The synthetic thyroid hormone, levothyroxine, protects cholinergic neurons in the hippocampus of naturally aged mice
2014-03-24AilingFuRumeiZhouXingranXu
Ailing Fu, Rumei Zhou, Xingran Xu
School of Pharmaceutical Sciences, Southwest University, Chongqing, China
The synthetic thyroid hormone, levothyroxine, protects cholinergic neurons in the hippocampus of naturally aged mice
Ailing Fu, Rumei Zhou, Xingran Xu
School of Pharmaceutical Sciences, Southwest University, Chongqing, China
Ailing Fu, School of Pharmaceutical Sciences, Southwest University,
Tiansheng Road, Beibei District,
Chongqing 400716, China,
Fuailing1008@hotmail.com.
The thyroid hormones, triiodothyronine and thyroxine, play important roles in cognitive function during the mammalian lifespan. However, thyroid hormones have not yet been used as a therapeutic agent for normal age-related cognitive deficits. In this study, CD-1 mice (aged 24 months) were intraperitoneally injected with levothyroxine (L-T4; 1.6 μg/kg per day) for 3 consecutive months. Our findings revealed a significant improvement in hippocampal cytoskeletal rearrangement of actin and an increase in serum hormone levels of L-T4-treated aged mice. Furthermore, the survival rate of these mice was dramatically increased from 60% to 93.3%. The Morris water maze task indicated that L-T4 restored impaired spatial memory in aged mice. Furthermore, level of choline acetyltransferase, acetylcholine, and superoxide dismutase were increased in these mice, thus suggesting that a possible mechanism by which L-T4 reversed cognitive impairment was caused by increased activity of these markers. Overall, supplement of low-dosage L-T4 may be a potential therapeutic strategy for normal age-related cognitive de fi cits.
nerve regeneration; brain injury; hippocampus; cholinergic neurons; levothyroxine; aging; learning and memory; survival rate; cognitive disorder; NSFC grant; neural regeneration
Funding: This work is supported by the National Natural Science Foundation of China, No. 81273416, and Fundamental Research Funds for the Central Universities, No. XDJK2013A030.
Fu AL, Zhou RM, Xu XR. The synthetic thyroid hormone, levothyroxine, protects cholinergic neurons in the hippocampus of naturally aged mice. Neural Regen Res. 2014;9(8):864-871.
Introduction
The human population around the world is rapidly aging. According to the Sixth National Census Data of 2010, 118,831,709 persons in China are 65 years or over, accounting for 8.87% of the total world population (National Bureau of Statistics of China)[1]. Because of the declining birthrate and increasing average life span, the world population over 65 years is expected to reach 22% or two billion people by 2050[2]. Age-associated cognitive decline involves de fi cits in learning and memory[3], thus lending to strategies for improving such facets of the aging population to improve life quality and survival.
The thyroid hormones, triiodothyronine and thyroxine, are multi-functional, playing pivotal roles in various physiological functions in mammals, such as the regulation of cellular differentiation during development and maintaining thermogenic and metabolic homeostasis in adults. Furthermore, thyroid hormones mediate important effects in the brain, and thus their deficiency contributes to a variety of brain-related pathologies. Dementia is a dominant characteristic in elderly hypothyroid patients, and a past history of thyroid dysfunction may be a risk factor for Alzheimer’s disease (AD)[4].
Oxidative stress is mainly responsible for cell damage, and is considered to be an important factor in aging and disease. Under physiological conditions, superoxide dismutase protects against superoxide anion, thereby preventing cell damage mediated by oxygen free radicals[5].
Acetylcholine is one of the major neurotransmitters involved in cognitive function[6]. The cytoskeleton maintains or changes cell physiology via the ordered rearrangement of actin protein and play a key role in controlling numerous physiological cell activities, including cell division, migration, endocytosis, ef fl ux function, apoptosis, in fl ammation, vesicle transport and gene expression[7]. Previous studies examining cellular substrates of age-related cognitive decline have focused on the hippocampus given its crucial structural and functional role in normal learning and memory, and its particular vulnerability to the aging process[8-9]. Furthermore, previous studies suggest that levothyroxine (L-T4) supports the development and maintenance of hippocampal cholinergic function[10-11]. L-T4 treatment reverses hypothyroidism-induced impairment of hippocampus-dependent cognition in thyroidectomized adult rats[12]. Therefore, we examined the changes of biochemical parameters and cytoskeletal markers in the hippocampus.
We have previously shown that the synthetic form of thyroxine, L-T4, prevents cognitive deficits and improves neurological function in an AD mouse model[13]. This study aimed to assess whether L-T4 improves cognitive impair-ment in memory-de fi cient aged mice by increasing superoxide dismutase activity, cholinergic function, and cytoskeleton rearrangement.
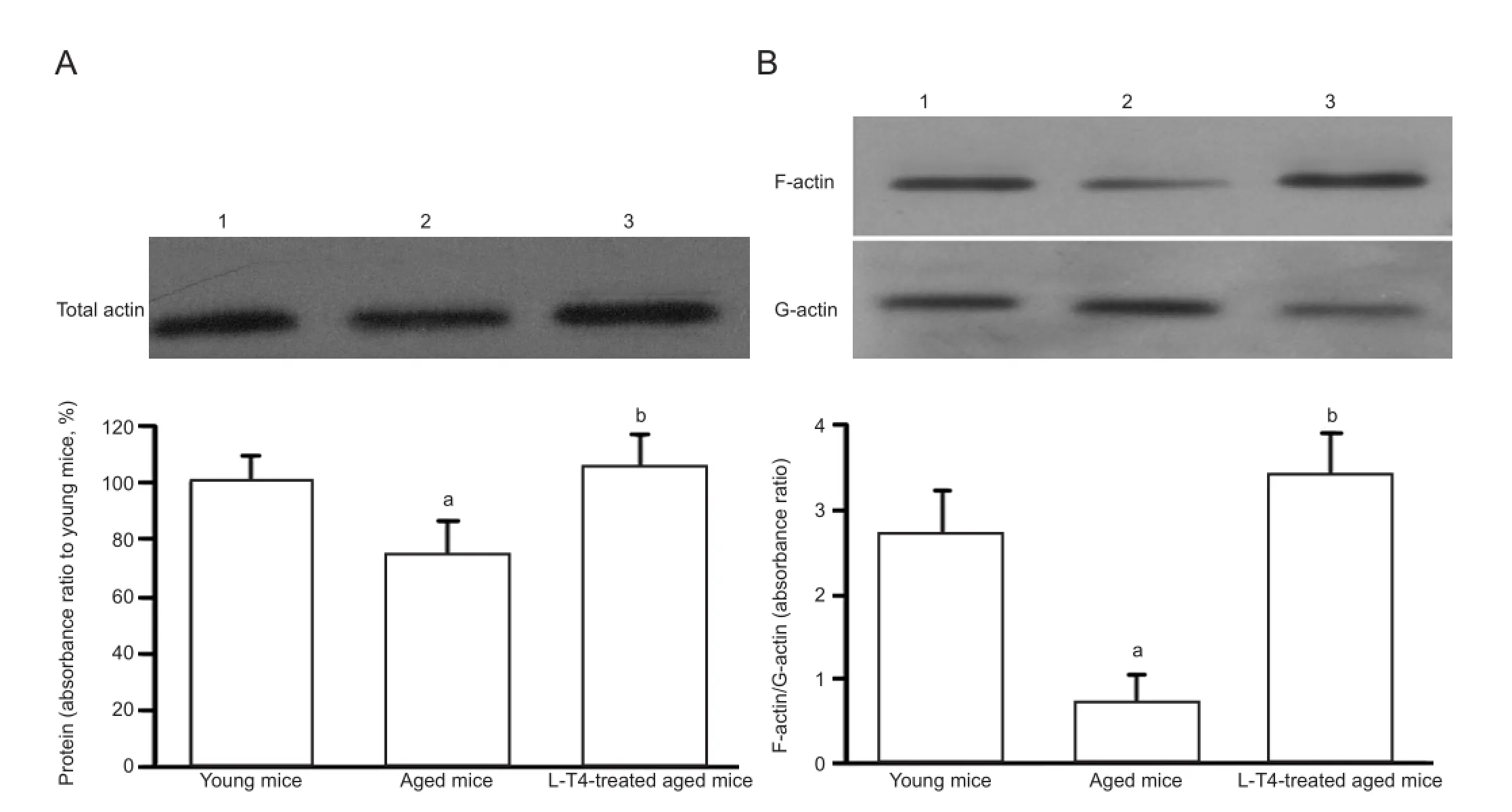
Figure 1 Levothyroxine (L-T4) treatment in aged mice increases the rearrangement of hippocampal actin.
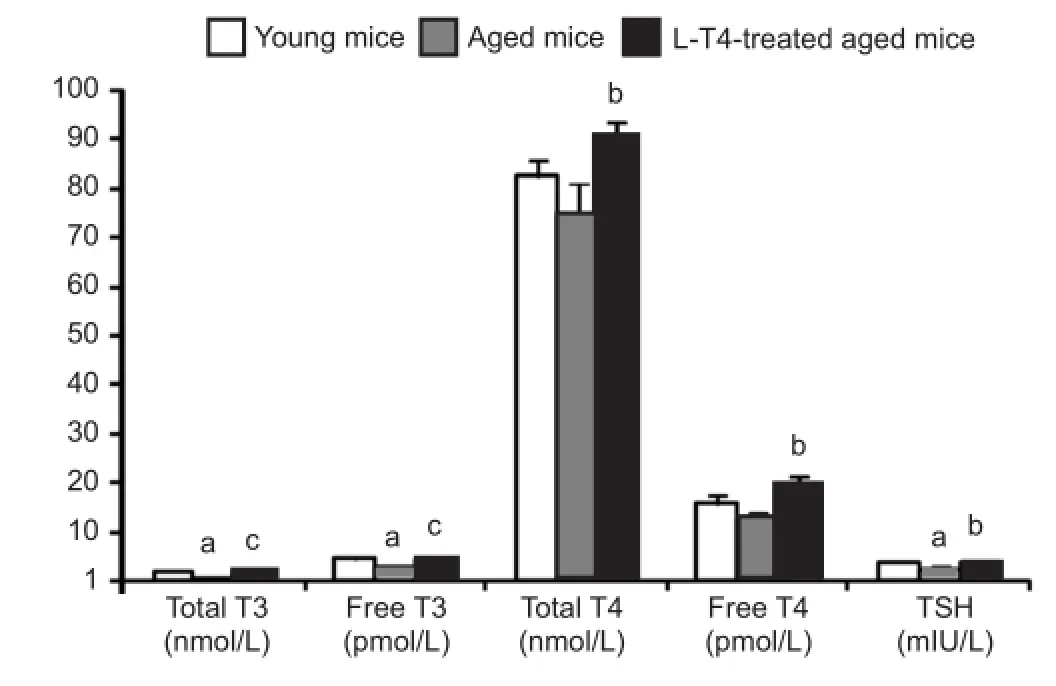
Figure 2 Levothyroxine(L-T4) treatment in aged mice increases serum hormone concentrations.
Results
Quantitative analysis of experimental animals
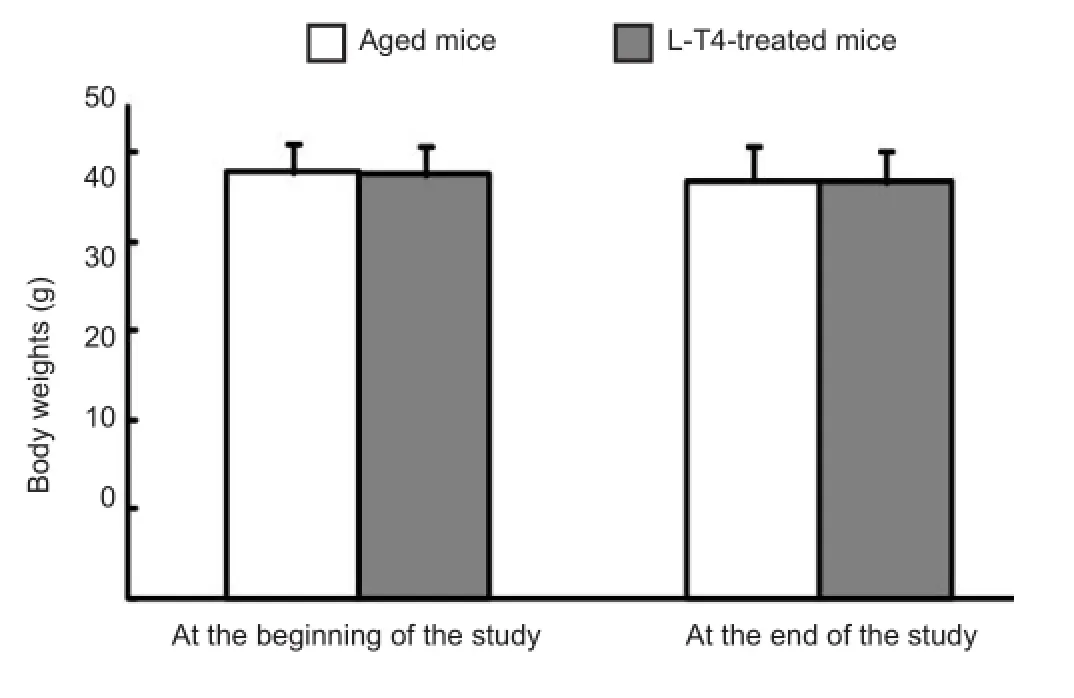
Figure 4 No difference in body weights of viable aged mice before and after levothyroxine (L-T4) treatment at the beginning of the study and at study completion.
Experiments were performed on three groups of CD-1 mice: (1) young (3 months old; n = 15) intraperitoneally (i.p.) treated with vehicle (saline), (2) aged (24 months old; n = 15) mice treated with vehicle, and (3) aged mice treated with L-T4 (i.p.) (n = 15).
L-T4 elevated the rearrangement of hippocampal actin in aged mice
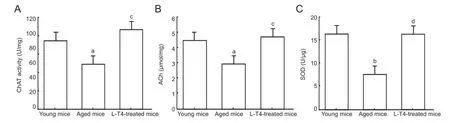
Figure 3 Levothyroxine (L-T4) treatment in aged mice increases the hippocampal levels of choline acetyltransferase (ChAT), acetylcholine (ACh), and superoxide dismutase (SOD).
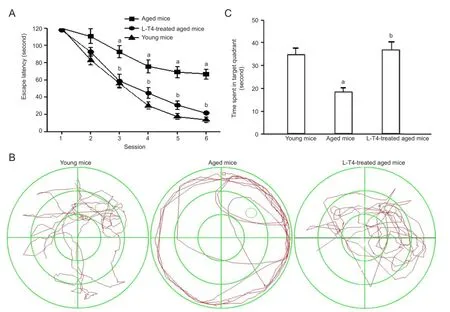
Figure 5 Levothyroxine (L-T4) improves spatial learning and memory in aged mice.
Actin is the major structural component of synapses, and has been shown to play a role in synaptic plasticity because of the dynamic transformations between G-actin and F-actin (i.e., actin rearrangement) via polymerization and depolymerization[14]. Because actin rearrangement plays an essential role in learning and memory[15]and L-T4 improves cognitive de fi cits in aged mice, we explored the effect of L-T4 on the rearrangement of actin in aged mice. Western blot analysis showed that hippocampal levels of total actin were not signi fi cantly altered between the three groups, despite a
tendency for levels to decrease in vehicle-treated aged mice (Figure 1). However, a signi fi cant (P < 0.05) decrease in the ratio of hippocampal F-actin/G-actin was observed in vehicle-treated aged mice compared with the young group (Figure 1). Furthermore, this ration was signi fi cantly (P < 0.01) elevated in aged mice treated with L-T4 compared with the vehicle-treated aged group.
L-T4 treatment elevated serum hormone concentrations in aged mice
Enzyme-linked immunosorbent assays (ELISA) revealed that serum levels of total triiodothyronine, free triiodothyronine, and thyroid stimulating hormone were markedly (P < 0.05) lower in vehicle-treated aged mice compared with the vehicle-treated young group (Figure 2). However, total and free thyroxine remained unchanged between both groups. L-T4 treatment in aged mice significantly increased the serum concentrations of total triiodothyronine (P < 0.01), free triiodothyronine (P < 0.01), total thyroxine (P < 0.05), free thyroxine (P < 0.05), and thyroid stimulating hormone (P < 0.05) compared with the vehicle-treated aged group. Furthermore, L-T4 did not result in subclinical hyperthyroidism (i.e., low thyroid stimulating hormone and normal thyroid hormones) in the aged mice (Figure 2).
L-T4 increased the levels of choline acetyltransferase (ChAT) and acetylcholine (ACh) in the hippocampus of aged mice
To identify the effects of L-T4 on cholinergic function in aged mice, we analyzed the levels of the cholinergic marker, ChAT[16], and ACh. Vehicle-treated aged mice exhibited a signi fi cantly (P < 0.05) lower level of hippocampal ChAT (Figure 3A) and ACh (Figure 3B) compared with vehicle-treated young mice. However, these levels were significantly (P < 0.05) increased in aged mice treated with L-T4 compared with the vehicle-treated aged group.
Superoxide dismutase (SOD) elevated in the hippocampus of aged mice treated with L-T4
SOD levels were significantly (P < 0.01) lower in vehicle-treated aged mice compared with the same treatment to young mice. However, L-T4 treatment in aged mice markedly (P < 0.01) increased the level of SOD compared with vehicle-treated aged mice (Figure 4C). These results showed that L-T4 increased the level of antioxidant enzyme.
L-T4 improved the survival rate of aged mice
A 3-month treatment of L-T4 increased the survival rate of aged mice from 60% (9/15) to 93% (14/15). Furthermore, no difference in the mean body weights of viable aged mice was found before and after L-T4 treatment at the beginning of the study and at study completion (Figure 4).
L-T4 increased spatial learning and memory in aged miceIn the Morris water maze task, the escape latency of L-T4-treated mice was significantly (P < 0.01) shorter than that of vehicle-treated aged mice at each training day from the third to the fi fth (Figure 5A). In the probe tests, the time spent in the target quadrant of L-T4-treated mice was significantly (P < 0.01) longer than the vehicle-treated aged group (Figure 5B, C). These results indicated that L-T4 treatment in aged mice improved cognitive de fi cits.
Discussion
All animals in late age have a mortality rate[17-18]. CD-1 mice have a high mortality rate at approximately 24 months of age (109 weeks, 66.4% and 63.3% for males and females, respectively) compared with younger mice (83 weeks, 32.6% and 28.6% for males and females, respectively)[19]. In the present study, aged (24 months old) male CD-1 mice had a 40% mortality rate during the 12-week experimental period (data not shown). However, mortality rate was dramatically decreased to 6.7% in aged mice treated with L-T4 (data not shown). This effect may have resulted from thyroid hormone-mediated activation of a multitude of metabolic processes essential for survival.
Several changes in thyroid function occur during aging[20]. Normal aging is associated with changes in the production of thyroid hormones and their metabolism[21]. Secretion of thyroxine and triiodothyronine is reduced in healthy aged individuals, and an age-dependent decline in circulating total and free triiodothyronine concentrations occur because of reduced peripheral conversion from thyroxine hence decreased secretion[21]. However, serum concentrations of total and free thyroxine remain relatively unchanged in these individuals because thyroxine degradation is reduced[22-23]. Thyroid stimulating hormone levels have long been regarded as the most sensitive indicator of thyroid function. Its serum concentration decreases in healthy aged individuals owing to its reduced secretion by the pituitary. Consistent with previous studies[4], fi ndings of the present study showed that serum levels of thyroid stimulating hormone and triiodothyronine (total and free) were decreased in aged mice. Aged animals also exhibit reduced cerebral and peripheral tissue responsiveness to thyroid hormones[24]. The affinity of thyroid hormones for their receptors is decreased during aging[25-26]. Therefore, the supplement of thyroid hormones may normalize their levels and ef fi ciencies, thereby restoring the energy and the metabolic function of cells, thus decreasing the mortality rate. However, the exact mechanisms will be further studied. Pharmacokinetic analyses have suggested that L-T4 crosses the blood-brain barrier[27-28], which is relatively impermeable to triiodothyronine. Up to 80% of triiodothyronine in the brain is produced locally via the conversion from thyroxine[29]. L-T4 administration increases both serum and brain concentrations of free thyroxine and triiodothyronine[29].
Normal aged humans, monkeys, rats, and mice exhibit spatial memory impairments compared with their younger counterparts[30-31]. Several studies have reported a decrease in learning and memory with age in CD-1 mice, as assessed by different behavioral tasks, such as the T-maze, 3D maze and Morris water maze[32-33]. Similar deficits are also observed in aged (18-20 months old) C57BL/6J and Kunming micecompared with young (3-4 months old) mice[34-36]. In the present study, the Morris water maze task showed that aged CD-1 mice had spatial learning memory de fi cits compared with their younger counterparts.
Thyroid hormones are necessary for regulating neural development and maintaining normal function of the central nervous system[37]. In animal experiments, L-T4 treatment signi fi cantly enhances the ability of animals to learn a spatial memory task[38-39]. In addition, clinical studies have shown that L-T4 replacement therapy in hypothyroid patients markedly improves their cognition and emotion[40-41]. Our present fi ndings suggest that L-T4 supplement at a safe dose of 1.6 μg/kg per day improves learning and memory in aged mice.
Consistent with previous reports[13,42], our results showed that ChAT, ACh, and SOD in the hippocampus were lower in aged mice, but were increased with L-T4 treatment.
The mechanism of action of thyroid hormones involves the uptake of triiodothyronine or thyroxine by the target cells to the cell nucleus, forming a complex with nuclear receptor protein thereby regulating the biological activities via transcription regulation. Therefore, thyroxine is considered to be a prohormone that yields metabolically active triiodothyronine via tissue deiodinase activities. This effect leads to the modulation of transcription of more than a hundred genes, including memory-related genes of the central nervous system[43-44]. L-T4 administration signi fi cantly increases the levels of memory-related proteins, such as ChAT, nerve growth factor, SOD, CAT and glutathione peroxidase[42]. Both the consolidation and retrieval of memory requires de novo protein synthesis, and thyroid hormones up-regulate the transcription of memory-related genes. Therefore, treatment of L-T4 to aged mice may increase memory-related protein synthesis, thus improving memory.
Nongenomic actions of thyroid hormones are caused by the lack of binding of the hormone to the intranuclear thyroid hormone receptor, and may be initiated in the plasma membrane or cytoplasm. Plasma membrane-initiated actions occur at the integrin αvβ3 receptor (inducing mitogen-activated protein kinase), affecting ion transport systems (such as the Na+/H+exchanger), or inducing complex cellular events (such as cell proliferation). L-T4 binds to the integrin receptor with greater affinity than triiodothyronine[45-46]. In the cytoplasm, thyroxine (but not triiodothyronine) acts on a truncated form of the nuclear thyroid hormone receptor α1 isoform, causing the conversion of soluble G-actin to F-actin, which is important for cell motility in glia and neurons[45-46]. Te actin cytoskeleton is abundant in pre- and post-synapses and is involved in the regulation of synaptic transmission at these sites, thus possibly changing synaptic efficacy[47-48]. Previous animal experiments have shown that actin rearrangement plays an essential role in learning and memory, and its disruption can be caused by the inhibition of the G-actin to F-actin conversion[49-50]. The transformation from G-actin to F-actin is significantly reduced during aging[49-50], and this effect may be associated with the disruption of actin regulatory proteins. Thyroxine promotes the conversion of G-actin to F-actin through its cytoplasmic receptor, which may explain why the ratio of F-actin/G-actin increased after L-T4 administration in the present study.
In summary, our findings suggest for the first time that L-T4 supplement is safe and effective for improving normal aged-related cognitive de fi cits.
Materials and Methods
Design
A randomized and controlled animal experiment.
Time and setting
The experiment was performed at the Department of Biopharmaceutics, School of Pharmaceutical Sciences, Southwest University, China, from March 2010 to December 2012.
Materials
Healthy male CD-1 mice were provided by the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China; license No. SCXK (Jing) 2006-2009). Their body weights were 32 ± 2.0 g for 3-month-old mice (young group; n = 15) and 45 ± 2.3 g for 24-month-old mice (aged group; n = 30). Animals were housed in plastic cages (420 mm × 240 mm × 170 mm) with free access to standard laboratory food and water and kept in a regulated environment 23 ± 1°C. All experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, published by the Ministry of Science and Technology of China[51].
Drugs
L-T4 (Sigma-Aldrich, St. Louis, MO, USA; 0.16 μg/mL) was dissolved in sodium hydroxide (pH 7.4).
Methods
L-T4 treatment
Aged mice were injected (i.p.) with 1.6 μg/kg L-T4[52]daily for 3 consecutive months. The aged control and young control mice were injected with 0.1 mL/g saline in parallel. The mortality of mice in each group was noted.
Morris water maze task for spatial learning and memory in mice
The Morris water maze apparatus (Chengdu Technology & Market Co., Ltd., Chengdu, Sichuan Province, China) was used to test spatial learning and memory, as previously described[53-54]. In brief, each training day consisted of four trials (two in the morning and two in the afternoon) on each training day for a total of 6 days. For each trial on the training day, the animals were randomly placed at four different starting positions at the junction between two adjacent quadrants (the east, north, west, or south poles of the maze). The animals were given 120 seconds to fi nd the submerged platform in opaque water. If an animal could not find the platform within this time-frame, it was guided to the platform. After mounting the platform, the animals were allowed to stay there for 30 seconds. The time that the mousetook to reach the platform was recorded as the escape latency. A probe test was performed 24 hours after the navigation test was completed[53-54]. The platform was removed from the pool, and the mice began from a unique starting location directly opposite the platform. During the probe trial, mice remained in the pool for 120 seconds. All trials were recorded with a digital camera using the computer software of the maze. During the navigation test, the escape latencies of all trials were recorded. Spatial learning was evaluated by calculating the mean escape latency from the four trials on each training day. Furthermore, time spent in the target quadrant was recorded during the probe trial. The ratio of time spent in the target quadrant within 120 seconds was also used to evaluate spatial memory.
Measurement of serum hormone concentrations by ELISA
Blood was collected from every animal after the Morris water maze task, and the serum was separated and stored at -70°C for later use[55]. Concentrations of thyroid stimulating hormone, triiodothyronine (total and free), and thyroxine (total and free) were determined by their respective ELISA kits (Adlitteram Diagnostic Laboratories, San Diego, CA, USA), according to the manufacturer’s instructions. All samples were performed in duplicate, and the intra- and inter-assay variations were less than 10%.
Biochemistry assays for hippocampal ChAT, ACh, and SOD
The assay methods were performed, as previously described[56]. Brie fl y, mice were decapitated, and the hippocampi[57]dissected and stored at -70°C for future use. Homogenate was prepared in cold saline (1,500 × g, 5 seconds, twice centrifugation with a 30 second interval) on ice. The level of ChAT was determined spectrophotometrically, according to Wolfgram[59-60]. The level of ACh was examined, according to Hestrin[58]. The level of SOD was determined by the xanthine oxidase method[59-60]. Protein concentration was determined using the BCA kit (Promage, Fitchburg, Wisconsin, USA). All biochemical parameters were normalized to the total homogenate protein.
Western blot analysis for actin
Subcellular fractionation and actin analysis was performed, as previously described[61-62]. Briefly, after decapitation, the hippocampus was rapidly removed and stored at -70°C until later use. Hippocampal homogenate in 0.32 mol/L sucrose buffer was centrifuged at 1,000 × g for 10 minutes, and the supernatant was further centrifuged at 10,000 × g for 15 minutes to obtain a crude synaptosome fraction (P1). Protein concentration of P1 solution was determined by the BCA kit (Promage). P1 was subsequently dissolved in hypo-osmotic buffer and centrifuged at 25,000 × g for 25 minutes to precipitate a synaptosomal membrane fraction (LP1). To separate F-actin and G-actin, LP1 was lysed in 100 μL of buffer A (1% Triton X-100, 20 mmol/L HEPES, 100 mmol/L NaCl, 2 mmol/L EDTA, 5 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L aprotinin, 1 mmol/L leupeptin, and 1 mmol/L phenylmethyl sulfonylfluoride, pH 7.2) for 1 hour, and then 50 μL of this solution was centrifuged at 10,000 × g for 20 minutes and the remaining volume (50 μL) was collected to determine total actin fraction. The pellet was dissolved in 50 μL of buffer B (15 mmol/L HEPES, 0.15 mmol/L NaCl, 1% SDS, 10 mmol/L EDTA, 1 mmol/L DTT, 5 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L aprotinin, 1 mmol/L leupeptin, and 1 mmol/L phenylmethyl sulfonyl fl uoride, pH 7.2) for 1 hour, and then centrifuged at 10,000 × g for 20 minutes. The G-actin fraction (the fi rst supernatant) and F-actin fraction (the second supernatant) were collected. Equal volume samples were used to detect immunoreactivity of total actin, F-actin, and G-actin by western blot analysis[63-64]. Briefly, samples (20 μg protein per lane) were subjected to SDS-polyacrylamide gel electrophoresis in a 12% gel, and then electrophoretically transferred to polyvinyl di fl ouride membranes (Amersham Pharmacia, Piscataway, NJ, USA). Membranes were blocked with 5% skimmed milk in 10 mmol/L Tris-HCl and 100 mmol/L NaCl (Tris-buffered saline; TBS), containing 0.01% Tween-20 (T; TBST) for 2 hours at room temperature and then respectively immersed with mouse anti-total actin, F-actin, or G-actin antibody (all at 1:5,000; Cell Signaling, Beverly, MA, USA) at 4°C overnight. Membranes were subsequently washed (twice, 15 minutes each) in TBST and incubated for 2 hours with horseradish peroxidase-conjugated goat anti-mouse IgG (1:1,000; Dingguo Biotechnology Company, Beijing, China). Signals were detected by the enhanced chemiluminescence system (Pierce, Rockford, IL, USA). Immunopositive signals were quanti fi ed by Quantity One software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data are expressed as mean ± SEM, and were analyzed by one-way analysis of variance followed by the Dunnett’s multiple comparisons post-hoc test. Data of mice that died naturally were excluded from the statistical analysis. Data were analyzed with SPSS 13.0 software (SPSS, Chicago, IL, USA). Signi fi cance was reached at values of P < 0.05 or P < 0.01.
Acknowledgments:We are grateful for Chen ZB (School of Pharmaceutical Sciences, Southwest University, Chongqing, China) for the suggestions of water maze test.
Author contributions:Fu AL conceived and designed the experiments and wrote the paper. Fu AL and Zhou RM performed the experiment and analyzed the data. Xu XR contributed reagents/materials/analysis tools. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Peer review:The present study firstly proposed that, levothyroxine improved the function of hippocampal neurons in aged rats, and this finding was confirmed by the levothyroxine action mechanism and molecular level of rat hippocampal structure. It is a novel, scientific, well-designed and clearly written paper.
[1] Zheng JW, Zhang SY, Yang C, et al. Current undergraduate and postgraduate dental education in China. J Dent Educ. 2013;77(1): 72-78.
[2] Abdullah B, Wolbring G. Analysis of Newspaper Coverage of active aging through the lens of the 2002 World Health Organization active ageing report: a policy framework and the 2010 Toronto charter for physical activity: a global call for action. Int J Environ Res Public Health. 2013;10(12):6799-6819.
[3] Graewe B, Lemos R, Ferreira C, et al. Impaired processing of 3D motion-de fi ned faces in mild cognitive impairment and healthy aging: an fMRI study. Cereb Cortex. 2013;23(10):2489-2499.
[4] Wijsman LW, de Craen AJ, Trompet S, et al. Subclinical thyroid dysfunction and cognitive decline in old age. PLoS One. 2013;8(3): e59199.
[5] Yang WN, Han H, Hu XD, et al. Te e ff ects of perindopril on cognitive impairment induced by d-galactose and aluminum trichloride via inhibition of acetylcholinesterase activity and oxidative stress. Pharmacol Biochem Behav. 2013;114-115:31-36.
[6] Xi Y, Wang M, Zhang W, et al. Neuronal damage, central cholinergic dysfunction and oxidative damage correlate with cognitive deficits in rats with chronic cerebral hypoperfusion. Neurobiol Learn Mem. 2013;109C:7-19.
[7] Han L, Wu XQ, Meng YF, et al. Listeria-induced host cellular actin cytoskeleton rearrangement and phospholipase D. Weishengwu Xuebao. 2006;46(5):852-855.
[8] Lee WH, Kumar A, Rani A, et al. In fl uence of viral vector-mediated delivery of superoxide dismutase and catalase to the hippocampus on spatial learning and memory during aging. Antioxid Redox Signal. 2012;16(4):339-350.
[9] Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+channels in senescent synaptic plasticity. Prog Neurobiol. 2012;96(3):283-303.
[10] Koohestani F, Brown CM, Meisami E. Postnatal growth hormone de fi ciency in growing rats causes marked decline in the activity of spinal cord acetylcholinesterase but not butyrylcholinesterase. Int J Dev Neurosci. 2012;30(7):578-583.
[11] Hefti F, Hartikka J, Bolger MB. E ff ect of thyroid hormone analogs on the activity of choline acetyltransferase in cultures of dissociated septal cells. Brain Res. 1986;375(2):413-416.
[12] Mori M, Ohshima K, Fukuda H, et al. Changes in the multiple components of rat pituitary TSH and TSH beta subunit following thyroidectomy. Acta Endocrinol (Copenh). 1984;105(1):49-56.
[13] Fu AL, Zhou CY, Chen X. Tyroid hormone prevents cognitive de ficit in a mouse model of Alzheimer’s disease. Neuropharmacology. 2010;58(4-5):722-729.
[14] Hou YY, Lu B, Li M, et al. Involvement of actin rearrangements within the amygdala and the dorsal hippocampus in aversive memories of drug withdrawal in acute morphine-dependent rats. J Neurosci. 2009;29(39):12244-12254.
[15] Bi AL, Wang Y, Li BQ, et al. Region-speci fi c involvement of actin rearrangement-related synaptic structure alterations in conditioned taste aversion memory. Learn Mem. 2010;17(9):420-427.
[16] Hall H, Cuellar-Baena S, Denisov V, et al. Development of NMR spectroscopic methods for dynamic detection of acetylcholine synthesis by choline acetyltransferase in hippocampal tissue. J Neurochem. 2013;124(3):336-346.
[17] Gavrilov LA, Gavrilova NS. Te Biology of Life Span: A Quantitative Approach. Chur, Switzerland: Harwood Academic Publishers. 1991.
[18] Vaupe JW. Trajectories of mortality at advanced ages. In: Wachter KW, Finch CE, eds. Between Zeus and the Salmon: the Biodemography of Longevity. Washington, DC: National Academy Press. 1997.
[19] Maita K, Hirano M, Harada T, et al. Mortality, major cause of moribundity, and spontaneous tumors in CD-1 mice. Toxicol Pathol. 1988;16(3):340-349.
[20] Maggio M, Dall’Aglio E, Lauretani F, et al. Te hormonal pathway to cognitive impairment in older men. J Nutr Health Aging. 2012; 16(1):40-54.
[21] Castellano CA, Laurin D, Langlois MF, et al. Tyroid function and cognition in the euthyroid elderly: a case-control study embedded in Quebec longitudinal study-NuAge. Psychoneuroendocrinology. 2013;38(9):1772-1776.
[22] Cao L, Wang F, Yang QG, et al. Reduced thyroid hormones with increased hippocampal SNAP-25 and Munc18-1 might involve cognitive impairment during aging. Behav Brain Res. 2012; 229(1): 131-137.
[23] Maggio M, Colizzi E, Fisichella A, et al. Stress hormones, sleep deprivation and cognition in older adults. Maturitas. 2013; 76(1): 22-44.
[24] Mooradian AD, Li J, Shah GN. Age-related changes in thyroid hormone responsive protein (THRP) expression in cerebral tissue of rats. Brain Res. 1998;793(1-2):302-304.
[25] De Nayer P, Rennotte B, Caucheteux D. Tyroid hormone receptors in brain and liver during ageing. Horm Metab Res. 1991; 23(1):12-14.
[26] Beydoun MA, Beydoun HA, Kitner-Triolo MH, et al. Tyroid hormones are associated with cognitive function: moderation by sex, race, and depressive symptoms. J Clin Endocrinol Metab. 2013; 98(8):3470-3481.
[27] Muzzio AM, Noyes PD, Stapleton HM, et al. Tissue distribution and thyroid hormone e ff ects on mRNA abundance for membrane transporters Mct8, Mct10, and organic anion-transporting polypeptides (Oatps) in a teleost fi sh. Comp Biochem Physiol A Mol Integr Physiol. 2014;167:77-89.
[28] Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035-3043.
[29] Tohyama K, Kusuhara H, Sugiyama Y. Involvement of multispecific organic anion transporter, Oatp14 (Slc21a14), in the transport of thyroxine across the blood-brain barrier. Endocrinology. 2004; 145(9):4384-4391.
[30] Kassem NA, Deane R, Segal MB, et al. Role of transthyretin in thyroxine transfer from cerebrospinal fl uid to brain and choroid plexus. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1310-1315.
[31] Salthouse TA, Mitchell DR, Palmon R. Memory and age di ff erences in spatial manipulation ability. Psychol Aging. 1989;4(4):480-486.
[32] Obermeyer S, Kolling T, Schaich A, et al. Di ff erences between old and young adults’ ability to recognize human faces underlie processing of horizontal information. Front Aging Neurosci. 2012;4:3.
[33] Foster TC, Defazio RA, Bizon JL. Characterizing cognitive aging of spatial and contextual memory in animal models. Front Aging Neurosci. 2012;4:12.
[34] Michalikova S, Ennaceur A, van Rensburg R, et al. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: e ff ects of low infrared light. Neurobiol Learn Mem. 2008;89(4):480-488.
[35] Magnusson KR, Scruggs B, Aniya J, et al. Age-related de fi cits in mice performing working memory tasks in a water maze. Behav Neurosci. 2003;117(3):485-495.
[36] Tong H, Chen GH, Liu RY, et al. Age-related learning and memory impairments in adult-onset hypothyroidism in Kunming mice. Physiol Behav. 2007;91(2-3):290-298.
[37] Patel J, Landers K, Li H, et al. Tyroid hormones and fetal neurological development. J Endocrinol. 2011;209(1):1-8.
[38] Alzoubi KH, Gerges NZ, Aleisa AM, et al. Levothyroxin restores hypothyroidism-induced impairment of hippocampus-dependent learning and memory: Behavioral, electrophysiological, and molecular studies. Hippocampus. 2009;19(1):66-78.
[39] Zamoner A, Heimfarth L, Oliveira Loureiro S, et al. Nongenomic actions of thyroxine modulate intermediate fi lament phosphorylation in cerebral cortex of rats. Neuroscience. 2008;156(3):640-652.
[40] Resta F, Triggiani V, Barile G, et al. Subclinical hypothyroidism and cognitive dysfunction in the elderly. Endocr Metab Immune Disord Drug Targets. 2012;12(3):260-267.
[41] Santos NC, Costa P, Ruano D, et al. Revisiting thyroid hormones in schizophrenia. J Tyroid Res. 2012;2012:569147.
[42] Smith JW, Evans AT, Costall B, et al. Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev. 2002;26(1):45-60.
[43] Davis PJ, Leonard JL, Davis FB. Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol. 2008;29(2):211-218.
[44] Moeller LC, Broecker-Preuss M. Transcriptional regulation by nonclassical action of thyroid hormone. Tyroid Res. 2011;4 Suppl 1:S6.
[45] Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic e fficacy. Nat Rev Neurosci. 2008; 9(5):344-356.
[46] Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25-55.
[47] Saura CA, Servián-Morilla E, Scholl FG. Presenilin/γ-secretase regulates neurexin processing at synapses. PLoS One. 2011; 6(4):e19430.
[48] Fischer A, Sananbenesi F, Schrick C, et al. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci. 2004;24(8):1962-1966.
[49] Nelson BS, Witty CF, Williamson EA, et al. A role for hippocampal actin rearrangement in object placement memory in female rats. Neurobiol Learn Mem. 2012;98(3):284-290.
[50] Motanis H, Maroun M. Di ff erential involvement of protein synthesis and actin rearrangement in the reacquisition of contextual fear conditioning. Hippocampus. 2012;22(3):494-500.
[51] Te Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animais. 2006-09-30.
[52] Iglesias R, Llobera M, Montoya E. Sequential changes in the pituitary thyroid axis after chronic TRH administration: e ff ects on euthyroid and thyroxine treated female rats. Acta Endocrinol (Copenh). 1985;109(2):237-242.
[53] Zhao BQ, Guo YR, Li XL, et al. Amelioration of dementia induced by Aβ 22-35 through rectal delivery of undecapeptide-hEGF to mouse brain. Int J Pharm. 2011;405(1-2):1-8.
[54] Zhou JP, Feng ZG, Yuan BL, et al. Transduced PTD-BDNF fusion protein protects against beta amyloid peptide-induced learning and memory de fi cits in mice. Brain Res. 2008;1191:12-19.
[55] Todini L, Malfatti A, Salimei E, et al. Measurement of thyroid hormones in donkey (Equus asinus) blood and milk: validation of ELISA kits and evaluation of sample collection, handling and storage. J Dairy Res. 2010;77(4):419-424.
[56] Fu AL, Dong ZH, Sun MJ. Protective e ff ect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory de fi cits in mice. Brain Res. 2006;1109(1):201-206.
[57] Franklin KBJ, Paxinos G. Te Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press. 2007.
[58] Dong Z, Fu A. Prevention of age-related memory de fi cit in transgenic mice by human choline acetyltransferase. Eur J Pharmacol. 2012;683(1-3):174-178.
[59] Sousa T, Pinho D, Morato M, et al. Role of superoxide and hydrogen peroxide in hypertension induced by an antagonist of adenosine receptors. Eur J Pharmacol. 2008;588(2-3):267-276.
[60] Alzoubi KH, Khabour OF, Salah HA, et al. Vitamin E prevents high-fat high-carbohydrates diet-induced memory impairment: the role of oxidative stress. Physiol Behav. 2013;119:72-78.
[61] Niedermayer T, Jégou A, Chièze L, et al. Intermittent depolymerization of actin fi laments is caused by photo-induced dimerization of actin protomers. Proc Natl Acad Sci U S A. 2012;109(27):10769-10774.
[62] Lamprecht R. Te roles of the actin cytoskeleton in fear memory formation. Front Behav Neurosci. 2011;5:39.
[63] Ailing F, Fan L, Li S, et al. Role of extracellular signal-regulated kinase signal transduction pathway in anxiety. J Psychiatr Res. 2008; 43(1):55-63.
[64] Fu A, Zhao Z, Gao F, et al. Cellular uptake mechanism and therapeutic utility of a novel peptide in targeted-delivery of proteins into neuronal cells. Pharm Res. 2013;30(8):2108-2117.
Copyedited by Mark F, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
Correction Announcement
Te information of the fi rst author Yanlin Bi of the article entitled “Adaptive and regulatory mechanisms in aged rats with postoperative cognitive dysfunction” published in Neural Regeneration Research [2014;9(5):534-539] was mistaken during proofreading.
The original information “Yanlin Bi1,2, Shuyun Liu2, Xinjuan Yu3, Mingshan Wu3, Yuelan Wang1” should be “Yanlin Bi1,3, Shuyun Liu2, Xinjuan Yu3, Mingshan Wu3, Yuelan Wang1”.
Hereby Certi fi ed!
Editorial Board of Neural Regeneration Research
10.4103/1673-5374.131602
http://www.nrronline.org/
Accepted: 2014-01-09
杂志排行
中国神经再生研究(英文版)的其它文章
- Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases
- Regulatory effects of anandamide on intracellular Ca2+concentration increase in trigeminal ganglion neurons
- Nasal mucosal inhalation of amyloid-beta peptide 3-10 defective adenovirus attenuates cytotoxicity induced by beta-amyloid (1-42)
- Similar effects of substance P on learning and memory function between hippocampus and striatal marginal division
- Fusion protein of single-chain variable domain fragments for treatment of myasthenia gravis
- Citalopram increases the differentiation ef fi cacy of bone marrow mesenchymal stem cells into neuronal-like cells
