;97:680-683.
40. Lee MC, Eatrides J, Chau A, Han G, Kiluk JV, Khakpour N, et al. Consequences of axillary ultrasound in patients with T2 or greater invasive breast cancers. Ann Surg Oncol 2011;18:72-77.
41. Chang MC, Crystal P, Colgan TJ.e evolving role of axillary lymph node fi ne-needle aspiration in the management of carcinoma of the breast. Cancer Cytopathol 2011;119:328-334.
42. García Fernández A, Fraile M, Giménez N, Reñe A, Torras M, Canales L, et al. Use of axillary ultrasound, ultrasound- fi ne needle aspiration biopsy and magnetic resonance imaging in the preoperative triage of breast cancer patients considered for sentinel node biopsy. Ultrasound Med Biol 2011;37:16-22.
44. Solon JG, Power C, Al-Azawi D, Duke D, Hill AD. Ultrasoundguided core biopsy: an e ff ective method of detecting axillary nodal metastases. J Am Coll Surg 2012;214:12-17.
46. Leenders MW, Broeders M, Croese C, Richir MC, Go HL, Langenhorst BL, et al. Ultrasound and fi ne needle aspiration cytology of axillary lymph nodes in breast cancer. To do or not to do? Breast 2012;21:578-583.
48. van Wely BJ, de Wilt JH, Schout PJ, Kooistra B, Wauters CA, Venderinck D, et al. Ultrasound-guided fi ne-needle aspiration of suspicious nodes in breast cancer patients; selecting patients with extensive nodal involvement. Breast Cancer Res Treat 2013;140:113-118.
49. Cools-Lartigue J, Sinclair A, Trabulsi N, Meguerditchian A, Mesurolle B, Fuhrer R, et al. Preoperative axillary ultrasound and fi ne-needle aspiration biopsy in the diagnosis of axillary metastases in patients with breast cancer: predictors of accuracy and futureimplications. Ann Surg Oncol 2013;20:819-827.
50. Amonkar SJ, Oates E, McLean L, Nicholson S. Pre-operative staging of the axilla in primary breast cancer. Byrede fi ning the abnormal appearing node can we reduce investigations without a ff ecting overall treatment? Breast 2013;22:1114-1118.
51. Topal U, Punar S, Tasdelen I, Adim SB. Role of ultrasound-guided core needle biopsy of axillary lymph nodes in the initial staging of breast carcinoma. Eur J Radiol 2005;56:382-385.
52. Joh JE, Han G, Kiluk JV, Laronga C, Khakpour N, Lee MC. Indications for axillary ultrasound use in breast cancer patients. Clin Breast Cancer 2012;12:433-437.
53. Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence aer sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010;252:426-432; discussion 432-3.
54. Dengel LT, Van Zee KJ, King TA, Stempel M, Cody HS, El-Tamer M, et al. Axillary dissection can be avoided in the majority of clinically node-negative patients undergoing breast-conserving therapy. Ann Surg Oncol 2014;21:22-27.
56. Sever A, Jones S, Cox K, Weeks J, Mills P, Jones P. Preoperative localization of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasonography in patients with breast cancer. Br J Surg 2009;96:1295-1299.
57. Sever AR, Mills P, Jones SE, Cox K, Weeks J, Fish D, et al. Preoperative sentinel node identi fi cation with ultrasound using microbubbles in patients with breast cancer. AJR Am J Roentgenol 2011;196:251-256.
58. Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation aer axillary UltraSouND). Breast 2012;21:678-681.
Cite this article as:Houssami N, Turner RM. Staging the axilla in women with breast cancer: the utility of preoperative ultrasoundguided needle biopsy. Cancer Biol Med 2014;11:69-77. doi: 10.7497/ j.issn.2095-3941.2014.02.001
Nehmat Houssami
E-mail: nehmath@med.usyd.edu.au
Received March 10, 2014; accepted March 26, 2014. Available at www.cancerbiomed.org
Copyright © 2014 by Cancer Biology & Medicine
杂志排行
Cancer Biology & Medicine的其它文章
- Inhibition of human esophageal squamous cell carcinomas by targeted silencing of tumor enhancer genes: an overview
- Research progress on the anticarcinogenic actions and mechanisms of ellagic acid
- Incidence and mortality of female breast cancer in the Asia-Paci fi c region
- Clinico-pathological signi fi cance of extra-nodal spread in special types of breast cancer
- Effects of postmastectomy radiotherapy on prognosis in different tumor stages of breast cancer patients with positive axillary lymph nodes
- Spindle cell carcinoma of the breast as complex cystic lesion: a case report
