Nasal mucosal inhalation of amyloid-beta peptide 3-10 defective adenovirus attenuates cytotoxicity induced by beta-amyloid (1-42)
2014-03-24TongziJiangWanshuGuoShaShaXiaonaXingRongGuoYunpengCao
Tongzi Jiang, Wanshu Guo, Sha Sha, Xiaona Xing, Rong Guo, Yunpeng Cao
Department of Neurology, First Af fi liated Hospital of China Medical University, Shenyang, Liaoning Province, China
Nasal mucosal inhalation of amyloid-beta peptide 3-10 defective adenovirus attenuates cytotoxicity induced by beta-amyloid (1-42)
Tongzi Jiang, Wanshu Guo, Sha Sha, Xiaona Xing, Rong Guo, Yunpeng Cao
Department of Neurology, First Af fi liated Hospital of China Medical University, Shenyang, Liaoning Province, China
Three-month-old Alzheimer’s disease model transgenic mice were immunized with Aβ1-42, Plp-Adenovirus [Ad]-X-CMV-(Aβ3-10)10-CpG [AdCpG-(Aβ3-10)10] or AdCpG virus fl uid via nasal mucosal inhalation, respectively. ELISA analysis of serum showed Aβ42antibody titers were signi fi cantly increased in mice immunized with Aβ1-42and AdCpG-(Aβ3-10)10. Concanavalin A and AdCpG-(Aβ3-10)10stimulation signi fi cantly increased the number of proliferating spleen cells cultured from AdCpG(Aβ3-10)10and Aβ42groups compared with the control group. In the AdCp-G(Aβ3-10)10group, levels of interleukin (IL)-4 and IL-10 were increased, while those of IL-2 and interferon-γ were decreased. In the Aβ42group, levels of IL-4, IL-10, IL-2 and interferon-γ were all increased. Experimental fi ndings indicate that AdCpG-(Aβ3-10)10vaccine can produce strong T helper 2 (T2) humoral immune responses in addition to the production of Aβ42antibody. Te cellular immunologic response was weak and avoided Aβ1-42-mediated cytotoxicity.
nerve regeneration; neurodegenerative disease; Alzheimer’s disease; immunotherapy; amyloid-beta peptide vaccine; cytokines; humoral immunity; inflammation; NSFC grant; neural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 30471927.
Jiang TZ, Guo WS, Sha S, Xing XN, Guo R, Cao YP. Nasal mucosal inhalation of amyloid-beta peptide 3-10 defective adenovirus attenuates cytotoxicity induced by beta-amyloid (1-42). Neural Regen Res. 2014;9(8):872-877.
Introduction
Current drug treatment for Alzheimer’s disease includes cholinergic inhibitors for the improvement of cognitive function, and N-methyl-D-aspartate receptor antagonists for medium and severe patients. However, these drugs only alleviate the symptoms of Alzheimer’s disease, and fail to a ff ect irreversible cognitive dysfunction and e ff ectively scavenge amyloid beta peptide (Aβ) in the brain. Aβ vaccines reduced and eliminated Aβ deposition in an Alzheimer’s disease transgenic mouse model, and signi fi cantly improved behavioral and cognitive impairment (Arendash et al., 2001a; Dodart et al., 2002). In 1999, the application of vaccine AN1792 (ELAN Pharmaceuticals, Inc., San Diego, CA, USA) in a phase IIa clinical trial was terminated owing to the presence of aseptic meningitis in 6% subjects, but an autopsy report found it e ff ectively scavanged brain Aβ plaques (Nicoll et al., 2003). AN1792-induced aseptic meningitis was caused by T cell-mediated autologous immune cellular responses (Town et al., 2002). T lymphocytes mediate cell-mediated immunity and immune regulatory effects. T helper cells can be classi fi ed into T helper (T)1 cells and T2 cells according to the type of cytokine produced. T1 cells secrete interferon-γ (IFN-γ) and IFN-β, for cell immunity, while T2 cells secrete interleukin (IL)-4 and IL-10 that function in humoral immunity. A safe and effective Aβ vaccine can reduce Th1 immune responses and increase Th2 immune responses, thus attenuating in fl ammation in the brain (Piras et al., 2014).
Numerous measures have been proposed to make the vaccine safe and e ff ective, such as selecting di ff erent amino acid fragments of Aβ1-42, using di ff erent adjuvants, and immune pathways (Serpente et al., 2014). A gene vaccine synthesized with an N-terminal amino acid fragment of Aβ1-42has attracted increasing attention, and Aβ1-6and Aβ1-15fragments have been investigated in animal experiments (Janus et al., 2000; Arendash et al., 2001b). However, it is important to avoid cellular immune responses caused by the Aβ vaccine. We constructed a recombinant defective adenovirus vaccine Plp-Adeno-X-CMV-(Aβ3-10)10-CpG [AdCpG-(Aβ3-10)10] using defective adenovirus carriers, by repeated synthesis of Aβ3-10that is bound to CpG (Guo et al., 2011). In this study, 3-month-old Alzheimer’s disease transgenic mice were immunized with B6.Cg-Tg(APPswe, PSEN1dE9)85Dbo/J via nasal mucosal inhalation, to observe serum Aβ antibody production and e ff ects on mousespleen cell in fl ammatory responses.
Materials and Methods
Vaccine and virus
Adenovirus AdCpG-(Aβ3-10)10was inserted into the target gene, and AdCpG virus without target gene adenovirus and adjuvant CpG were provided by our research group as previously described (Guo et al., 2011).
Immunization of transgenic mice
Eighteen double transgenic mice B6.Cg-Tg(APPswe, PSEN1dE9)85Dbo/J, aged 3 months, 9 males and 9 females, weighing 220-280 g, were provided by the Experimental Animals Center of China Medical University, China (license No. SCXK (Liao) 2008-0005). The experiment was performed under approval of the Experimental Animal Ethics Committee, the First Affiliated Hospital of China Medical University, China. The mice were randomly divided into three groups: Aβ1-42group, AdCpG group and Aβ3-10group. Te mice of all three groups were nasally inhalated with 20 μL Aβ1-42(Sigma, St. Louis, MO, USA), AdCpG virus (containing 1010vector particles, equivalent to 108pfu virus) or Ad-CpG-(Aβ3-10)10(1010vector particles, equivalent to 108pfu virus) (Morgan et al., 2000). Nasal mucosal immunization was administered to mice every 3 weeks, for a total of eight immunizations.
Preparation of blood specimens
Tail vein blood (0.3 mL) was collected when mice were aged 3 months (1 week before immunization), 6 months (1 week after the fourth immunization), and 7.5 months (1 week after the sixth immunization). Cardiac blood (2 mL) was collected at the age of 10 months (4 weeks after the eighth immunization). The collected blood samples were placed at room temperature for 2 hours, and centrifuged at 4°C at 2,500 r/min, for 20 minutes. The serum was stored until further use.
Indirect enzyme-linked immunosorbent assay (ELISA) detection of serum anti-Aβ42antibody concentration
One hundred μL Aβ1-42(5 mL/L; AnaSpect, Fremont, CA, USA) was coated onto 96-well plates and incubated overnight at 4°C. The plate was then rinsed with PBS containing 0.05% Tween-20, three times, and incubated with 200 μL blocking buffer per well (PBS containing 0.5% fetal bovine serum and 0.05% Tween-20) at room temperature for 1 hour. Then, the buffer solution was discarded, the plate was rinsed with PBS (containing 0.05% Tween-20) three times, and incubated with mouse quantitive anti-Aβ1-16monoclonal antibody (100, 30, 10, 3, 1, 0 μg/L; Covance, Princeton, NJ, USA) at 4°C overnight. The serum and standard antibodies were removed, the plate was rinsed with PBS (containing 0.05% Tween-20) three times, and incubated with anti-mouse IgG (1:2,000; Thermo Fisher Biochemical Products Co., Ltd.,) at room temperature for 1 hour. The secondary antibody was removed, and the plate was rinsed five times and incubated with 3,3′,5,5′-tetramethylbenzidine (100 μL per well) at room temperature for 15 minutes, until the dye was visible. Terminating solution (100 μL) was added to each well, and the absorbance at 450 nm was calculated using a microplate reader (BioTex, Winooski, VT, USA).
In vitro culture of spleen cells after immunization
Ten-month-old mice were euthanized under anesthesia (10% chloral hydrate) and splenic tissue was harvested and placed in a petri dish containing RPMI-1640 medium (Termo Fisher Biochemical Products Co., Ltd., Beijing, China). Spleen tissue was sheared and ground to obtain a cell suspension. Tis was centrifuged at 4°C at 1,000 r/min (centrifugal radius of 12.5 cm) for 10 minutes, and the supernatant was discarded. Erythrocyte lysis bu ff er (3 mL; Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) was added to the cells for 5 minutes and mixed with RPMI 1640 medium. Te cells were re-suspended by centrifugation at 4°C at 1,000 r/min for 10 minutes, twice. Te precipitates were added with RPMI 1640 medium containing 10% fetal bovine serum. Te cell density was adjusted to 5 × 106/mL. Te cells were then incubated into the 96-well plates containing 2 μg/μL concanavalin A (Beijing Dingguo Changsheng Biotechnology Co., Ltd.) and 20 μg/μL AdCpG-(Aβ3-10)10, in a CO2incubator for 72 hours.
MTT assay forin vitroproliferation rate of spleen cells after immunization
Spleen cells were cultured in vitro and incubated with 20 μL MTT solution per well (5 mg/mL; Beijing Dingguo Changsheng Biotechnology Co., Ltd.) for an additional 4 hours, and centrifuged at 2,000 r/min (centrifugal radius of 12.5 cm) at 4°C for 10 minutes. The supernatant was removed and 150 μL DMSO was added to each well. The absorbance value at 492 nm was detected by ELISA (Corning, Steuben County, NY, USA).
ELISA detection of IFN-γ, IL-2, IL-4, and IL-10 levels in spleen cell culture medium
Different cytokines (IFN-γ, IL-2, IL-4, IL-10) were added to the microporous plate (Corning) and were incubated with 100 μL standard sample at different concentrations (GD Animal Health service, Deventer, Netherland) and 50 μL biotinylated antibody working solution (Thermo Fisher Biochemical Products Co., Ltd.) at 20-25°C for 120 minutes. All solutions in the wells were discarded and 100 μL enzyme conjugate working solution (Thermo Fisher Biochemical Products Co., Ltd.) was added to each well except for the blank, and incubated at 20-25°C for 30 minutes. Subseqently, 50 μL each of chromogenic agents A and B (Shanghai Baoman Bio-Technology Co., Ltd., Shanghai, China) were added and developed in the dark at 37°C for 10 minutes. Ten the reaction was terminated. Te absorbance value of each well at 450 nm was measured with a microplate reader. IFN-γ, IL-2, IL-4, and IL-10 content was obtained by comparison with the standard curve.
Statistical analysis
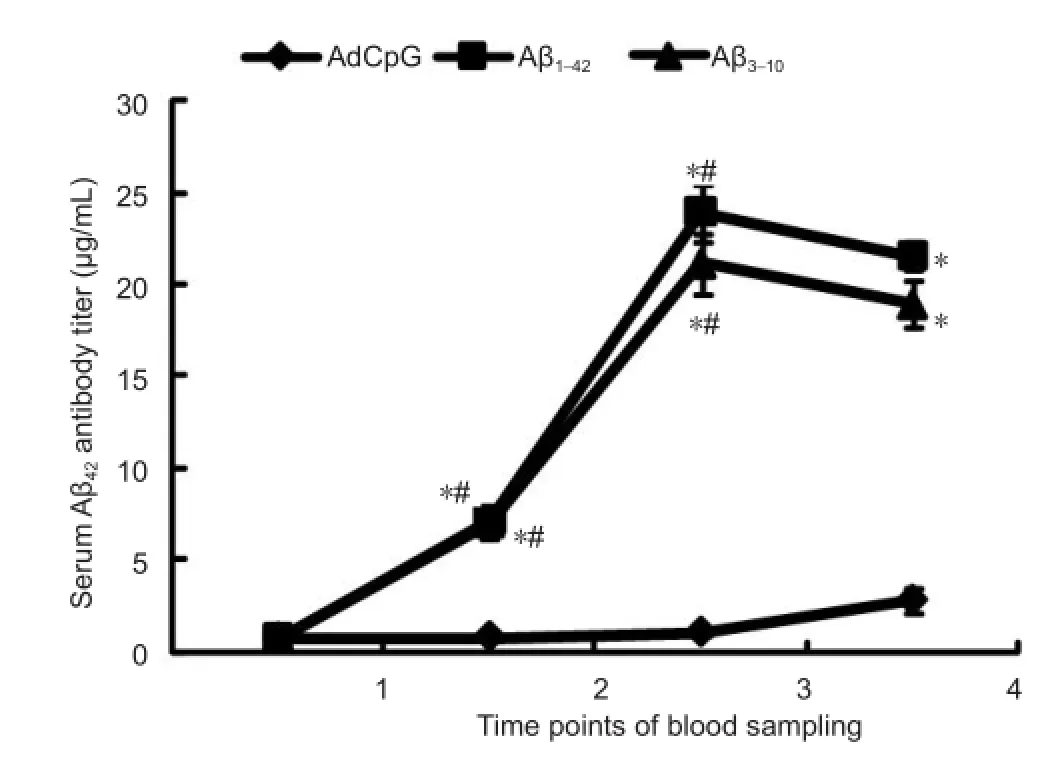
Figure 1 Changes in serum Aβ42antibody titers in transgenic mice after nasal mucosal immunization measured by enzyme-linked immunosorbent assay (ELISA).
Results
Serum anti-Aβ42antibody titers increased after immunization in transgenic mice
ELISA results showed that low serum Aβ42antibody levels could be measured before immunization. As the time after immunization increased, serum Aβ42antibody concentrations in the Aβ1-42group and Aβ3-10group gradually increased (P < 0.05). Tis increase was not statistically signi ficant after the sixth and eighth immunizations (P > 0.05). In the AdCpG group, the serum Aβ42antibody concentration was unchanged during immunization. Compared with the AdCpG group, the Aβ1-42and Aβ3-10groups showed signi ficantly higher serum Aβ42antibody concentrations (P < 0.05). There was no significant difference between the Aβ1-42and Aβ3-10groups (P > 0.05; Figure 1).
Nasal mucosal inhalation of AdCpG-(Aβ3-10)10promoted the in vitro proliferation of mouse spleen cellsAfter AdCpG-(Aβ3-10)10and Aβ1-42immunization, mouse spleen cells were cultured in vitro. MTT assay showed that stimulation with concanavalin A or AdCpG-(Aβ3-10)10, significantly increased the number of spleen cells cultured in the Aβ1-42and Aβ3-10groups compared with the AdCpG group (P < 0.05), but there was no signi fi cant di ff erence between the Aβ1-42and Aβ3-10groups (P > 0.05; Figure 2).
Effect of nasal mucosal inhalation of AdCpG-(Aβ3-10)10on IFN-γ, IL-2, IL-4 and IL-10 levels in spleen cell culture medium
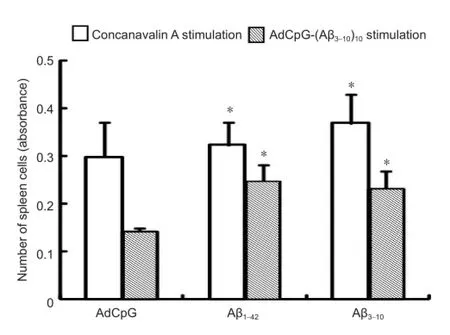
Figure 2 Proliferation of mouse spleen cells cultured in vitro after immunization (MTT assay).
ELISA results showed that after concanavalin A stimulation, IFN-γ and IL-2 levels in the culture medium of Aβ42immunized spleen cells (Aβ1-42group and Aβ3-10group) were significantly decreased compared with the AdCpG group (P < 0.05), whereas IL-4 and IL-10 levels showed no significant change. There was no significant difference in cytokine levels between the Aβ1-42and Aβ3-10groups (P > 0.05). After AdCpG-(Aβ3-10)10stimulation, IFN-γ, IL-2, IL-4 and IL-10 levels in the culture medium of Aβ42immunized spleen cells (Aβ1-42group and Aβ3-10group) were significantly increased compared with the AdCpG group (P < 0.05). IL-4 and IL-10 levels in the culture medium of AdCpG-(Aβ3-10)10immunized spleen cells were increased (P < 0.05), while IFN-γ and IL-2 levels were decreased (P < 0.05; Figure 3).
Discussion
Schenk et al. (2002) showed for the fi rst time that Aβ42peptide vaccine reduced Aβ deposition and scavenged senile plaques in the brain of PDAPP mice, opening a new field for the immunotherapy for Alzheimer’s disease. Subsequent studies have mostly supported the fi ndings; for example Aβ immunization improved learning and memory functions in Alzheimer’s disease transgenic mice (McLaurin et al., 2002). Furthermore, the presence of serum antibodies after immunization e ff ectively inhibited Aβ fi ber aggregation (McLaurin et al., 2002). Other studies have demonstrated the e ff ect of immunotherapy against Alzheimer’s disease. A phase I clinical trial using QS21 as an adjuvant for Aβ42peptide vaccine induced an immune response that generated Aβ antibodies that bound with amyloid-like plaques, thus signi fi cantly improving cognitive function in patients. Unfortunately, 6% of patients developed undersiable symptoms of cerebrospinal meningitis in a phase II clinical trial of the AN1792 vaccine (Hock et al., 2003), and the clinical trial was discontinued. A study by Dodart et al. (2002) con fi rmed aseptic meningitis occurred because of cellular immune responses mediated by T cells, which can be avoided.
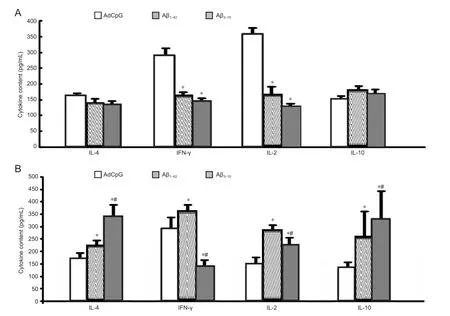
Figure 3 Di ff erential cytokine expression in mouse spleen cells after stimulation with concanavalin A (A) and AdCpG-(Aβ3-10)10(B) by enzyme-linked immunosorbent assay.
Different types of cellular immune responses (Th1 and Th2) mediate either immune regulatory or inflammatory processes, and various types of T cell responses are important for developing Alzheimer’s disease immunotherapeutic strategies. Extracellular pathogen infection (or antigen immunity) is mainly prevented by antibody and complement reactions as these antigens preferentially induce the di ff erentiation of T2 cells, whereas intracellular infections (or antigen) are dominated by T1 responses. Infectious pathogen antigens are scavenged by activated macrophages or microglia in the central nervous system. Te T1 response mainly promotes the secretion of IL-2 and IFN-γ (Orgogozo et al., 2003), whereas Th2 responses produce IL-4 and IL-10, the expression of CD40, and the synthesis of IgG1, IgG3 and IgE antibodies from B cells. In addition, T2 responses stimulate human B cells to produce IgG2, IgG4, IgA, IgE and other antibody isotypes that remove extracellular Aβ with no damage to surrounding tissues or cells. Terefore, a crucial strategy for treating Alzheimer’s disease is to reduce T1 immune responses and enhance T2 immune responses.
A variety of methods have been proposed to develop a safe vaccine for Alzheimer’s disease, including the synthesis of Aβ peptide chain amino acid sequences, adjuvants, vectors and immune system pathways. However, these methods a ff ect the vaccine’s efficacy. Kim et al. (2007) prepared a novel vaccine with Aβ1-6and Pseudomonas exotoxin A receptor delivered by adenoviral vector, and found that nasal mucosal immunization of this vaccine reduced brain Aβ plaques, induced T2 responses, and inhibited T1 responses in transgenic mice. Movsesyan et al. (2008) demonstrated that prophylactic immunization with DNA epitopes vaccine could induce strong Th2 immune responses and generate high concentrations of Aβ antibodies with cases of meningitis observed. Frenkel et al. (2001) elucidated that Aβ3-6is the epitope that prevents Aβ accumulation, and the lack of the 3rdamino acid signi fi cantly decreased the affinity of Aβ antibodies. It was also shown that the CpG funtion of activating the immune system in animals is mainly mediated by B lymphocyte proliferation and the secretion of cytokines from activated monocytes (Vellas et al., 2009). Accumulating evidence has demonstrated the application of adenoviral DNA as an immune adjuvant and therapeuticdrug (Ballas et al., 1996). Tus, it is feasible to use a defective adenovirus as the vector for nasal immunization (Lemere and Masliah, 2010). Furthermore, it is simple to use, is low cost, has a longer duration of expression, and causes less trauma.
1.4 口腔黏膜炎治疗效果评价[13] 显效:7 d内溃疡面完全愈合、口腔黏膜完整或溃疡面愈合2/3以上、疼痛完全消失;有效:7 d内创面缩小、肉芽组织正常生长、疼痛消失或减轻、无炎性分泌物;无效:创面无变化或扩大、疼痛不减轻。评估时特别注意舌下、舌系带、上下唇内侧和口咽部黏膜。
Based on the above, we chose the N-terminal 3-10 amino acids of Aβ1-42as a macromolecular antigen, in an attempt to generate humoral immunity and reduce cell-mediated immunity. In addition, CpG functions as an adjuvant to increase the immune e ff ects of (Aβ3-10)10antigen and overcome immune tolerance. Te aim of this study was to induce Aβ antibodies and induce Th2 immune responses using the constructed AdCpG-(Aβ3-10)10. Lemere et al. (2009) found that Aβ immunotherapy effectively prevented neurological damage in the brain of Alzheimer’s disease patients, before Aβ aggregation. Therefore, we immunized 3-month-old transgenic mice with AdCpG-(Aβ3-10)10before senile plaques formed.
We examined the plasma of 6-month-old transgenic mice by ELISA and observed the successful induction of Aβ42antibodies at high concentrations. At 9 months of age (after the eighth immunization), plasma antibodies could still be detected, and the measured concentration was similar to that after the fourth immunization. Tis evidence indicated that a high concentration of antibodies was obtained after four immunizations, and were maintained at a high concentration after multiple immunizations. After Aβ42vaccine immunization, under the stimulation of antigen or concanavalin A, the number of proliferative spleen cells cultured in vitro was signi fi cantly increased, and the secretion of IL-2, IFN-γ, IL-4 and IL-10 was also increased. This evidence indicated that Aβ42peptides contain T cell and B cell epitopes, and induce both Th1 and Th2 responses. After mice were immunized with the AdCpG-(Aβ3-10)10vaccine, the number of proliferative spleen cells and IL-4 and IL-10 levels were increased, while IL-2 and IFN-γ levels were reduced. Therefore, the Aβ3-10subunit is B cell epitope antigen, that stimulates T2 responses to activate cellular immune responses.
This study demonstrated that the constructed Ad-CpG-(Aβ3-10)10could induce high concentrations of Aβ antibodies in young transgenic mice after immunization, stimulate Th2 responses and reduce Th1 responses. When 3-month-old double transgenic mice B6.Cg-Tg(APPswe, PSEN1dE9) were nasal immunized with AdCpG-(Aβ3-10)10vaccine, IL-4 and IL-10 levels were significantly increased, indicating that the vaccine induced Th2 humoral immune responses, and no apparent cellular immune responses were observed. Tis evidence indicated the safety of the vaccine. We further detected serum Aβ antibody titers before, during and after immunization by ELISA, and found that high levels of serum antibody were present in AdCpG-(Aβ3-10)10immunized mice. AdCpG-(Aβ3-10)10, as a second generation vaccine for Alzheimer’s disease, could induce high levels of Aβ antibodies in young transgenic mice, produce T2 responses, and reduce T1 responses.
Author contributions:Jiang TZ was responsible for the study design, collecting the data, and writing the manuscript. Guo WS, Sha S, Xing XN and Guo R implemented the study. Cao YP evaluated the study. Jiang TZ and Cao YP were responsible for the manuscript. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Arendash GW, Gordon MN, Diamond DM, Austin LA, Hatcher JM, Jantzen P, DiCarlo G, Wilcock D, Morgan D (2001a) Behavioral assessment of Alzheimer’s transgenic mice following long-term Aβ vaccination: task speci fi city and correlations between Aβ deposition and spatial memory. DNA Cell Biol 20:737-744.
Arendash GW, Gordon MN, Diamond DM, Austin LA, Hatcher JM, Jantzen P, DiCarlo G, Wilcock D, Morgan D (2001b) Behavioral assessment of Alzheimer’s transgenic mice following long-term Abeta vaccination: task speci fi city and correlations between Abeta deposition and spatial memory. DNA Cell Biol 20:737-744.
Ballas ZK, Rasmussen WL, Krieg AM (1996) Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol 157:1840-1845.
Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM (2002) Immunization reverses memory de fi cits without reducing brain Aβ burden in Alzheimer’s disease model. Nat Neurosci 5:452-457.
Frenkel D, Kariv N, Solomon B (2001) Generation of auto-antibodies towards Alzheimer’s disease vaccination. Vaccine 19:2615-2619.
Guo R, Huang K, Jiang TZ, Li J, Li Y, Xing XN, Cao YP (2011) Induced T2 dominant immune response in APPswe, PSEN1dE9 transgenic mice after nasal immunization with an adenoviral vector encoding 10 tandem repeats of beta-amyloid 3-10. Neural Regen Res 6:2005-2012.
Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Müller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJF, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM (2003) Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron 38:547-554.
Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D (2000) Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature 408:979-982.
Kim HD, Tahara K, Maxwell JA, Lalonde R, Fukuiwa T, Fujihashi K, Van Kampen KR, Kong FK, Tang DC, Fukuchi K (2007) Nasal inoculation of an adenovirus vector encoding 11 tandem repeats of Abeta1-6 upregulates IL-10 expression and reduces amyloid load in a Mo/Hu APPswe PS1dE9 mouse model of Alzheimer’s disease. J Gene Med 9:88-98.
Lemere CA (2009) Developing novel immunogens for a safe and e ff ective Alzheimer’s disease vaccine. Prog Brain Res 175:83-93.
Lemere CA, Masliah E (2010) Can Alzheimer disease be prevented by amyloid-β immunotherapy? Nat Rev Neurol 6:108-119.
McLaurin J, Cecal R, Kierstead M, Tian X, Phinney AL, Manea M, French J, Lambermon MH, Darabie AA, Brown ME (2002) Terapeutically e ff ective antibodies against amyloid-β peptide target amyloid-β residues 4-10 and inhibit cytotoxicity and fi brillogenesis. Nat Med 8:1263-1269.
Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Du ff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, W AG (2000) Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature 408:982-985.
Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, Head E, Biragyn A, Cribbs DH, Agadjanyan MG (2008) Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine-a novel immunotherapeutic strategy. PLoS One 3:e2124.
Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO (2003) Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat Med 9:448-452.
Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C (2003) Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology 61:46-54.
Piras S, Furfaro AL, Piccini A, Passalacqua M, Borghi R, Carminati E, Parodi A, Colombo L, Salmona M, Pronzato MA, Marinari UM, Tabaton M, Nitti M (2014) Monomeric Aβ1-42 and RAGE: key players in neuronal di ff erentiation. Neurobiol Aging 35:1301-1308.
Schenk D (2002) Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci 3:824-828.
Serpente M, Bonsi R, Scarpini E, Galimberti D (2014) Innate immune system and in fl ammation in Alzheimer’s disease: from pathogenesis to treatment. Neuroimmunomodulation 21:79-87.
Town T, Vendrame M, Patel A, Poetter D, DelleDonne A, Mori T, Smeed R, Crawford F, Klein T, Tan J, Mullan M (2002) Reduced T1 and enhanced T2 immunity after immunization with Alzheimer’s beta-amyloid(1-42). J Neuroimmunol 132:49-59.
Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, Tompkins C, Leibman C, Pomfret M, Grundman M (2009) Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr Alzheimer Res 6:144-151.
Copyedited by Croxfod L, Norman C, Cao J, He Z, Yu J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.131605
Yunpeng Cao, Ph.D., Department of Neurology, First Affiliated Hospital of China Medical University, Shenyang 110005, Liaoning Province, China
http://www.nrronline.org/
Accepted: 2014-02-25
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases
- Regulatory effects of anandamide on intracellular Ca2+concentration increase in trigeminal ganglion neurons
- The synthetic thyroid hormone, levothyroxine, protects cholinergic neurons in the hippocampus of naturally aged mice
- Similar effects of substance P on learning and memory function between hippocampus and striatal marginal division
- Fusion protein of single-chain variable domain fragments for treatment of myasthenia gravis
- Citalopram increases the differentiation ef fi cacy of bone marrow mesenchymal stem cells into neuronal-like cells
